Abstract
A wide diversity of free-living organisms show increases in mortality rates and/or decreases in reproductive success with advancing age. However, the physiological mechanisms underlying these demographic patterns of senescence are poorly understood. Immunosenescence, the age-related deterioration of immune function, is well documented in humans and laboratory models, and often leads to increased morbidity and mortality due to disease. However, we know very little about immunosenescence in free-living organisms. Here, we studied immunosenescence in a free-living population of tree swallows, Tachycineta bicolor, assessing three components of the immune system and using both in vivo and in vitro immunological tests. Immune function in tree swallow females showed a complex pattern with age; acquired T-cell mediated immunity declined with age, but neither acquired nor innate humoral immunity did. In vitro lymphocyte proliferation stimulated by T-cell mitogens decreased with age, suggesting that reduced T-cell function might be one mechanism underlying the immunosenescence pattern of in vivo cell-mediated response recently described for this same population. Our results provide the most thorough description of immunosenescence patterns and mechanisms in a free-living vertebrate population to date. Future research should focus on the ecological implications of immunosenescence and the potential causes of variation in patterns among species.
Keywords: immunocompetence, lymphocyte proliferation, natural antibodies, phytohemagglutinin test, ageing
1. Introduction
Until recently, it was assumed that individuals in the wild were highly unlikely to show signs of senescence (Holmes & Austad 1995; Kirkwood & Austad 2000); however, we now know that a wide diversity of free-living populations show age-related increases in mortality rates and/or decreases in reproductive success indicative of senescence (e.g. Promislow 1991; Holmes et al. 2001; Bonduriansky & Brassil 2002; Bryant & Reznick 2004; Morbey et al. 2005). Less clear are the physiological mechanisms underlying these demographic patterns (Reznick et al. 2004; Bronikowski & Promislow 2005). Physiological senescence is a multifaceted phenomenon involving irreversible deterioration of many organ systems in the body (Rose 1991). One main survival-related system affected by age is the immune system, consisting of complex defence mechanisms evolved in response to ubiquitous threats from parasites and diseases (Roitt et al. 1998). In humans, efficient function of the immune system is essential for survival, and immunosenescence, the deterioration of immune function with age, results in increased risk of infection, autoimmune disease and cancer (Miller 1996; Effros 2003) that lead to higher morbidity and mortality among the old (Pawelec et al. 2002).
The vertebrate immune system can be divided into two main arms: innate immunity (the first line of defence) and acquired immunity. Both arms can be further subdivided into humoral and cell-mediated components (Roitt et al. 1998). Most knowledge about immunosenescence comes from studies of humans and mammalian laboratory models, which suggest that the ageing immune system is characterized by altered activity of most of its components (reviewed by Miller 1996; Grubeck-Loebenstein & Wick 2002). Decline occurs in acquired cell-mediated immunity (reviewed by Aspinall 2003) and acquired humoral immunity (reviewed by Weksler & Szabo 2000), but the latter is thought to be caused by the former, given that important aspects of B-cell function depend on helper T-cell function (Pawelec et al. 2002). Regarding innate immunity, certain components may decline with age (e.g. function of phagocytes, Pawelec et al. 1998), although others may actually increase with age (e.g. inflammation, Franceschi et al. 2000).
Recent studies on ecoimmunology suggest that immunosenescence may be a common phenomenon in free-living animals, including both invertebrates (Adamo et al. 2001; Kurtz 2002) and vertebrates (Cichon et al. 2003; Lozano & Lank 2003; Saino et al. 2003; Haussmann et al. 2005; Ujvari & Madsen 2006). However, we still know very little about immunosenescence patterns, mechanisms and their implications in the wild, including whether immunosenescence is a causal factor in demographic senescence patterns (Saino et al. 2003). In particular, studies to date on free-living vertebrates (with the exception of humans) have measured only one component of the immune system in a given species (either acquired cell-mediated or humoral immunity) and information on innate immunity is lacking altogether. In addition, previous studies have used only in vivo assays of immune function that involve complex integrated responses by several immune effectors and therefore preclude elucidation of specific mechanisms underlying the observed immune patterns (Silliman & Wang 2006).
In this study, we examine immunosenescence patterns and mechanisms in a free-living population, using tree swallows (Tachycineta bicolor, Viellot 1808) as our model system. Tree swallows are secondary cavity-nesting aerial insectivores members of the family Hirundinidae (Robertson et al. 1992). We assessed three components of the immune system (acquired cell-mediated immunity, acquired humoral immunity and innate humoral immunity) and used in vitro assays, in addition to in vivo assays, to shed light on potential mechanisms underlying the immunosenescence patterns.
We focused on two basic questions: (i) how the different immune components behave with age and (ii) what mechanisms underlie the immunosenescence patterns. With respect to the latter, we know that tree swallows in our study population show immunosenescence in their in vivo acquired cell-mediated immune response assessed by the standard phytohemagglutinin (PHA) skin test (Haussmann et al. 2005). This test is the most widely used estimate of cell-mediated immune function in studies of wild birds and has been used as a general index of in vivo T-lymphocyte activation and proliferative potential (reviewed by Fairbrother et al. 2004). However, the response to PHA also involves innate components such as heterophils, macrophages and basophils (Goto et al. 1978; Martin et al. 2006), suggesting that the reduced swelling in older individuals could be due to reduced T-lymphocyte activation and proliferative potential, reduced recruitment of cells of the innate immune system, or both. We therefore used in vitro assays to assess whether the observed age-related decline of in vivo cell-mediated response is associated with reduced T-lymphocyte activation and proliferation. In addition, we measured in vivo acquired humoral immunity mediated by B-lymphocytes to determine whether this component also declines with age in tree swallows, and if so, whether the decline is associated with reduced in vitro B-lymphocyte activation and proliferation.
2. Material and methods
(a) Study population
We conducted this study in a tree swallow nest-box population in Tompkins County, NY (42°29′ N, 76°27′ W), which has been studied since 1985 (Winkler & Allen 1996; Winkler et al. 2004) and provided an adequate number of known-age individuals. Several factors besides age can affect immune responses. Individuals with higher body mass and/or body condition tend to mount stronger responses (e.g. Saino et al. 1997; Navarro et al. 2003). In tree swallows, individuals that start breeding earlier show stronger immune responses than later breeders (Hasselquist et al. 2001; Ardia 2005). In addition, stress can influence immune responses through elevated corticosterone (CORT) levels (reviewed by Padgett & Glaser 2003). Therefore, to assess the unique contribution of age to variation in immune responses and enhance the detectability of immunosenescence patterns, we statistically controlled for these potentially confounding factors (see below).
(b) Field sample collection
During the 2005 breeding season, we monitored nests daily to determine clutch completion date. We captured 45 females at their nest-boxes between 05:00 and 13:00 h on 3 June 2005. At this time, females were in late incubation (last 4 days) or early nestling period (first 4 days). Females ranged from 1 to 10 years of age, as determined by their ringing history. We knew exact ages for 26 females, and for the remaining, age was a minimum estimate based on each female's plumage in the year she was first ringed. We collected blood (approx. 200 μl) by jugular venipuncture and immediately 100 μl were diluted 1 : 1 in AIM-V lymphocyte medium supplemented with 25 mM HEPES, 2 mM l-glutamine and 50 μg ml−1 gentamicin (all from Life Technologies, Rockville, MD) and stored on ice for use in the lymphocyte proliferation assay the following morning. The remainder of the blood (approx. 100 μl) was transferred into capillary tubes for use in the humoral immunity assays (both innate and acquired) and for measuring CORT concentration. We stored capillary tubes on ice until centrifugation, after which we isolated the plasma for storage at −20°C. Females were weighed to the nearest 0.1 g and head–bill length (a measure of structural body size) was measured to the nearest 0.1 mm to estimate a body condition index (Schulte-Hostedde et al. 2005). Finally, we injected females intraperitoneally with 100 μl of a 2% suspension of sheep red blood cells (SRBC, HemoStat Laboratories, Dixon, CA) in phosphate buffered saline (PBS) and then released them. Following Ardia et al. (2003), females were recaptured 8 days later and a second blood sample (approx. 60 μl) was obtained to determine anti-SRBC antibody production.
(c) Immune function assays
In vitro lymphocyte proliferation—we used a whole-blood mitogenic stimulation assay (Cunnick et al. 1994) that we optimized for use in tree swallows; parameters we report provided the highest proliferation responses for tree swallow blood. We had previously determined that response of blood cells kept on ice for 24 h did not differ from that of fresh cells (paired samples t-test: t=0.516, p=0.616, n=12). In the laboratory, blood samples were further diluted to 1 : 20 using supplemented AIM-V lymphocyte medium (same as above) and 50 μl were dispensed into 96-well microplates containing 50 μl of mitogen (stimulated wells) or 50 μl of medium (non-stimulated wells). We stimulated lymphocytes using standard mitogens used in poultry immunology (e.g. Hovi et al. 1978; Cunnick et al. 1994): two T-cell mitogens, phytohaemagglutinin (PHA, 60 μg ml−1) and concanavalin A (ConA, 60 μg ml−1) and one B-cell mitogen, lipopolysaccharide from Salmonella typhimurium (LPS, 100 μg ml−1), all from Sigma (St Louis, MO). Blood cultures were incubated in a 7% CO2, 41°C humidified atmosphere for a total of 48 (LPS plates) or 72 h (PHA and ConA plates). Plates were pulsed with tritiated [3H] thymidine (0.5 μCi/well) for the last 24 h, then harvested onto glass-fibre filters using a cell harvester (Combi Cell Harvester, Skatron Instruments, Sterling, VA) and counted in a scintillation counter. Each sample was tested in triplicate for each mitogen and the medium and the counts per minute (c.p.m.) for triplicates were averaged. The proliferative response was expressed as a stimulation index (SI) calculated by dividing the mean c.p.m. of mitogen-stimulated wells by the mean c.p.m. of non-stimulated wells (Cunnick et al. 1994).
In vivo acquired humoral immune response (SRBC test)—we quantified antibody production by B-lymphocytes in response to immunization with SRBC following Ardia et al. (2003). We heat inactivated plasma (56°C for 30 min) and placed 10 μl in the first well of a 96-well plate. Next, we serially diluted samples starting with 10 μl plasma in 10 μl PBS along the row. Then, 10 μl of a 2% SRBC suspension was added to each well and plates were incubated at 37°C for 90 min. Titres (SRBC Ab titres) are expressed as the log2 of the highest dilution factor of plasma that showed hemagglutination. We recorded half scores between two titres when the termination of hemagglutination was intermediate. All plasma samples were run in duplicate and average titres were used. Pre- and post-immunization samples, as well as negative and positive controls, were run in each plate. None of the pre-immunization samples showed hemagglutination.
Constitutive innate humoral immunity—we used a haemolysis–hemagglutination assay developed in birds (Matson et al. 2005) to assess the levels of natural antibodies (NAbs) in peripheral blood and complement-mediated cell lysis. Lysis of cells probably results from the interaction between NAbs and complement, while hemagglutination depends only on NAbs (Matson et al. 2005). We performed a base-2 serial dilution of plasma in PBS as described for the SRBC test, except that plasma was not heat-inactivated in order to preserve complement proteins. Next, we added 10 μl of a 2% suspension of rabbit red blood cells (RRBC, HemoStat Laboratories) to each well and incubated plates at 37°C for 90 min. The scoring of hemagglutination (NAb titre) and lysis titres was performed as described by Matson et al. (2005). Titres are expressed as the log2 of the highest dilution factor of plasma that showed each response. We recorded half scores between two titres when the termination of hemagglutination or lysis was intermediate. All plasma samples were run in duplicate and average titres were used. Negative and positive controls were run in each plate.
(d) Corticosterone assay
We quantified CORT level in plasma to control for its concentration at the time of blood sampling and determine its effect on immune responses. All samples were run in triplicate in a single radioimmunoassay (no. 07-120102, MP Biomedicals, Irvine, CA) that has been validated for use in passerines (Washburn et al. 2002). Intra-assay coefficient of variation was 9.7%.
(e) Statistical analyses
All variables were checked for normality and log10 transformed when necessary. Lysis titres were not normally distributed even after transformation, so we used non-parametric tests (Spearman's rank correlation) when analysing this variable. We used multiple regression analyses to determine the relationships between immune function and age while controlling for potential confounding factors (clutch completion date, body mass, body condition index and CORT concentration). Body condition index and body mass were highly correlated (r=0.910, p<0.0001, n=45), therefore we only present the results for models using body mass. We used backward elimination to determine the final regression models, using α=0.05 and 0.1 as entry and removal probabilities, respectively. Sample sizes vary among analyses because we could not perform all assays in some samples due to limited blood volume. All statistical analyses were performed using SPSS v. 13.
3. Results
Neither time of day nor breeding stage explained significant variation in immune responses (all p>0.05). CORT levels increased with handling time (r=0.543, p=0.011, n=31), but after controlling for handling time, CORT levels were not significantly correlated with age (rp=0.005, p=0.980, n=31).
(a) Acquired cell-mediated immunity
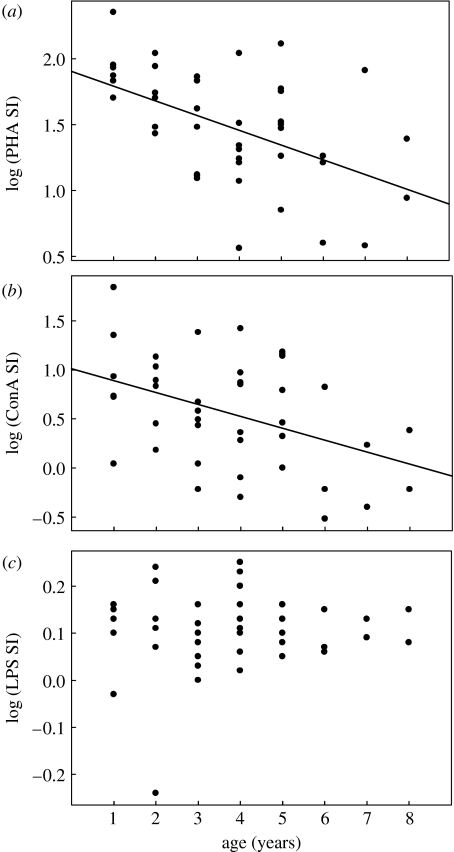
In vitro lymphocyte proliferation in response to T-cell mitogens (PHA and ConA) decreased with age among adult females (figure 1a,b). The final regression model for PHA included age as the only significant explanatory variable (r=−0.503, p=0.001, n=43), whereas the final model for ConA included age (rp=−0.490, p=0.001, n=43) and body mass (rp=−0.414, p=0.006, n=43). Forcing CORT concentration and/or clutch completion date into the above models did not change the relationships.
Figure 1.
In vitro lymphocyte proliferation responses of adult female tree swallows as a function of age (n=43 in all cases). (a) Lymphocyte proliferation in response to PHA (PHA SI; r=−0.503, p=0.001, regression line: log y=−0.112 log x+1.9), (b) lymphocyte proliferation in response to ConA (ConA SI; r=−0.422, p=0.005, regression line: log y=−0.121 log x+1.0), (c) lymphocyte proliferation in response to LPS (LPS SI; r=−0.079, p=0.613).
(b) Acquired humoral immunity
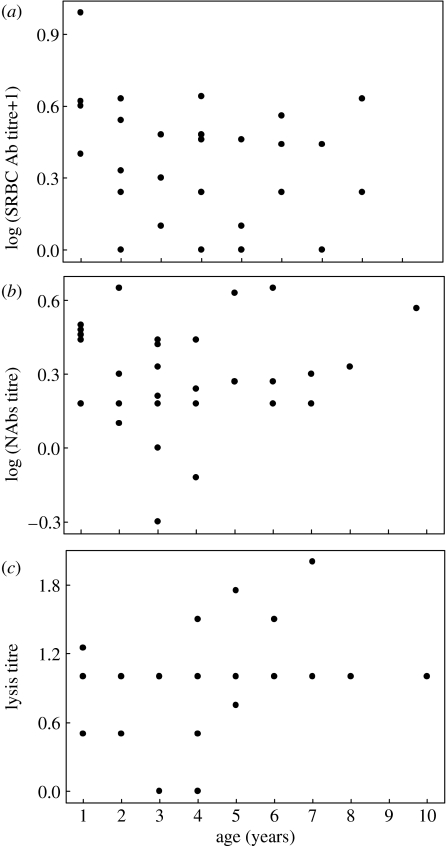
In vitro lymphocyte proliferation in response to the B-cell mitogen (LPS) did not change with female age (figure 1c, r=0.079, p=0.613, n=43). The final regression model for LPS included CORT concentration as the only explanatory variable (r=−0.443, p=0.013, n=31), with higher CORT concentrations associated with lower proliferation. However, forcing body mass and/or clutch completion date into the model rendered that relationship non-significant. In vivo antibody response to SRBC did not change significantly with age (figure 2a). None of the variables explained significant variation among adult females in this immune response.
Figure 2.
Humoral immune responses of adult female tree swallows as a function of age. (a) Acquired humoral response to SRBC challenge (SRBC Ab titre; r=−0.273, p=0.120, n=33). Note: 1 was added to the argument of the logarithm to show titres=0 corresponding to undiluted plasma, (b) levels of natural antibodies (NAbs titre; r=0.111, p=0.531, n=34), (c) complement-mediated cell lysis (Lysis titre; Spearman r=0.065, p=0.724, n=32).
(c) Innate immunity
The two measures of innate humoral immunity (NAbs titre and lysis titre) did not change with age among female tree swallows (figure 2b,c, respectively). The final regression model for NAbs titre included only CORT concentration (r=−0.401, p=0.031, n=29), with higher concentrations associated with lower titres. Forcing body mass and/or clutch completion date into the model, however, rendered that relationship non-significant. No significant model was found for lysis titre.
4. Discussion
In vitro proliferative response to T-cell mitogens (PHA/ConA) decreased with age in free-living adult female tree swallows ranging from 1 to 8 years of age, with the oldest individuals showing a response 84% weaker than the youngest. Indeed, age was the main predictor of variation in in vitro response to PHA/ConA among individuals, with the potential confounding variables (i.e. body mass, clutch completion date and CORT concentration) explaining little or no variation. An age-related decrease in T-lymphocyte proliferative ability in response to mitogens is a hallmark of immunosenescence in humans and mammalian laboratory models (Effros et al. 1994; Miller 1996; Franceschi et al. 2000), and together with other parameters of T-lymphocyte function, can predict survival among old individuals, suggesting an association between longevity and well-preserved immunity (reviewed by Pawelec et al. 2002). To our knowledge, this immunosenescence pattern had never been documented in a non-mammalian vertebrate or any free-living population with the exception of humans.
Haussmann et al. (2005) reported immunosenescence in this same tree swallow population based on the PHA skin test, with older individuals mounting weaker responses (i.e. smaller swellings) than younger ones. Data from this study support the hypothesis that reduced activation and/or proliferative potential of T-lymphocytes from older tree swallows is one mechanism underlying the pattern of immunosenescence in the PHA skin test response. The importance of T-cell function in the in vivo response to PHA challenge has been experimentally demonstrated in chickens thymectomized as neonates, which show a 50% reduction in swelling responses compared with controls (Goto et al. 1978). Since T-cells are not the only cells involved in the PHA skin response, future studies should assess the potential contribution of other immune components to the age-related reduction in swelling. In particular, studies of the histology of the skin swelling response to PHA (e.g. Martin et al. 2006) in young and old individuals could help determine the relative contribution of lymphocytes versus innate components such as heterophils, macrophages and basophils.
Ecoimmunology studies have shown that increased reproductive effort results in reduced immune function (see Schmid-Hempel 2003 for review). Therefore, an alternative explanation for the decline in immune function with age is a shift in resource allocation from self-maintenance to reproductive effort as individuals get older (Cichon et al. 2003). This scenario predicts that during the non-reproductive period any age differences in immune function would vanish (Cichon et al. 2003), which would not be the case if immunosenescence were due to a progressive and unavoidable deterioration of the immune system. Studies with laboratory mammals and humans indicate that immunosenescence occurs even in the absence of reproduction; however, reproductive costs may indeed accelerate immunosenescence (Helle et al. 2004). Future studies in ecoimmunology should focus on understanding the interaction between immunosenescence and reproductive effort in free-living organisms.
Female tree swallows did not show immunosenescence in our measured aspects of acquired humoral immunity. This result differs from two recent studies in free-living passerine birds. Antibody responses against Newcastle disease virus vaccine (NDV) declined with age in female barn swallows (Hirundo rustica) ranging from 1 to 3 years old (Saino et al. 2003), and older female collared flycatchers (Ficedula albicollis; 5–6 years old) mounted weaker responses to SRBC than younger ones (less than 4 years old). We did not find evidence of immunosenescence in acquired humoral immunity in tree swallows, even when analysing a broader range of ages (i.e. 1–10 years old) and performing an in vitro assay of lymphocyte proliferation in addition to the in vivo antibody response to immunization.
An interesting aspect of our results on acquired humoral immunity is that we did not find an age-related reduction in the production of antibodies against SRBC by B-cells, which is dependent on T-helper cells (a subtype of T-cell), despite finding reduced response to T-cell mitogens with age. One possible explanation for this result is that an increase in B-cell responsiveness with age compensated for the decrease in T-cell responsiveness resulting in no net difference in antibody production across ages. However, this seems unlikely as we did not find support for increased B-cell responsiveness with age through our in vitro test, and we are not aware of any study demonstrating increased B-cell function with age. Another possible explanation is that the response to SRBC does not decline with age because rather than T-helper cells, it is T-cytotoxic cells that show reduced response with advancing age in the in vitro assay, as has been shown in humans (Grossmann et al. 1989).
Different patterns of immunosenescence among species are likely the consequence of differences in demography and selection, possibly reflecting the link between life history and physiology (Ricklefs & Wikelski 2002). Indeed, avian species with higher survival rates and longer lifespans show a slower senescent decline of in vivo PHA response than species with the opposite life-history traits (Haussmann et al. 2005). North American tree swallows appear to have higher annual survival rates (50%, Robertson et al. 1992) and maximum recorded lifespans (12 years, Robertson & Rendell 2001) than those reported for the European barn swallow (35–40%, 7 years, Saino et al. 2003) and collared flycatcher (35–40%, 8 years, Cichon et al. 2003) populations on which immunosenescence data were reported; therefore, we would predict slower senescence in tree swallows. In addition, differences in diversity and/or abundance of parasites in the environment can favour different levels of investment in immune function and/or different immune strategies (Zuk & Stoehr 2002; Schmid-Hempel 2003; Lindstrom et al. 2004), but this has not yet been studied in relation to comparative patterns of immunosenescence.
Finally, tree swallows did not show immunosenescence in either aspect of innate humoral immunity measured. Neither the level of circulating NAbs against RRBC nor complement-mediated cell lysis showed a decline with age. It remains to be determined whether cellular aspects of innate immunity, such as the function of phagocytes (Pawelec et al. 1998), show immunosenescence in this species.
In summary, we present the most thorough study of immunosenescence patterns in a free-living population to date. Immune function of tree swallows shows a complex age-related pattern with some aspects of immune function declining with age and others remaining unchanged. Our results in a free-living bird coincide with general patterns in humans and mammalian laboratory models that show that acquired immunity declines more pervasively with age than does innate immunity (Franceschi et al. 2000) and that T-cell function is more affected than B-cell function (Pawelec et al. 2002). Our study also provides evidence for a potential mechanism underlying immunosenescence of the in vivo response to PHA, the most widely used immune test in ecological immunology. Finally, our data also suggest that species might differ not only in the rate of senescence of a given immune component (Haussmann et al. 2005), but also in which components are more affected by age. An important next step for ecoimmunology is to understand the ecological and evolutionary implications of immunosenescence patterns in the wild.
Acknowledgments
We thank David Vleck, Noah Hamm, David Cerasale and Scott Haber for their field assistance and Tom Martin, Anne Bronikowski, David Vleck and three anonymous reviewers for valuable comments on previous versions of this manuscript. This work was supported by a William Clark graduate student award in Ecology and Evolutionary Biology from Iowa State University to M.G.P. and by NSF IBN-013437 to D.W.W. All work with swallows was made in accordance with standard animal care protocols and approved by Iowa State University Animal Care and Use Committee.
References
- Adamo S.A, Jensen M, Younger M. Changes in lifetime immunocompetence in male and female Gryllus texensis (formerly G-integer): trade-offs between immunity and reproduction. Anim. Behav. 2001;62:417–425. doi:10.1006/anbe.2001.1786 [Google Scholar]
- Ardia D.R. Individual quality mediates trade-offs between reproductive effort and immune function in tree swallows. J. Anim. Ecol. 2005;74:517–524. [Google Scholar]
- Ardia D.R, Schat K.A, Winkler D.W. Reproductive effort reduces long-term immune function in breeding tree swallows (Tachycineta bicolor) Proc. R. Soc. B. 2003;270:1679–1683. doi: 10.1098/rspb.2003.2424. doi:10.1098/rspb.2003.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall R. Age-related changes in the function of T cells. Microsc. Res. Tech. 2003;62:508–513. doi: 10.1002/jemt.10412. doi:10.1002/jemt.10412 [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Brassil C.E. Rapid and costly ageing in wild male flies. Nature. 2002;420:377–377. doi: 10.1038/420377a. doi:10.1038/420377a [DOI] [PubMed] [Google Scholar]
- Bronikowski A.M, Promislow D.E.L. Testing evolutionary theories of aging in wild populations. Trends Ecol. Evol. 2005;20:271–273. doi: 10.1016/j.tree.2005.03.011. doi:10.1016/j.tree.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Bryant M.J, Reznick D. Comparative studies of senescence in natural populations of guppies. Am. Nat. 2004;163:55–68. doi: 10.1086/380650. doi:10.1086/380650 [DOI] [PubMed] [Google Scholar]
- Cichon M, Sendecka J, Gustafsson L. Age-related decline in humoral immune function in Collared Flycatchers. J. Evol. Biol. 2003;16:1205–1210. doi: 10.1046/j.1420-9101.2003.00611.x. doi:10.1046/j.1420-9101.2003.00611.x [DOI] [PubMed] [Google Scholar]
- Cunnick J.E, Kojic L.D, Hughes R.A. Stress-induced changes in immune function are associated with increased production of an interleukin-1-like factor in young domestic-fowl. Brain Behav. Immun. 1994;8:123–136. doi: 10.1006/brbi.1994.1012. doi:10.1006/brbi.1994.1012 [DOI] [PubMed] [Google Scholar]
- Effros R.B. Genetic alterations in the ageing immune system: impact on infection and cancer. Mech. Ageing Dev. 2003;124:71–77. doi: 10.1016/s0047-6374(02)00171-9. doi:10.1016/S0047-6374(02)00171-9 [DOI] [PubMed] [Google Scholar]
- Effros R.B, Boucher N, Porter V, Zhu X.M, Spaulding C, Walford R.L, Kronenberg M, Cohen D, Schachter F. Decline in Cd28(+) T-cells in centenarians and in long-term T-cell cultures—a possible cause for both in-vivo and in-vitro immunosenescence. Exp. Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. doi:10.1016/0531-5565(94)90073-6 [DOI] [PubMed] [Google Scholar]
- Fairbrother A, Smits J, Grasman K.A. Avian immunotoxicology. J. Toxicol. Environ. Health B Crit. Rev. 2004;7:105–137. doi: 10.1080/10937400490258873. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. doi:10.1016/S0264-410X(99)00513-7 [DOI] [PubMed] [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. Suppression of phytohemagglutinin skin-response in thymectomized chickens. Poult. Sci. 1978;57:246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Grossmann A, Ledbetter J.A, Rabinovitch P.S. Reduced proliferation in lymphocytes-T in aged humans is predominantly in the CD8+ subset, and is unrelated to defects in transmembrane signaling which are predominantly in the CD4+ subset. Exp. Cell Res. 1989;180:367–382. doi: 10.1016/0014-4827(89)90064-5. doi:10.1016/0014-4827(89)90064-5 [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv. Immunol. 2002;80:243–284. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Wasson M.F, Winkler D.W. Humoral immunocompetence correlates with date of egg-laying and reflects work load in female tree swallows. Behav. Ecol. 2001;12:93–97. doi:10.1093/beheco/12.4.457 [Google Scholar]
- Haussmann M.F, Winkler D.W, Huntington C.E, Vleck D, Sanneman C.E, Hanley D, Vleck C.M. Cell-mediated immunosenescence in birds. Oecologia. 2005;145:270–275. doi: 10.1007/s00442-005-0123-3. doi:10.1007/s00442-005-0123-3 [DOI] [PubMed] [Google Scholar]
- Helle S, Lummaa V, Jokela J. Accelerated immunosenescence in preindustrial twin mothers. Proc. Natl Acad. Sci. USA. 2004;101:12 391–12 396. doi: 10.1073/pnas.0402215101. doi:10.1073/pnas.0402215101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.J, Austad S.N. The evolution of avian senescence patterns—implications for understanding primary aging processes. Am. Zool. 1995;35:307–317. [Google Scholar]
- Holmes D.J, Fluckiger R, Austad S.N. Comparative biology of aging in birds: an update. Exp. Gerontol. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. doi:10.1016/S0531-5565(00)00247-3 [DOI] [PubMed] [Google Scholar]
- Hovi T, Suni J, Hortling L, Vaheri A. Stimulation of chicken lymphocytes by T- and B- cell mitogens. Cell. Immun. 1978;39:70–78. doi: 10.1016/0008-8749(78)90084-9. doi:10.1016/0008-8749(78)90084-9 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L, Austad S.N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. doi:10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- Kurtz J. Phagocytosis by invertebrate hemocytes: causes of individual variation in Panorpa vulgaris scorpionflies. Microsc. Res. Tech. 2002;57:456–468. doi: 10.1002/jemt.10099. doi:10.1002/jemt.10099 [DOI] [PubMed] [Google Scholar]
- Lindstrom K.M, Foufopoulos J, Parn H, Wikelski M. Immunological investments reflect parasite abundance in island populations of Darwin's finches. Proc. R. Soc. B. 2004;271:1513–1519. doi: 10.1098/rspb.2004.2752. doi:10.1098/rspb.2004.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G.A, Lank D.B. Seasonal trade-offs in cell-mediated immunosenescence in ruffs (Philomachus pugnax) Proc. R. Soc. B. 2003;270:1203–1208. doi: 10.1098/rspb.2002.2309. doi:10.1098/rspb.2002.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.B, Han P, Lewittes J, Kuhlman J.R, Klasing K.C, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct. Ecol. 2006;20:290–299. doi:10.1111/j.1365-2435.2006.01094.x [Google Scholar]
- Matson K.D, Ricklefs R.E, Klasing K.C. A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 2005;29:275–286. doi: 10.1016/j.dci.2004.07.006. doi:10.1016/j.dci.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Miller R.A. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. doi:10.1126/science.273.5271.70 [DOI] [PubMed] [Google Scholar]
- Morbey Y.E, Brassil C.E, Hendry A.P. Rapid senescence in Pacific salmon. Am. Nat. 2005;166:556–568. doi: 10.1086/491720. doi:10.1086/491720 [DOI] [PubMed] [Google Scholar]
- Navarro C, Marzal A, De Lope F, Møller A.P. Dynamics of an immune response in house sparrows Passer domesticus in relation to time of day, body condition and blood parasite infection. Oikos. 2003;101:291–298. doi:10.1034/j.1600-0706.2003.11663.x [Google Scholar]
- Padgett D.A, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. doi:10.1016/S1471-4906(03)00173-X [DOI] [PubMed] [Google Scholar]
- Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J. Leukoc. Biol. 1998;64:703–712. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- Pawelec G, et al. T cells and aging. Front. Biosci. 2002;7:1056–1183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L. Senescence in natural populations of mammals—a comparative study. Evolution. 1991;45:1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x. doi:10.2307/2409837 [DOI] [PubMed] [Google Scholar]
- Reznick D.N, Bryant M.J, Roff D, Ghalambor C.K, Ghalambor D.E. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. doi:10.1038/nature02936 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E, Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Robertson R.J, Rendell W.B. A long-term study of reproductive performance in tree swallows: the influence of age and senescence on output. J. Anim. Ecol. 2001;70:1014–1031. doi:10.1046/j.0021-8790.2001.00555.x [Google Scholar]
- Robertson R.J, Stuchbury B.J, Cohen R.R. Tree swallow (Tachycineta bicolor) In: Poole A, Stettenheim P, Gill F, editors. Birds of North America. vol. 11. Academy of Natural Sciences; American Ornithologists' Union; Philadelphia, PA; Washington, DC: 1992. pp. 1–28. [Google Scholar]
- Roitt I, Brostoff J, Male D. Mosby; London, UK: 1998. Immunology. [Google Scholar]
- Rose M.R. Oxford University Press; New York, NY: 1991. Evolutionary biology of aging. [Google Scholar]
- Saino N, Bolzern A.M, Moller A.P. Immunocompetence, ornamentation, and viability of male barn swallows (Hirundo rustica) Proc. Natl Acad. Sci. USA. 1997;94:549–552. doi: 10.1073/pnas.94.2.549. doi:10.1073/pnas.94.2.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Ferrari R.P, Romano M, Rubolini D, Møller A.P. Humoral immune response in relation to senescence, sex and sexual ornamentation in the barn swallow (Hirundo rustica) J. Evol. Biol. 2003;16:1127–1134. doi: 10.1046/j.1420-9101.2003.00616.x. doi:10.1046/j.1420-9101.2003.00616.x [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. doi:10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde A.I, Zinner B, Millar J.S, Hickling G.J. Restitution of mass–size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Silliman C.C, Wang M. The merits of in vitro versus in vivo modeling in investigation of the immune system. Environ. Toxicol. Pharmacol. 2006;21:123–134. doi: 10.1016/j.etap.2005.07.002. doi:10.1016/j.etap.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Ujvari B, Madsen T. Age, parasites, and condition affect humoral immune response in tropical pythons. Behav. Ecol. 2006;17:20–24. doi:10.1093/beheco/ari091 [Google Scholar]
- Washburn B.E, Morris D.L, Millspaugh J.J, Faaborg J, Schulz J.H. Using a commercially available radioimmunoassay to quantify corticosterone in avian plasma. Condor. 2002;104:558–563. doi:10.1650/0010-5422(2002)104[0558:UACART]2.0.CO;2 [Google Scholar]
- Weksler M.E, Szabo P. The effect of age on the B-Cell repertoire. J. Clin. Immunol. 2000;20:240–249. doi: 10.1023/a:1006659401385. doi:10.1023/A:1006659401385 [DOI] [PubMed] [Google Scholar]
- Winkler D.W, Allen P.E. The seasonal decline in tree swallow clutch size: physiological constraint or strategic adjustment? Ecology. 1996;77:922–932. doi:10.2307/2265512 [Google Scholar]
- Winkler D.W, Wrege P.H, Allen P.E, Kast T.L, Senesac P, Wasson M.F, Llambias P.E, Ferretti V, Sullivan P.J. Breeding dispersal and philopatry in the tree swallow. Condor. 2004;106:768–776. doi:10.1650/7634 [Google Scholar]
- Zuk M, Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]