Abstract
Many unpalatable butterfly species use coloration to signal their distastefulness to birds, but motion cues may also be crucial to ward off predatory attacks. In previous research, captive passion-vine butterflies Heliconius mimetic in colour pattern were also mimetic in motion. Here, I investigate whether wing motion changes with the flight demands of different behaviours. If birds select for wing motion as a warning signal, aposematic butterflies should maintain wing motion independently of behavioural context. Members of one mimicry group (Heliconius cydno and Heliconius sapho) beat their wings more slowly and their wing strokes were more asymmetric than their sister-species (Heliconius melpomene and Heliconius erato, respectively), which were members of another mimicry group having a quick and steady wing motion. Within mimicry groups, wing beat frequency declined as its role in generating lift also declined in different behavioural contexts. In contrast, asymmetry of the stroke was not associated with wing beat frequency or behavioural context—strong indication that birds process and store the Fourier motion energy of butterfly wings. Although direct evidence that birds respond to subtle differences in butterfly wing motion is lacking, birds appear to generalize a motion pattern as much as they encounter members of a mimicry group in different behavioural contexts.
Keywords: locomotor mimicry, insect flight, mimetic behaviour, mutualism, bird vision, Müllerian mimicry
1. Introduction
Wing pigmentation can cause an insect to blend into its background environment (Cuthill et al. 2005) or serve to warningly signal that they are unprofitable to predators. Mimicry between species in wing coloration has been a central theme of evolutionary theory for more than a century (Bates 1862; Mu¨ller 1879; Sheppard et al. 1985). In his classic paper, Bates (1862) suggested that mimicry should extend to motion, but the potential for subtle similarities between mimetic butterfly species in locomotor behaviour that are invisible to humans have only recently been recognized (Srygley & Ellington 1999a). Heliconius butterflies engage in a variety of daily activities including foraging for pollen (Gilbert 1972) and nectar at flowers, searching for hostplants, courting and congregating at specific sites to roost overnight (Mallet & Gilbert 1995). In this paper, I investigate the components of wing motion as potential warning signals of butterflies in the behavioural contexts constituent of their daily activities.
Natural selection will favour the predator's generalization of signals associated with survival to include background contexts other than those in which they are learned (Bouton 1993). If birds that perceive and memorize motion forage between environments, then wing motion would serve butterflies optimally if it were independent of environmental and behavioural contexts. However in courtship, species-specific signals might be particularly important for females to distinguish a male of their own species from a co-mimic. Selection on courtship behaviours might then reinforce differences between colour mimics (Jiggins et al. 2002).
2. Material and methods
Sympatric in a lowland tropical rainforest of Panama, two distasteful, brightly coloured species Heliconius cydno chioneus and Heliconius melpomene rosina are members of the sylvaniform lineage (Lee et al. 1992), and two other distasteful, brightly coloured species Heliconius sapho candidus and Heliconius erato petiverana are members of the pupal-mating lineage (so-called because males often mate with females while they emerge from their pupal cases). Although H. cydno and H. melpomene are sister-species, H. cydno appears like H. sapho of the pupal-mating lineage, and H. melpomene appears like H. erato (figure 1). Hence in a remarkable case of Mu¨llerian mimicry, sister species have diverged in coloration from one another and converged on the coloration of one of the members of the second pair of sister-species. This divergence of sisters and convergence of co-mimics (co-mimics are the distasteful species that comprise a mimicry group) permit us to partition variation in wing motion among the effect of arising from distinct lineages and that of being subject to a different selective regime, made evident a priori by the colour mimicry group to which each individual belongs.
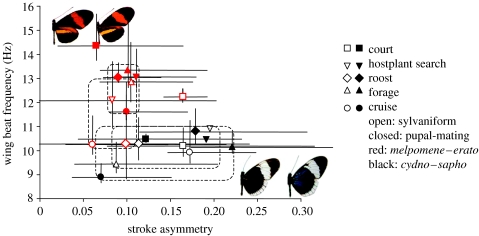
Figure 1.
The difference among sister species and mimicry groups in asymmetry ratio and wing beat frequency is largely independent of behavioural contexts. Heliconius butterflies were filmed conducting different flight behaviours which are generally associated with different flight speeds, but only cruising was significantly different in wing beat frequency and none were different in asymmetry ratio. The dashed lines encircle the behaviours of H. melpomene (open, red symbols) and H. erato (closed, red symbols), except courting. The dashed and dotted lines encircle H. cydno (open, black symbols) and H. sapho (closed, black symbols). The H. melpomene–H. erato mimicry group has high wing beat frequencies and low asymmetry ratios, whereas the H. cydno–H. sapho group has low wing beat frequencies and high asymmetry ratios. Sister species H. melpomene and H. cydno are pictured on the left of each mimicry group, and pupal-maters H. erato and H. sapho are on the right. To the left or below each point are the standard errors, and to the right or above each point are the standard deviations for the two wing motion parameters.
Heliconius co-mimics roost in aggregations in the evening. From these roosts they cruise between flowers where they forage for pollen and nectar principally in the morning hours. Near midday, females begin to search for passion-vine Passiflora hostplants to lay their eggs, whereas males search for and court females. Although pupal-mating males search for female pupae and await their emergence, they also seek unmated and previously mated females. In the late afternoon, butterflies aggregate at roost sites of dead twigs and flutter slowly in the vicinity prior to hanging upside down to rest.
These five flight behaviours have rather distinct forward velocities. Males hover in courtship; females fly very slowly to search for hostplants. In the late afternoon when near the roost, butterflies fly slowly and repeatedly land on dead twigs. Forward velocities when foraging at flowers vary from nearly hovering over flowers to flying at moderate speeds between them. Cruising represents the fastest velocities. At slower velocities, air circulation to offset body weight must be generated by the flapping wings, whereas at faster velocities, airflow across the wings provide the circulation necessary to generate lift with less physical effort (Srygley & Ellington 1999b). Since wing beat frequency should decrease with flight speed, I predicted that the order of wing beat frequency from high to low would be: court; hostplant search; roost; forage and cruise. Lacking an aerodynamic function, asymmetry ratio (i.e. deviation from a sinusoidal wing stroke) should not vary with flight speed. However, it may differ in courtship if it is a component of the mating signal.
Over a 1-year period (September 2001–September 2002) and between June and August 2005, I filmed butterflies with a Redlake Motionmeter highspeed video camera (generally at 250 frames s−1). They were filmed foraging at flowers, searching for hostplants, courting, and roosting both in natural outdoor settings in lowland rainforest edges and in a large, black plastic net insectary with a white reinforced-nylon roof in Gamboa, Panama or in a black plastic screen insectary housed within a glass greenhouse in San Juan, Puerto Rico (H. erato only). Butterflies were filmed cruising in natural outdoor settings without prior handling or over Lake Gatún following release over the lake (which forms a part of the Panama Canal). Once the insect began to fly towards shore, I pursued the insect in a boat and filmed it from the bow as it flew. Wing beat frequency and elevation of the wing on the stroke plane are best observed when the wing is moving in a plane perpendicular to the camera lens. Hence, I generally held the camera level to the ground and aimed the lens along the body axis. Sequences in which the insect's anterior–posterior body axis remained close to a vertical plane parallel to the camera lens were selected for digitization. For those insects that were digitized in more than one context, I randomly selected a single sequence. A total of 99 sequences among the four species and five behaviours were analysed (see table in electronic supplementary material).
I calculated the elevation of the insect's wings in each frame of the sequence. Heliconius butterflies flap both wing pairs at the same time such that the downstroke and upstroke begin together. Assuming the forewing tips were at the same elevation relative to their stroke planes, I calculated wing elevation independent of the insect's roll away from vertical. Wing elevation data for an entire sequence were smoothed with a Fourier analysis (Srygley & Ellington 1999a). I calculated the root mean square (RMS) difference between the observed values for wing elevation and those predicted by a Fourier series that fit the fundamental (first harmonic) searching for a minimum RMS while varying wing beat frequency by ±0.1 Hz. At this wing beat frequency, I fit the second, third and fourth harmonic to the observed data to find the series that minimized the RMS. I then varied wing beat frequency again by ±0.1 Hz steps to assure that the wing beat frequency and the series were the best fit to the data. The asymmetry ratio was calculated as one less the Fourier coefficient of the fundamental divided by the sum of the Fourier coefficients for the fundamental and any higher harmonics included in the series. Hence, the asymmetry ratio is zero when the elevation of the wing plotted over time is sinusoidal and best explained by the fundamental without any higher harmonics. As higher harmonics enter, the elevation of the wing varies away from sinusoidal and the stroke becomes more asymmetric.
3. Results
The lineage from which an individual arose was a marginal factor determining wing motion, whereas the mimicry group to which it was a member was highly significant (multivariate ANOVA, table 1). Sylvaniforms had, on an average, a lower wing beat frequency than pupal-maters, but there was no apparent difference in asymmetric motion of the wings among the lineages (Univariate ANOVA's, table 1). Above and beyond this effect of common descent, the mimicry group comprising H. cydno and H. sapho had, on an average, a lower wing beat frequency and more asymmetric strokes than that comprising H. melpomene and H. erato (figure 1 and univariate tests, table 1). Thus, wing beat frequency and subtle motions of the wing within the strokes are associated with colour mimicry. Wing beat frequency has an aerodynamic function, but may also serve as a cue to the birds, whereas asymmetric motion of the wings has no known aerodynamic function and is most likely to be a component of the mimetic signals.
Table 1.
Two-way MANOVA for the dependent variables wing-beat frequency and asymmetry ratio.
| univariate F-tests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| multivariate test statistics | wing beat frequency | asymmetry ratio | ||||||||
| source of variation | Wilks' lambda | F | d.f. | p | % explained | F | p | % explained | F | p |
| lineage | 0.928 | 3.014 | 2, 78 | 0.0548 | 3.3 | 6.058 | 0.0160 | 0.0 | 0.000 | 0.9899 |
| mimicry | 0.612 | 24.717 | 2, 78 | <0.0001 | 26.0 | 47.352 | <0.0001 | 5.1 | 5.227 | 0.0249 |
| behaviour | 0.737 | 3.217 | 8, 156 | 0.0021 | 13.3 | 9.022 | 0.0003 | 2.3 | 0.585 | 0.6747 |
| lineage×mimicry | 0.927 | 3.064 | 2, 78 | 0.0523 | 3.4 | 6.163 | 0.0152 | 0.2 | 0.196 | 0.6589 |
| lineage×behaviour | 0.878 | 1.312 | 8, 156 | 0.2411 | 2.4 | 1.074 | 0.3751 | 6.0 | 1.533 | 0.2008 |
| mimicry×behaviour | 0.873 | 1.367 | 8, 156 | 0.2151 | 5.7 | 2.645 | 0.0395 | 0.6 | 0.164 | 0.9559 |
| three-way interaction | 0.843 | 1.738 | 8, 156 | 0.0940 | 2.4 | 1.057 | 0.3836 | 9.0 | 2.314 | 0.0647 |
Wing motion varied significantly with behaviours conducted by Heliconius (MANOVA, table 1). Wing beat frequencies were significantly different among behaviours, but asymmetry ratios did not differ (univariate tests, table 1). In the a posteriori multiple comparison of the means, only cruising butterflies (figure 2) had significantly lower wing beat frequencies relative to the other behaviours. For comparison, a sample movie of each species foraging and the corresponding Fourier reconstruction of the wave form may be downloaded from the electronic supplementary material. The order with respect to the means was: hostplant search (12.4 Hz)a; court (12.3 Hz)a; roost (12.1 Hz)a; forage (11.4 Hz)a; and cruise (10.4 Hz)b (different superscripts denote significant differences at p<0.05).
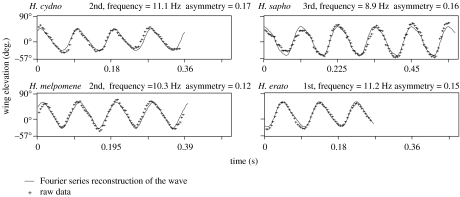
Figure 2.
Fourier reconstruction of the wing strokes for cruising Heliconius. Rows constitute mimicry groups and columns constitute sister species. In the title for each graph, I denote the largest harmonic required to minimize the root mean square difference between the data and the wave form as first, second or third; the wing beat frequency, and the asymmetry ratio, which is the deviation of the wing motion from a sinusoidal wave form (i.e. the variation in the motion explained by the addition of the harmonics). Wing elevation is 0° when horizontal, 90° when fully elevated and negative when below horizontal. The axis for time varies depending on the wing beat frequency.
The interactions of mimicry and behaviour, and lineage and behaviour were not significant overall (MANOVA, table 1). Hence, there was no evidence in this study that butterflies within mimicry groups or within lineages behaved differently when courting. However, in figure 1, courting H. erato and H. melpomene males are well separated in wing motion from that during other behaviours. In addition, they are well separated from one another, whereas H. sapho and H. cydno are more similar to one another when courting. There was a marginal effect of the interaction of lineage and mimicry. Wing beat frequency was influenced by this interaction more so than asymmetry ratio (univariate tests, table 1).
4. Discussion
Wing beat frequency varied with behavioural context as predicted from its functional role in generating lift to hold the insect aloft. Other than differences between hovering and forward flight in hawkmoths (Willmott & Ellington 1997), there has been little research on whether wing kinematics vary from one behavioural context to another in any insect species. In addition to its functional role, wing beat frequency was consistently associated with mimicry, and so it may serve as an important cue of palatability to predators. Differences in wing beat frequency might be attributed to differences in body temperature between mimicry groups (Srygley & Chai 1990), but from an earlier study conducted in artificial environments, these two Heliconius mimicry groups differed in wing beat frequency although there were no differences in body temperature among the species (Srygley 1999a). In addition, one would predict a different order of wing beat frequencies with behaviours. If wing beat frequency were attributed to body temperature, then cruising would be predicted to have the fastest wing beat frequencies and roosting the slowest, contrary to what was observed. The ability of birds to perceive differences in wing beat frequency of the order of those exhibited by the Heliconius mimicry groups has not been investigated previously, but starlings (Sturnus vulgaris), a generalist insectivore, were capable of accurately measuring the passage of time in a foraging situation to the minimum tested time-interval (0.8 s, Brunner et al. 1992).
Asymmetry ratio was consistently associated with mimicry, and in contrast to wing beat frequency, it was independent of behavioural context or handling effects. It was also independent of wing beat frequency (r=−0.157, p=0.123), which further indicates that asymmetry ratio is not important to vortex generation for lift (Srygley & Thomas 2002). Unconstrained by flight behaviour and wing kinematics, variation from nearly sinusoidal to more asymmetric wing motion is more likely to evolve as a result of direct selection on signalling behaviours by predators. Although only 5% of the variance in asymmetry ratio was explained by mimicry, when combined with wing beat frequency, 42% of the variance in wing kinematics discriminated mimicry groups. Thus, it is probably not asymmetry ratio alone, but the combination of asymmetry ratio and wing beat frequency that is learned by predators. Asymmetry ratio is a reliable signal that can be generalized across ecological contexts. Thus, it may serve as the signature which verifies similarities in wing beat frequency among mimics. Taken together, the wave may serve to identify the mimics before the birds can see details in colour pattern, much as friends may be identified by their manner of walking before details in facial features are evident (Cutting 1977; Jukisch et al. 2006). One component of the signal may enhance learning of a second stimulus and evoke a stronger reaction than if a single stimulus was learned on its own (Rowe & Guilford 1996; Srygley 1999b).
I have inferred from the convergence of wing motion within mimicry groups that birds not only distinguish subtle differences in wing motion but also select for them in association with mimicry. However to date, no direct evidence that birds respond to subtle differences in wing motion exists. Both theoretical models and empirical evidence indicate that motion is perceived as the Fourier energy of a luminance pattern moving in space (Adelson & Bergen 1985; Wilson et al. 1992). In pigeons (Columba livia), neurons in the basal optic nucleus are most sensitive to vertical translation (Wylie et al. 1998). These neurons respond to the leading edge of a single dark object moving through their excitatory field (Wang et al. 2000) as well as large-field motion. In contrast, pigeon tectal neurons sensitive to velocity respond preferentially to horizontal motion, whereas tectal neurons sensitive to acceleration are direction insensitive. In pigeons, acceleration-encoding neurons in the optic tectum respond to sinusoidal modulation of velocity (Cao et al. 2004), and in Anolis lizards, the optic tectum is stimulated by sinusoidal motion such as the head-bobbing display that males perform (Persons et al. 1999). Both the basal optic nucleus and the tectum are involved in the optokinetic response which holds objects steady in the visual field. Since birds often catch butterflies by the flapping wings, they must visually process the wing's acceleration to predict its future position. Visual processing must be at least 2–4 times faster than the human eye (Srygley & Ellington 1999a). Birds process vertical motion of the wing in the basal optic nucleus and acceleration in the optic tectum. These regions interact and probably operate in coordination for optokinesis (Wang et al. 2000).
I have assumed that spectral composition, i.e. the frequency components of the wave, are processed by the optic system and that there is no information in the phase differences between the harmonics. In the human auditory system, sound waves composed of the same Fourier coefficients but at different phase angles are perceived to be the same (Traux 1999), and yet we are not incapable of detecting differences in phase. Binaural comparison of sound arrival to the ears is a phase comparison used to determine the direction of a sound source. Our understanding of avian vision is limited, and the importance of temporal information in the higher harmonics remains equivocal. Carefully designed, conditioning experiments are needed to tease apart the abilities of insectivorous birds to discriminate spectral and temporal information in waves characteristic of the wing motion of butterflies and other insects.
Convergence of wing beat frequency and asymmetry ratio within mimicry groups suggests that visual processing of Fourier motion energy of the wings is the first step which allows birds to compare differences in wing beat frequency and wing acceleration. The combination of coloration and behavioural traits associated with locomotor mimicry represents a relatively unexplored facet of the adaptive peaks towards which mimetic prey converge as a result of the selection pressure applied by avian predators (Srygley & Ellington 1999b; Srygley 2004). A peak shift in predator cognition, resulting in a shift in the prey's adaptive landscape, is enhanced by multispecies communities (Yachi & Higashi 1998) and multicomponent signals (Rowe & Guilford 1996). The benefit of Müllerian mimicry is also enhanced by the former (Beatty et al. 2004), but we do not yet know if it is enhanced by the latter.
Acknowledgements
A Senior Postdoctoral Fellowship from the Smithsonian Institution, an equipment grant from the Royal Society London, and a research grant from the Association for the Study of Animal Behaviour supported the research. I thank M. Speed for commenting on the manuscript, C. Ellington for advice on studying insect flight, E. Leigh and the staff at Smithsonian Tropical Research Institute for hosting me in Panama, and C. Jiggins and O. McMillan for permitting me to film Heliconius in their insectaries. The Autoridad Nacional del Ambiente (ANAM) granted permission to conduct the research in Panama. The Korean Science and Engineering Federation (KOSEF) Brain Pool program supported the preparation of the manuscript while I was a Visiting Professor in the Behaviour and Ecology Laboratory at Seoul National University.
Supplementary Material
Refer to the footnotes for abbreviations and the formula for wing motion
These selected high speed (250 fps) videos of butterflies foraging in natural settings, for which the first frame is shown on the right in the Powerpoint file (and the video can be seen by downloading the .mov or .dv file with the name of the species), arerendered into graphs of the Fourier wave form of the wing motion on the left (Srygley, R. B. & Ellington, C. P. 1999 Discrimination of flying mimetic, passion-vine butterflies Heliconius. Proc. R. Soc. B 266, 2137–2140). H. sapho and H. erato are both pupal-mating sister species, whereas H. cydno and H. melpomene are sylvaniform sister species (Lee, C. S., McCool, B. A., Moore, J. L., Hillis, D. M. & Gilbert, L. E. 1992 Phylogenetic study of heliconiine butterflies using restriction analysis of their ribosomal RNA genes. Zool. J. Linn. Soc. 106, 17–31). Within each lineage, butterflies have diverged in wing beat frequency and asymmetric motion of the wings to converge on their respective co-mimics
Fourier reconstructions of the wing motion of foraging Heliconius butterflies
Fourier reconstructions of the wing motion of foraging Heliconius butterflies
Fourier reconstructions of the wing motion of foraging Heliconius butterflies
References
- Adelson E.H, Bergen J.R. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A. 1985;2:284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Bates H.W. Contributions to an insect fauna of the Amazon valley Lepidoptera: Heliconidae. Trans. Linn. Soc. 1862;23:495–566. [Google Scholar]
- Beatty C.D, Belrinckx K, Sherratt T.H. The evolution of Müllerian mimicry in multispecies communities. Nature. 2004;431:63–66. doi: 10.1038/nature02818. doi:10.1038/nature02818 [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. doi:10.1037/0033-2909.114.1.80 [DOI] [PubMed] [Google Scholar]
- Brunner D, Kacelnik A, Gibbon J. Optimal foraging and timing processes in the starling Sturnus vulgaris: effect of inter-capture interval. Anim. Behav. 1992;44:597–561. doi:10.1016/S0003-3472(05)80289-1 [Google Scholar]
- Cao P, Gu Y, Wang S.R. Visual neurons in the pigeon brain encode the acceleration of stimulus motion. J. Neurosci. 2004;24:7690–7698. doi: 10.1523/JNEUROSCI.2384-04.2004. doi:10.1523/JNEUROSCI.2384-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Parraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Cutting J.C. Recognizing friends by their walk: gait perception without familiarity cues. Bull. Psychon. Soc. 1977;9:353–356. [Google Scholar]
- Gilbert L.E. Pollen feeding and reproductive biology of Heliconius butterflies. Proc. Natl Acad. Sci. USA. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. doi:10.1073/pnas.69.6.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiggins C.D, Estrada C, Rodrigues A. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 2002;17:680–691. doi: 10.1111/j.1420-9101.2004.00675.x. doi:10.1111/j.1420-9101.2004.00675.x [DOI] [PubMed] [Google Scholar]
- Jukisch D, Daum I, Troje N.F. Self recognition versus recognition of others by biological motion: viewpoint-dependent effects. Perception. 2006;35:911–920. doi: 10.1068/p5540. doi:10.1068/p5540 [DOI] [PubMed] [Google Scholar]
- Lee C.S, McCool B.A, Moore J.L, Hillis D.M, Gilbert L.E. Phylogenetic study of heliconiine butterflies using restriction analysis of their ribosomal RNA genes. Zool. J. Linn. Soc. 1992;106:17–31. [Google Scholar]
- Mallet J, Gilbert L.E. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biol. J. Linn. Soc. 1995;55:159–180. doi:10.1006/bijl.1995.0034 [Google Scholar]
- Müller F. Ituna and Thyridia; a remarkable case of mimicry in butterflies. Proc. Entomol. Soc. 1879;1879:20–29. (translated by R. Mendola) [Google Scholar]
- Persons M.H, Fleishman L.J, Frye M.A, Stimphil M.E. Sensory response patterns and the evolution of visual design in anoline lizards. J. Comp. Physiol. A. 1999;184:585–607. doi:10.1007/s003590050358 [Google Scholar]
- Rowe C, Guilford T. Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature. 1996;383:520–522. doi:10.1038/383520a0 [Google Scholar]
- Sheppard P.M, Turner J.R.G, Brown K.S, Benson W.W, Singer M.C. Genetics and the evolution of Muellerian mimicry in Heliconius butterflies. Phil. Trans. R. Soc. B. 1985;308:433–610. [Google Scholar]
- Srygley R.B. Locomotor mimicry in Heliconius butterflies: contrast analyses of flight morphology and kinematics. Phil. Trans. R. Soc. B. 1999a;354:203–214. doi:10.1098/rstb.1999.0372 [Google Scholar]
- Srygley R.B. Incorporating motion into investigations of mimicry. Evol. Ecol. 1999;13:691–708. doi:10.1023/A:1011046202928 [Google Scholar]
- Srygley R.B. The aerodynamic costs of warning signals in palatable, mimetic butterflies and their distasteful models. Proc. R. Soc. B. 2004;271:589–594. doi: 10.1098/rspb.2003.2627. doi:10.1098/rspb.2003.2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srygley R.B, Chai P. Predation and the elevation of thoracic temperature in brightly-colored, Neotropical butterflies. Am. Nat. 1990;135:766–787. doi:10.1086/285073 [Google Scholar]
- Srygley R.B, Ellington C.P. Discrimination of flying, mimetic passion-vine butterflies Heliconius. Proc. R. Soc. B. 1999;266:2137–2140. doi:10.1098/rspb.1999.0899 [Google Scholar]
- Srygley R.B, Ellington C.P. Estimating the relative fitness of local adaptive peaks: the aerodynamic costs of flight in mimetic passion-vine butterflies Heliconius. Proc. R. Soc. B. 1999;266:2239–2245. doi:10.1098/rspb.1999.0914 [Google Scholar]
- Srygley R.B, Thomas A.L.R. Unconventional lift-generating mechanisms in free-flying butterflies. Nature. 2002;420:660–664. doi: 10.1038/nature01223. doi:10.1038/nature01223 [DOI] [PubMed] [Google Scholar]
- Traux B. Cambridge Street Publishing; British Columbia, Canada: 1999. Handbook for acoustic ecology. [Google Scholar]
- Wang Y, Gu Y, Wang S.R. Feature detection of visual neurons in the nucleus of the basal optic root in pigeons. Brain Res. Bull. 2000;51:165–169. doi: 10.1016/s0361-9230(99)00220-8. doi:10.1016/S0361-9230(99)00220-8 [DOI] [PubMed] [Google Scholar]
- Willmott A.P, Ellington C.P. The mechanics of flight in the hawkmoth Manduca sexta II. Aerodynamic consequences of kinematic and morphological variation. J. Exp. Biol. 1997;200:2723–2745. doi: 10.1242/jeb.200.21.2723. [DOI] [PubMed] [Google Scholar]
- Wilson H.R, Ferrera V.P, Yo C. A psychophysically motivated model for two-dimensional motion perception. Vis. Neurosci. 1992;9:79–97. doi: 10.1017/s0952523800006386. [DOI] [PubMed] [Google Scholar]
- Wylie D.R, Bischof W.F, Frost B.J. Common reference frame for neural coding of translational and rotational optic flow. Nature. 1998;392:278–282. doi: 10.1038/32648. doi:10.1038/32648 [DOI] [PubMed] [Google Scholar]
- Yachi S, Higashi M. The evolution of warning signals. Nature. 1998;394:882–884. doi:10.1038/29751 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Refer to the footnotes for abbreviations and the formula for wing motion
These selected high speed (250 fps) videos of butterflies foraging in natural settings, for which the first frame is shown on the right in the Powerpoint file (and the video can be seen by downloading the .mov or .dv file with the name of the species), arerendered into graphs of the Fourier wave form of the wing motion on the left (Srygley, R. B. & Ellington, C. P. 1999 Discrimination of flying mimetic, passion-vine butterflies Heliconius. Proc. R. Soc. B 266, 2137–2140). H. sapho and H. erato are both pupal-mating sister species, whereas H. cydno and H. melpomene are sylvaniform sister species (Lee, C. S., McCool, B. A., Moore, J. L., Hillis, D. M. & Gilbert, L. E. 1992 Phylogenetic study of heliconiine butterflies using restriction analysis of their ribosomal RNA genes. Zool. J. Linn. Soc. 106, 17–31). Within each lineage, butterflies have diverged in wing beat frequency and asymmetric motion of the wings to converge on their respective co-mimics
Fourier reconstructions of the wing motion of foraging Heliconius butterflies
Fourier reconstructions of the wing motion of foraging Heliconius butterflies
Fourier reconstructions of the wing motion of foraging Heliconius butterflies