Abstract
Our previous study documented expression of a male-transmitted cytochrome c oxidase subunit II protein (MCOX2), with a C-terminus extension (MCOX2e), in unionoidean bivalve testes and sperm mitochondria. Here, we present evidence demonstrating that MCOX2 is seasonally expressed in testis, with a peak shortly before fertilization that is independent of sperm density. MCOX2 is localized to the inner and outer sperm mitochondrial membranes and the MCOX2 antibody’s epitope is conserved across >65 million years of evolution. We also demonstrate the presence of male-transmitted mtDNA and season-specific MCOX2 spatial variation in ovaries. We hypothesize that MCOX2 plays a role in reproduction through gamete maturation, fertilization and/or embryogenesis.
Keywords: cytochrome c oxidase subunit II, COX2, DUI, C-terminus extension, mtDNA, male-transmitted mitochondria, Unionoidea
1. Introduction
Mitochondrial DNA (mtDNA) inheritance in some bivalves involves distinct maternal and paternal transmission routes and divergent gender-associated mtDNA genomes. Therefore, doubly uniparental inheritance (DUI) of mtDNA is a genetic transmission system that violates the rule of standard maternal inheritance (SMI) of organelles [1]. DUI was discovered in Mytilus and our brief overview is based principally on that genus. In lineages with DUI, mothers transmit mitochondria containing female-transmitted mtDNA (F-type) to both sons and daughters (identical to mothers in SMI systems). Unlike SMI, fathers also transmit mitochondria, containing paternally inherited mtDNA (M-type), to their sons and daughters. However, shortly after fertilization, embryos destined to become female either lose this M-type mtDNA (true homoplasmy) or the amount of M-type mtDNA declines to low levels (functional homoplasmy) [2, 3]. Relatively low levels of M-type are often present in the somatic tissues of males, but it is abundant in testicular tissue [4, 5]. The observed lack of deletions and premature stop codons in M genomes is inconsistent with the hypothesis that M genomes are selfish genetic elements [6]. Specialization for selectively advantageous male reproductive functionality has been offered as an ultimate explanation for the maintenance of male-transmitted mt genomes, with sperm competition hypothesized as the proximate driving force [7, 8]. An alternative model postulates a causal relationship between the retention of paternally transmitted mitochondria and sex determination with the latter being controlled by nuclear genes in the mother [9].
In animals, cytochrome c oxidase subunit II (COX2) is a mitochondrially encoded protein functioning in the inner mt membrane. It belongs to the respiratory chain terminal enzymatic complex (IV) and facilitates transfer of electrons from cytochrome c to molecular oxygen [10]. The M genomes of unionoidean bivalves possess a unique, rapidly evolving coding 3′ extension of cox2, resulting in an ~80% increase in gene length over that in the corresponding F genomes [11, 12]. Using Venustaconcha ellipsiformis as a model system, we demonstrated that the MCOX2e coding extension is expressed as multiple transmembrane helices (TMHs) [13]. Based on the taxonomic conservation of the Mcox2e gene region and the tissue distribution of the extended MCOX2 protein in V. ellipsiformis, we hypothesized functional significance for MCOX2e.
In the present study, we (1) investigated in detail the tissue distribution of MCOX2 in both male and female V. ellipsiformis, (2) studied the seasonal aspects of MCOX2 expression in V. ellipsiformis, (3) utilized immunohistochemistry (IHC) to localize MCOX2 in male and female gonads, (4) identified the sub-cellular location of MCOX2 in sperm, using immuno electron microscopy (IEM) and (5) analyzed testes protein extracts from representatives of six additional genera to determine if MCOX2e is expressed in unionoidean bivalve species other than V. ellipsiformis. Our results indicate, for the first time, a role for a mitochondrion-encoded protein, MCOX2, in male and female reproduction.
2. Methods
2.1. DNA extraction and PCR analyses
DNA extraction and general PCR protocols were performed as described in [14]. The M genome-specific primer pair MCOI 22F and HCO-700dy2, which amplifies the 5′ half of Mcox1 in V. ellipsiformis (716 bp amplicon) [14], was used to assess M-type mtDNA presence/absence in V. ellipsiformis DNA extracts. Amplicons were detected in 2% NuSieve GTG (Cambrex Bio Science Rockland, Inc., Rockland, ME) agarose gels post-stained with SYBR Green® (Invitrogen Corp., Carlsbad, CA).
2.2. Protein sample preparation and Western blots
Male and female tissues stored at −70°C were homogenized in RIPA lysis buffer containing proteolytic inhibitors. Tissue preparations were separated by 12% SDS-PAGE and Western blotting was performed as described in [13].
2.3. Immunohistochemistry
Bivalve tissues were fixed in 1.5% formaldehyde in phosphate-buffered saline (PBS) at 4°C overnight. Immunostaining was performed as described in [13].
2.4. Electron microscopy
IEM (Immunoelectron Microscopy)
Bivalve tissues were fixed in 1.0% glutaraldehyde and 4.0% paraformaldehyde in 0.1M phosphate buffer, pH 7.2, for 4 h and washed 3 times for 10 min in buffer. Tissues were sectioned (70nm), placed on nickel grids and incubated for 15 min in a low molecular weight blocking solution (0.05% glycine in PBS), followed by 30 min in a high molecular weight blocking solution (goat block solution, Electron Microscopy Sciences, Fort Washington, PA) and a 5 min wash in incubation buffer (10mM phosphate buffer, 150 mM NaCl, pH 7.4 with 0.2% BSA-c and 15 mM NaN3). Sections were then incubated with the MCOX2primary antibody (1: 200; [13]) in incubation buffer for 1 h. After 3, 5 min incubation buffer washes, grids were placed in gold solution (10 nm gold in goat anti rabbit serum, diluted 1: 20 in incubation buffer, Electron Microscopy Sciences, Fort Washington, PA) for 2 h. Six, 5 min incubation buffer washes were followed by 3, 5 min. PBS washes. Sections were post fixed in 2.0% glutaraldehyde in PBS for 5 min, washed in PBS for 5 min and 5 times in dH20 for 2 min each. Sections were post stained in uranyl acetate for 15 min and lead citrate for 1 min and viewed with a JEOL 100 CX TEM.
SEM (Scanning Electron Microscopy)
Bivalve tissues were prepared for SEM in 2.5% glutaraldehyde in mussel buffer [15] followed by four phosphate buffer washes and post-fixation in 1.0% OsO4 in the latter buffer. After four phosphate buffer washes, the tissue was dehydrated in a graded series of acetone, critical point dried, mounted on aluminum stubs, sputter coated with gold, and viewed in a JEOL JEM5400 SEM.
3. Results
3.1 Presence of M-type mtDNA in Venustaconcha ellipsiformis ovaries
Using the M genome-specific primer pair, we tested for the presence of M-type genomes in V. ellipsiformis gonadal tissues. The 716 bp Mcox1 amplicons obtained using template DNA from female gonads indicate the presence of M genomes in this tissue (Fig. 1). Mcox1 amplicons derived from testicular DNA were detectable using ethidium bromide staining but the amplicons derived from ovarian DNA necessitated visualization with the more sensitive SYBR Green staining technique.
Figure 1. Presence of M-type mtDNA in Venustaconcha ellipsiformis female gonad.
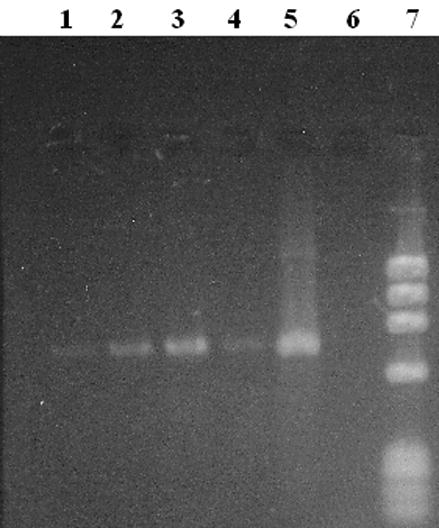
A SYBR Green stained, 2% agarose gel displaying Mcox1 amplicons (716 bp) obtained from V. ellipsiformis female (lanes 1–4) and male (positive control, lane 5) gonad DNA extractions. Twenty microliters (of 50 μl total reaction volumes) was loaded in lanes 1–4 & 6 with 5 μl loaded in lane 5. A negative control is in lane 6 and a phiX174/HaeIII size marker in lane 7.
3.2 Seasonal expression of MCOX2 in testes and ovaries
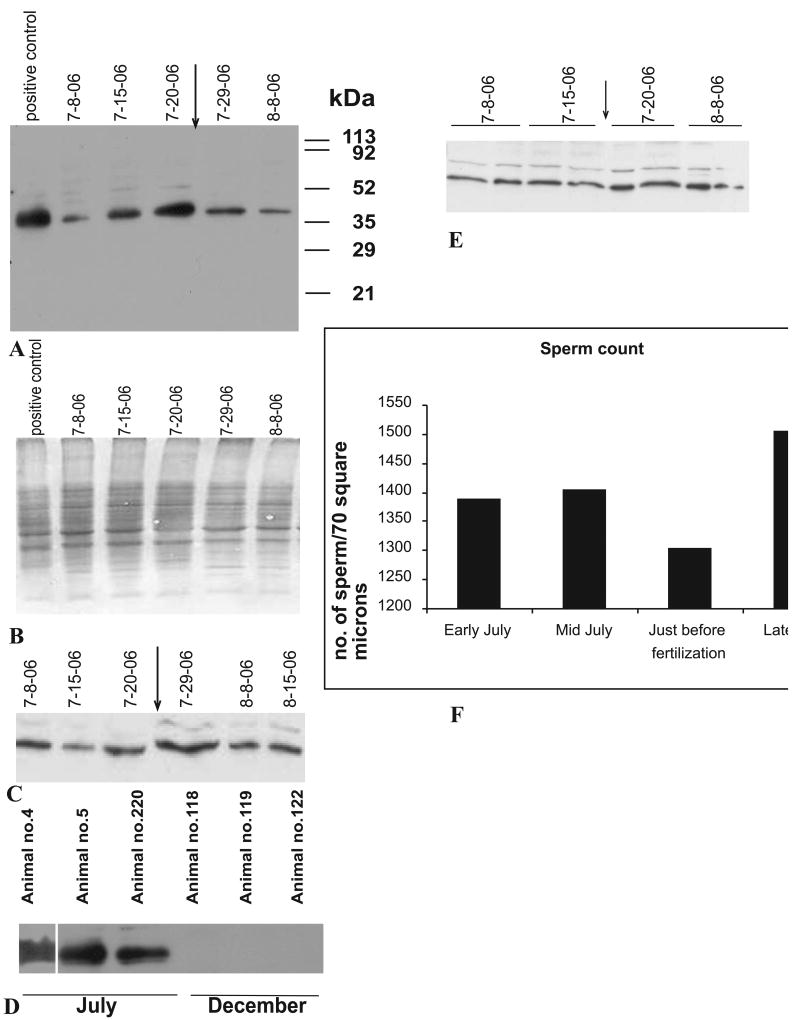
To examine the functional aspects of MCOX2, we investigated its expression in a year-long study of V. ellipsiformis, which produces one brood of young per year. Testes MCOX2 expression was higher during the summer months, with maximum expression observed just prior to fertilization (determined by the appearance of brooded embryos in females [Fig. 2A]). Testicular MCOX2 expression decreased following the appearance of brooded embryos and little to no expression was observed in all non-summer months (Fig. 2A, D). Male somatic tissues (e.g., mantle) and female ovary showed no seasonal pattern in MCOX2 expression (Fig. 2C and 2E, respectively) suggesting that the pre-fertilization spike in MCOX2 expression occurs only in male gonads. Testicular sperm densities, estimated for 18 individuals from 7 μm paraffin sections stained with hematoxylin and eosin, varied little (Fig. 2F) over four sampling dates (covering the 8 July 2006 to 25 July 2006 time period). Additionally, all testicular (N=20) and ovarian (N=8) tissues analyzed via western blotting tested positive for the MCOX2 protein.
Figure 2. Western blots demonstrating the seasonal aspects of MCOX2 expression in Venustaconcha ellipsiformis gonadal and somatic tissues.
(A) Illustrates the testicular MCOX2 expression phenotype in V. ellipsiformis tissues collected on multiple dates before and after the initiation (indicated by arrow) of female brooding of embryos in 2006. The positions of marker proteins are shown (in kDa). (B) SDS gel electrophoresis image of (A). (C) Expression of MCOX2 in mantle tissue from male V. ellipsiformis from the same time points as given in (A). (D) MCOX2 expression phenotypes from three mid-July 2006- vs. three December 2005-collected testes samples. (E) Expression of MCOX2 in V. ellipsiformis ovaries from the same time points (two animals per time point) as in (A). All lanes in (A), (B), (C), (D) and (E) were loaded with an equivalent amount of protein. (F) Graphical representation of mean testicular sperm counts from a total of 18 individuals over four sampling dates.
3.3 Immunohistochemistry of testes and ovaries demonstrates unique spatial distribution of MCOX2
To better understand the spatial expression of MCOX2, we examined MCOX2 in testes and ovaries using immunohistochemistry. Testicular MCOX2 expression showed distinct spatial and temporal patterns (Fig. 3A, B, C). Approximately five weeks prior to fertilization, MCOX2 expression was weak in the testicular acini (arrow pointing to acinar wall) despite the presence of numerous sperm in the acinar lumen (Figs. 3A, D). Two weeks prior to fertilization, MCOX2 expression was strong (Fig. 3B, E) in sperm and acinar walls (arrow), which is in agreement with western blot data (Fig. 2A) and localization of MCOX2 to sperm mitochondria [13]. Approximately 10 days post-fertilization, testicular MCOX2 expression was reduced, despite the presence of sperm in the acini (Figs. 3C, F), and principally appeared in the acinar walls (arrow).
Figure 3. Spatial distribution of MCOX2 in male and female gonads.
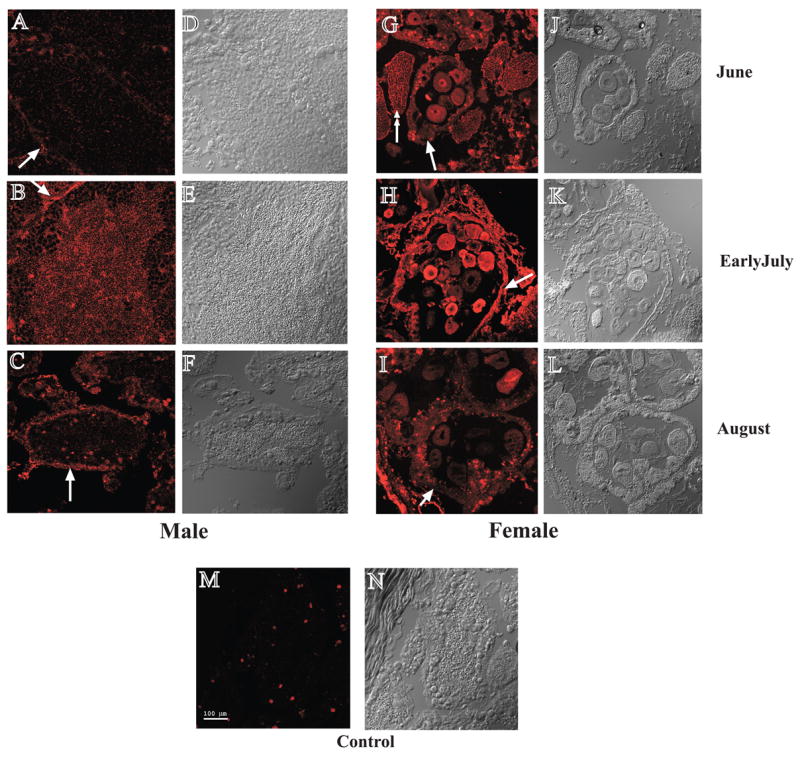
(A-C) MCOX2 expression in male gonads at three time points. Note: distribution of MCOX2 changes from before to after fertilization period with its expression peaking just before fertilization (see Fig. 2 for fertilization timing). Images on the right (D-F) are the corresponding brightfield DIC images of the confocal immunofluorescence images on the left. (G-I) Spatial distribution of MCOX2 in female gonads, at three time points showing maximum expression of MCOX2 in the cytoplasm and nucleoplasm of eggs just prior to fertilization. Images on the right (J-L) are the corresponding brightfield DIC images of the confocal immunofluorescence images on the left. (M-N) Only relatively small, localized areas demonstrated immunoreactivity in male gonads using only secondary antibody. Bar (A-N) = 100 μm
In ovaries, MCOX2 expression remained relatively constant (Fig. 2E) but the spatial distribution appeared to change over the three IHC time points (Fig. 3G, H, I). At the earliest time point, MCOX2 expression was uniform across the ovarian tissue sections (Fig. 3G) with the protein found in eggs, acinar walls (arrow) and muscle fibers (double arrow). Just prior to fertilization, MCOX2 displayed a more heterogeneous localization and was expressed in ovarian acinar walls (arrow) and in some eggs (Fig. 3H). Other eggs displayed low or undetectable levels of MCOX2 expression. After fertilization, high levels of MCOX2 expression were localized in acinar walls (arrow) and in a small number of eggs (Fig. 3I). In eggs, MCOX2 was found in either the cytoplasm, nucleoplasm or in both locations but never in the nucleolus.
3.4 Electron microscopy indicates multiple sub-cellular locations for MCOX2 in sperm
We identified the sub-cellular location of MCOX2 in mature sperm from three unionoidean bivalve species: V. ellipsiformis, Fusconaia subrotunda and Plethobasus cyphyus. Unionoidean spermatozoa contain five large, spherical, radially arrayed mitochondria, which are not covered by a sheath (Fig. 4A). Spermatozoan mitochondria consistently displayed identical MCOX2 antibody-mediated immunogold labeling in our IEM experiments (Fig. 4B). Staining was observed in both the outer and the inner mitochondrial membranes (Fig. 4B), but no signal was detected with the secondary antibody alone (Fig. 4C).
Figure 4. EM photomicrographs demonstrating unionoidean bivalve sperm morphology and the sub-cellular location of MCOX2 in sperm mitochondria.
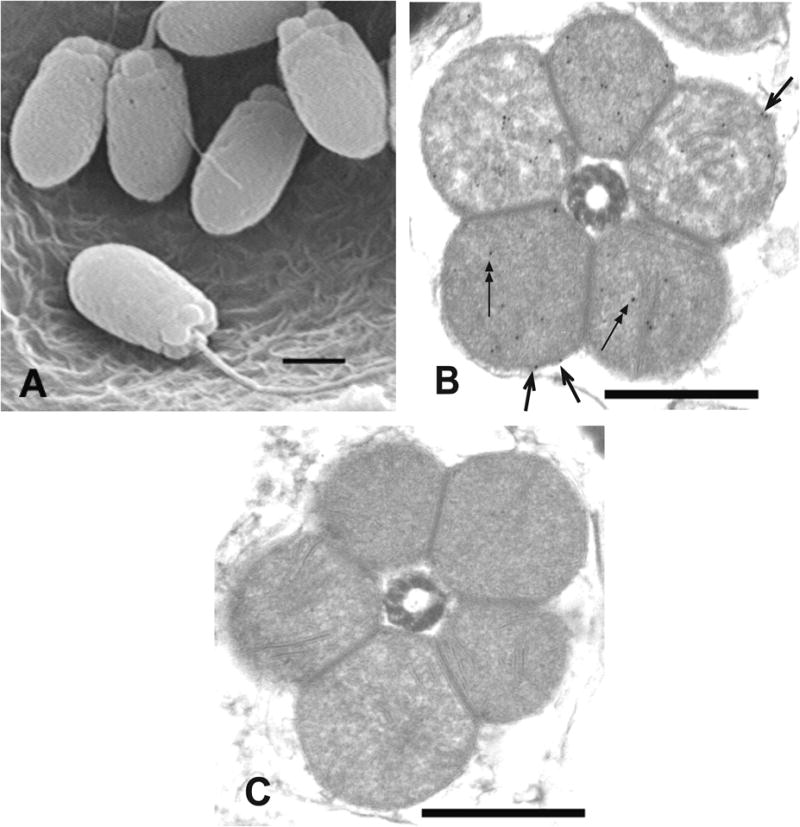
(A) The general morphology of unionoidean bivalve spermatozoa (SEM of Plethobasus cyphyus sperm; scale bar = 1μm). (B) experimental IEM photomicrograph which displays immunogold labeling, using the MCOX2 primary antibody, in both the inner (two headed arrows) and outer (single headed arrows) mt membranes in a cross-section of the mitochondrial “ring” from Fusconaia subrotunda sperm, and (C) a negative control IEM photomicrograph from F. subrotunda sperm (both IEM scale bars = 0.5μm).
3.5 The MCOX2 antibody epitope is conserved across nine unionoidean bivalve genera
To examine if MCOX2 expression is present in other unionoidean bivalve genera, we performed Western blot analysis on testicular extracts from representatives of six additional genera (Fig. 5). The MCOX2 protein was observed around the 36 kDa region in the six additional genera surveyed (Fig. 5). The observed unequal band intensities are likely due to interspecifically variable antibody-epitope affinities and that most tissue samples were collected irrespective of reproductive season.
Figure 5. Evidence for expression of MCOX2 in male gonads from nine unionoidean bivalve genera.
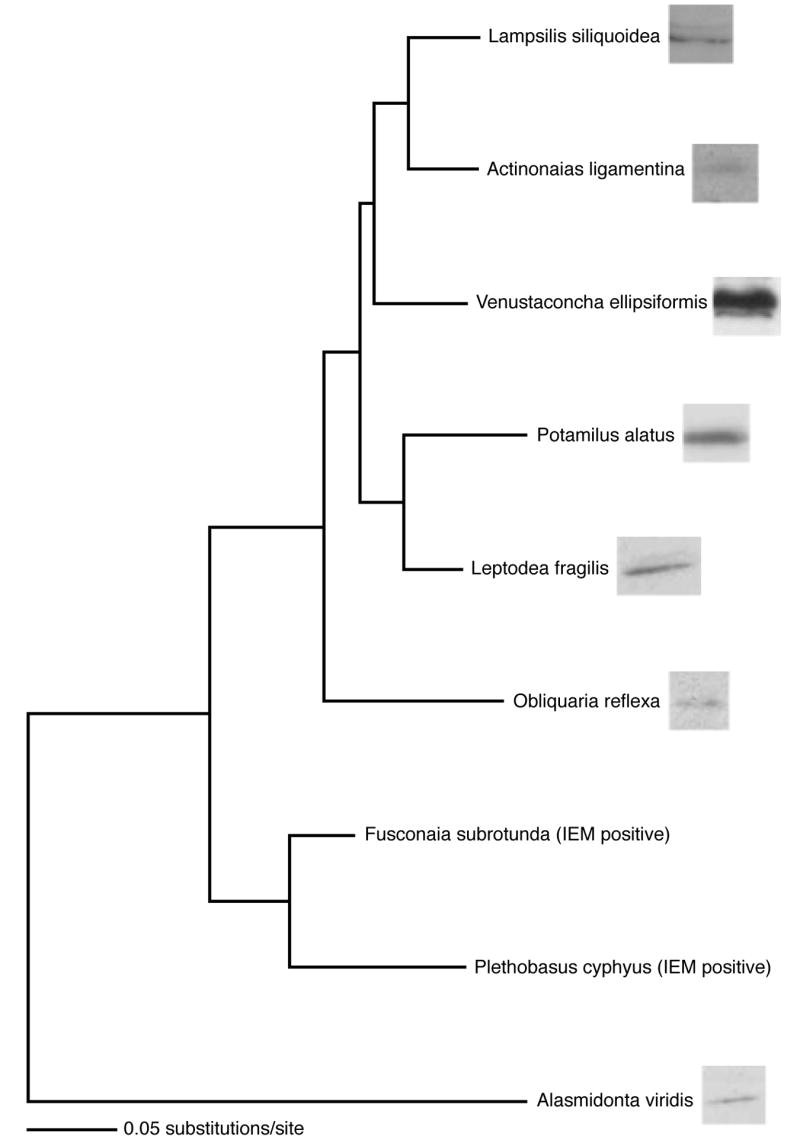
Evolutionary tree depicting species relationships and maximum likelihood-based patristic distances, using Fcox1 DNA sequences, among the nine genera represented in this study (tree after Campbell et al. [24]). Western blot analysis lanes are presented after the species names and show MCOX2 expression in male gonads representing seven genera. In Fusconaia and Plethobasus, MCOX2 expression is evidenced by IEM results. The divergence of these genera occurred over an evolutionary history >65 myr. Equal amounts of protein were loaded in all lanes.
4. Discussion
Our seasonal study of MCOX2 expression and histological localization in V. ellipsiformis provides insights into the functional aspects of MCOX2 (Figures 2 & 3). The seasonal peak in testicular MCOX2 expression (both WB and IHC data) immediately prior to fertilization and independent of sperm density, its low expression in testes during the winter months (= non-reproductive season) and the low, uniform expression in male somatic tissues suggests that MCOX2 functions in male reproduction (e.g., sperm maturation, fertilization and/or embryogenesis). The apparent over-expression of COX2 during spermatogenesis in rats and humans [16, 17], as well as in bivalves, suggests that COX2’s involvement in male reproduction may be a relatively ancient and general phenomenon.
The presence of M-type mtDNA and MCOX2 in V. ellipsiformis ovaries, as well as the former in Mytilus galloprovincialis ovaries [3], suggests that adult females in DUI systems are heteroplasmic for the F- and M-type mt genomes. Thus, M genomes derived from their fathers’ sperm are not completely lost in adult females, but rather persist and are expressed in ovarian tissue. Whether the “native” M-type mtDNA located in ovaries is typically transmitted to progeny [3] or represents an evolutionary dead-end is unclear. Our previous failure [13] to detect the presence of MCOX2 in female gonadal tissues was likely due to the small number of samples analyzed and the spatial heterogeneity of MCOX2 localization in ovarian tissue. Although the overall level of MCOX2 expression in female gonad does not fluctuate substantially over the oogenenic cycle, MCOX2 spatial distribution does vary with the timing of reproduction. The localization of MCOX2 in eggs occurs immediately prior to fertilization and is consistent with a reproductive role for MCOX2 in females.
As expected, our IEM findings support an inner mt membrane function for MCOX2. These observations are consistent with the hypothesis that unionoidean bivalve MCOX2 participates in electron transport chain (ETC) processes. Surprisingly, our IEM experiments also indicate an outer mt membrane location for MCOX2 (Fig. 4), consistent with a novel, non-ETC function for the protein in sperm mitochondria. Given the exposed, outer membrane location of MCOX2 (Fig. 4) and the TMH structure of the MCOX2e region [13], the protein may serve to “tag” sperm-derived mitochondria to enable gender-specific mt localizations similar to that observed in Mytilus [18, 19]. The conservation of the MCOX2 antibody’s epitope (located in the MCOX2e C-terminus tail) over >65 myr of evolutionary history (Fig. 5) is consistent with the hypothesis that the mt surface tagging involves the C-terminus tail of MCOX2e.
Given our evidence for spatial and temporal heterogeneity of MCOX2 expression, we can address the hypotheses regarding the maintenance of male-transmitted genomes under DUI. The “specialization for selectively advantageous male reproductive functionality” hypothesis is unlikely given the biparental maximal just before fertilization gamete expression phenotypes of MCOX2. An obligate functionality for MCOX2 in eggs would oppose any selective pressure on the M genomes to increase male fitness at the expense of female fitness. Alternatively, the “participates in sex determination” hypothesis for the maintenance of male-transmitted genomes is still viable.
Overall, these findings suggest opportunities for new insights into the evolutionary processes responsible for the origin and maintenance of organellar genetic transmission systems. The novel expression and localization patterns of MCOX2 and the over-expression of COX2 [16, 17] are consistent with the ability of mtDNA-encoded gene products to participate in non-ETC processes [20–22], including germ line formation [23]. Experimental studies, in conjunction with evolutionary genetic analyses, are needed to further clarify the functions of the MCOX2 protein and the mt transmission genetics of DUI-containing species.
Acknowledgments
We would like to thank Mike Model (Department of Biological Sciences, KSU) for his assistance in Confocal Microscopy. This work was supported by grants from NIH (HD38520, to S.V.), NSF (DEB0237175, to W.R.H.), NSERC (Discovery Grant 217175 to D.T.S.) and a Saginaw Valley State University Professional Growth Grant (to S.P.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breton S, Beaupré HD, Stewart DT, Hoeh WR, Blier PU. The unusual system of doubly uniparental inheritance of mtDNA: isn’t one enough? Trends in Genetics. 2007;23:465–474. doi: 10.1016/j.tig.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland B, Stewart D, Kenchington ER, Zouros E. The fate of paternal mitochondrial DNA in developing female mussels, Mytilus edulis: Implications for the mechanism of doubly uniparental inheritance of mitochondrial DNA. Genetics. 1998;148:341–347. doi: 10.1093/genetics/148.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obata M, Sano N, Kawamura K, Komaru A. Inheritance of two M type mitochondrial DNA from sperm and unfertilized eggs to offspring in Mytilus galloprovincialis. Development, Growth and Diff. 2007;49:335–344. doi: 10.1111/j.1440-169X.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 4.Garrido-Ramos MA, Stewart DT, Sutherland BW, Zouros E. The distribution of male-transmitted and female-transmitted mitochondrial DNA types in somatic tissues of blue mussels: Implications for the operation of doubly uniparental inheritance of mitochondrial DNA. Genome. 1998;41:818–824. [Google Scholar]
- 5.Dalziel AC, Stewart DT. Tissue-specific expression of male-transmitted mitochondrial DNA and its implications for rates of molecular evolution in Mytilus mussels (Bivalvia: Mytilidae) Genome. 2002;45:348–355. doi: 10.1139/g01-159. [DOI] [PubMed] [Google Scholar]
- 6.Mizi A, Zouros E, Moschonas N, Rodakis GC. The complete maternal and paternal mitochondrial genomes of the Mediterranean mussel Mytilus galloprovincialis: implications for the doubly uniparental inheritances mode of mtDNA. Mol Biol Evol. 2005;22:952–967. doi: 10.1093/molbev/msi079. [DOI] [PubMed] [Google Scholar]
- 7.Zeh JA, Zeh DW. Maternal inheritance, sexual conflict and the maladapted male. Trends in Genetics. 2005;21:281–286. doi: 10.1016/j.tig.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Belknap Press; Massachusetts: 2006. pp. 1–632. [Google Scholar]
- 9.Saavedra C, Reyero MI, Zouros E. Male-dependent doubly uniparental inheritance of mitochondrial DNA and female-dependent sex-ratio in the mussel Mytilus galloprovincialis. Genetics. 1997;145:1073–1082. doi: 10.1093/genetics/145.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritcher OMH, Ludwig B. Cytochrome c oxidase – structure, function, and physiology of a redox-driven molecular machine. Rev Physiol Biochem Pharmacol. 2003;147:47–74. doi: 10.1007/s10254-003-0006-0. [DOI] [PubMed] [Google Scholar]
- 11.Curole JP, Kocher TD. Ancient sex-specific extension of the cytochrome c oxidase II gene in bivalves and the fidelity of doubly-uniparental inheritance. Mol Biol Evol. 2002;19:1323–1328. doi: 10.1093/oxfordjournals.molbev.a004193. [DOI] [PubMed] [Google Scholar]
- 12.Curole JP, Kocher TD. Evolution of a unique mitotype-specific protein coding extension of the cytochrome c oxidase II gene in freshwater mussels (Bivalvia: Unionoida) Mol Biol Evol. 2005;61:381–389. doi: 10.1007/s00239-004-0192-7. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti R, Walker JM, Stewart DT, Trdan RJ, Vijayaraghavan S, Curole JP, Hoeh WR. Presence of a unique male-specific extension of C-terminus to the cytochrome c oxidase subunit II protein coded by the male-transmitted mitochondrial genome of Venustaconcha ellipsiformis (Bivalvia: Unionoidea) FEBS Lett. 2006;580:862–866. doi: 10.1016/j.febslet.2005.12.104. [DOI] [PubMed] [Google Scholar]
- 14.Walker JM, Bogan AE, Bonfiglio EA, Campbell DC, Christian AD, Curole JP, Harris JH, Wojtecki RJ, Hoeh WR. Primers for amplifying the hypervariable, male-transmitted COII-COI junction region in amblemine freshwater mussels (Bivalvia: Unionoidea: Ambleminae) Molecular Ecology Notes. 2007;7:489–491. [Google Scholar]
- 15.Misamore MJ, Lynn JW. Role of the cytoskeleton in sperm entry during fertilization in the freshwater bivalve Dreissena polymorpha. Biological Bulletin. 2000;199:144–156. doi: 10.2307/1542874. [DOI] [PubMed] [Google Scholar]
- 16.Saunders PTK, Millar MR, West AP, Sharpe RM. Mitochondrial cytochrome c oxidase II messenger ribonucleic acid is expressed in pachytene spermatocytes at high levels and in a stage-dependent manner during spermatogenesis in the rat. Biol of Reprod. 1993;48:57–67. doi: 10.1095/biolreprod48.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Liang G, Zhang DX, Wang LJ, Sha YS, Zhang JC, Miao SY, Zong SD, Wang LF, Koide SS. Identification of differentially expressed genes of primary spermatocyte against round spermatid isolated from human testis using the laser capture microdissection technique. Cell Res. 2004;14(6):507–512. doi: 10.1038/sj.cr.7290254. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Kenchington E, Zouros E. Differential segregation patterns of sperm mitochondria in embryos of the blue mussel (Mytilus edulis) Genetics. 2004;166:883–894. doi: 10.1534/genetics.166.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogswell AT, Kenchington ELR, Zouros E. Segregation of sperm mitochondria in two- and four cell embryos of the blue mussel Mytilus edulis: implications for the mechanism of doubly uniparental inheritance of mitochondrial DNA. Genome. 2006;49:799–807. doi: 10.1139/g06-036. [DOI] [PubMed] [Google Scholar]
- 20.Loveland B, Wang CR, Yonekawa H, Hermel E, Lindahl KF. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990;60:971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- 21.Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondria l protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci USA. 2004;101:6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadacharan SK, Singh B, Bowes T, Gupta RS. Localization of mitochondrial DNA encoded cytochrome c oxidase subunits I and II in rat pancreatic zymogen granules and pituitary growth hormone granules. Histochem Cell Biol. 2005;124:409–421. doi: 10.1007/s00418-005-0056-2. [DOI] [PubMed] [Google Scholar]
- 23.Iida T, Kobayashi S. Essential role of mitochondrially encoded large rRNA for germ-line formation Drosophila embryos. Proc Natl Acad Sci USA. 1998;95:11274–11278. doi: 10.1073/pnas.95.19.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell DC, Serb JM, Minton RL, Lydeard C. Phylogeny of North American amblemines (Bivalvia, Unionoida): prodigious polyphyly proves pervasive across genera. Invert Biol. 2005;124:131–164. [Google Scholar]