Abstract
Conspecific sperm precedence (CSP) has been identified as an important post-copulatory, pre-zygotic mechanism that can act to reduce gene flow between populations. The evolution of CSP is thought to have arisen as a by-product of male and female coevolution in response to intraspecific post-copulatory sexual selection. However, little is known about the mechanisms that generate CSP. When Callosobruchus subinnotatus females copulate with both C. subinnotatus and Callosobruchus maculatus males, regardless of mating order, the majority of eggs are fertilized by conspecific sperm. The low number of heterospecific fertilizations does not result from general differences in the viability of sperm in the female reproductive tract, as heterospecific sperm fertilized equivalent numbers of eggs as conspecific sperm in the absence of sperm competition. Instead, CSP results from disadvantages to heterospecific sperm that are manifest only when in competition with conspecific sperm. CSP in C. subinnotatus appears to result from two, not mutually exclusive, mechanisms. First, conspecific sperm are better able to displace heterospecific sperm from female storage. Second, conspecific sperm achieve disproportionately higher numbers of fertilizations relative to their proportional representation in the fertilization set. Thus, we provide evidence of differential sperm use from the female spermatheca.
Keywords: conspecific sperm precedence, post-copulatory sexual selection, sperm selection, Callosobruchus
1. Introduction
Conspecific sperm precedence (CSP), defined as the favoured utilization of sperm from conspecific males in fertilization when both conspecifics and heterospecifics have inseminated a female (Howard 1999), has been implicated as an important isolating mechanism between closely related species (Howard et al. 1998, 1999). Essentially, studies demonstrating CSP show that females ‘discriminate’ between conspecific and heterospecific sperm at fertilization. Thus, when a female copulates with both a conspecific and heterospecific male, the majority of its subsequent eggs are fertilized by conspecific sperm, regardless of male mating order. This typically differs from intraspecific sperm competition studies, in which the majority of a female's subsequent eggs are fertilized by sperm from the last-mating male (Simmons & Siva-Jothy 1998; Simmons 2001). So far, CSP has been demonstrated in: ladybirds (Nakano 1985); grasshoppers (Bella et al. 1992); flour beetles (Wade et al. 1994; Fricke & Arnqvist 2004); ground crickets (Gregory & Howard 1994); and Drosophila species (Price 1997). Analogous processes have also been reported in other groups: conspecific pollen precedence in plants (Arnold et al. 1993; Rieseberg et al. 1995) and conspecific sperm/egg recognition in broadcast spawning marine invertebrates (Palumbi 1998).
It has been suggested that CSP evolves as a result of the rapid and divergent coevolution of male and female reproductive traits, driven by intraspecific post-copulatory sexual selection (Rice 1996; Howard et al. 1998, 1999). In allopatry, the reproductive traits of populations diverge to the extent that when populations come into secondary contact, a degree of pre-zygotic reproductive incompatibility in the form of CSP is evident. This hypothesis suggests that the mechanisms that facilitate CSP are an extension of the basic mechanisms affecting the outcome of intraspecific sperm competition.
Demonstration of CSP reveals little about the mechanisms that facilitate it. The fertilization success of heterospecific males may simply be suppressed by mechanisms that operate irrespective of whether a female also copulates with a conspecific male. Relative to conspecific sperm, heterospecific sperm may be transferred and/or stored in fewer numbers, be more easily lost from storage, lose function in the ‘alien’ female reproductive tract or fail to bind to receptor sites on the ‘alien’ egg (Howard 1999). However, in a number of cases, heterospecific sperm appear to function quite normally within the female reproductive tract, and the competitive inferiority of heterospecific sperm is revealed only after females have also copulated with a conspecific male. Sperm of heterospecific males within female sperm storage organs may be particularly vulnerable to displacement or incapacitation by a later-mating conspecific male. In a similar vein, heterospecific males and/or their sperm may be poorly adapted to overcome defences protecting conspecific sperm already in storage. For example, when female Drosophila simulans copulate with conspecific males after initially copulating with heterospecific Drosophila mauritiana males, components of the conspecific seminal fluid act to physically displace heterospecific sperm from storage. However, if the conspecific male copulates first, components of the seminal fluid appear to inhibit the function of heterospecific sperm transferred later (Price et al. 2000).
Here, we investigate the mechanisms of CSP in Callosobruchus subinnotatus (Fabricius) and Callosobruchus maculatus (Pic), whereby C. subinnotatus is a sister species to the group containing C. maculatus (Tuda et al. 2006). Both are cosmopolitan pests of stored legumes that occasionally occur together in seed stores (Haines 1991; Mbata 1992; Lale & Vidal 2001). Although nothing is reported about hybridization in the wild, asymmetric hybridization readily occurs in the laboratory: C. maculatus males mate with C. subinnotatus females but the reciprocal cross does not occur (P. F. Rugman-Jones, personal observation). This is probably the result of a component in the sex pheromone produced by C. maculatus females which inhibits the male C. subinnotatus sexual response (Mbata et al. 2000).
In order to reveal the mechanisms of CSP, Price et al. (2000) suggest that patterns of sperm storage and use after single heterospecific or conspecific insemination be compared to patterns seen after double inseminations (i.e. by a heterospecific and a conspecific male). This protocol requires the ability to discriminate between heterospecific and conspecific sperm. In Callosobruchus, a difference in sperm length between C. maculatus and C. subinnotatus (Rugman-Jones 2003) affords us the opportunity to make such empirical observations on the fate of sperm within the reproductive tract of a single female. Furthermore, since C. maculatus has been used as a model organism for evaluating factors that affect intraspecific sperm competition (Eady 1991, 1994a,b, 1995; Eady & Tubman 1996; Wilson et al. 1997; Brown & Eady 2001; Eady et al. 2004), identifying the mechanisms that facilitate CSP in this genus may enlighten our understanding of intraspecific sperm competition mechanisms.
2. Material and methods
(a) Insect stocks and matings
The C. subinnotatus beetles used in this study were derived from a Nigerian population in culture at the University of Sunderland for six months and previously in culture at the Universities of Sheffield and Leicester. The C. maculatus beetles used were derived from a South Indian population, in culture at Sunderland for approximately 3 years. Stock cultures of both species were maintained on black-eyed beans, Vigna unguiculata (L.), in a constant temperature environment at 27°C and 35% R.H., with a 12 L : 12 D photoperiod.
All study copulations took place in individual perspex pots, under constant environmental conditions at 27°C and 35% R.H., and during a time window 1–4 h before the onset of photophase. Mating behaviour in C. subinnotatus is strongly influenced by photoperiod and peaks during this ‘window’ (Mbata et al. 1997). At the time of their first copulation, all females were virgin and 24–48 h post-eclosion. All copulating males were virgin and 48–72 h post-eclosion. Males and females were randomly paired and allowed 45 min to initiate copulation. Copulatory behaviour of both species is very similar. The male approaches a female from behind, climbing onto her back and ‘drumming’ his antennae on her elytra. At this point, unwilling females can kick the males off or simply walk away. However, when genital contact is achieved (i.e. copulation begins), the male stops ‘drumming’ and raises his antennae in the air. Copulation ends when the female ‘kicks’ the male free (Eady 1994a). Thus, the start and end of copulation can be unambiguously assigned. All copulations were observed under red lighting and copulating pairs were separated immediately upon the end of copulation. Females failing to mate were discarded: under these conditions, approximately 80% of virgin females mated. All beetles were maintained under the culture conditions throughout the study and where stated, females were provided with black-eyed beans as oviposition substrate. Elytron length was recorded as a measure of male and female body size (Wilson & Hill 1989).
(b) Viability of the interspecific cross
Female C. subinnotatus were mated to two conspecific or two heterospecific males, allowing 48 h between copulations, during which, females were isolated with 30 beans. A refractory period of 48 h was required before the majority of females would remate (Rugman-Jones 2003). Immediately following the second mating, females were isolated with 30 fresh beans and allowed to oviposit until their natural deaths. Female lifetime fecundity was recorded and the viability of the cross estimated as percentage of egg hatch as determined by the colour of the eggs 7 days after female death. Hatching larvae burrow through the underside of the egg directly into the bean filling the vacated egg with very obvious white frass; thus, infertile eggs remain clear while fertile eggs appear opaque.
Fertility of the hybrid offspring was quantified by two methods. First, 50 hybrids of each sex were collected, isolated in a culture box with 100 g of beans and allowed to freely copulate and oviposit. Four weeks later, fertile and sterile eggs were counted. In a second experiment, a further 40 hybrids of each sex were permitted a single copulation with either a pure-species C. maculatus or C. subinnotatus of the opposite sex. Immediately following the copulation, each female (hybrid and pure-species) was isolated with 30 beans and allowed to oviposit until its natural death. Fertile and sterile eggs were then counted.
(c) Conspecific sperm precedence
The proportion of eggs fertilized by the second male to mate (P2) was estimated using the sterile male technique (Boorman & Parker 1976). Callosobruchus subinnotatus females were mated sequentially to a conspecific then an irradiated heterospecific (C. maculatus) male, or vice versa. The extent of last-male sperm precedence was also determined for females mated sequentially to two conspecific or two heterospecific males (in both instances the second male having been irradiated). Males were irradiated with a 12 krad dose of γ radiation from a cobalt60 source, 24 h prior to mating, inducing 96.5% sterility. Females were isolated with 15 beans and allowed to oviposit for 48 h between copulations, then transferred to 30 fresh beans following the second copulation and allowed to oviposit until their natural deaths. All eggs were then counted and paternity estimates made based on the numbers of fertile and sterile eggs laid after the second copulation (Boorman & Parker 1976).
(d) Number of sperm entering and remaining in the spermatheca
At some point after the spermatophore is transferred to the female, sperm migrate from the bursa copulatrix into the spermatheca. In C. maculatus, this begins 5 min after insemination and the capacity of the spermatheca is reached approximately 1–2 h later (Eady 1994a,b). By counting the number of sperm in the spermatheca at different times following copulation, the number of sperm entering and remaining in storage can be determined.
Callosobruchus subinnotatus females were permitted a single copulation with a conspecific or heterospecific male and immediately isolated with 20 cowpeas as oviposition sites. The number of sperm in the spermatheca of approximately half of the females in each treatment was determined 2–4 h post-copulation. Females were euthanized and then immediately dissected in insect saline. Individual spermathecae were dissected free and transferred to cavity slides containing 40 μl insect saline. Sperm were expelled through the severed spermathecal duct by gently squeezing the spermatheca with watchmakers' forceps in a series of soft pulses, taking care not to rupture the spermatheca. This process was repeated until no sperm were visible in the spermatheca. Finally, the spermatheca was ruptured and the solution mixed for 2 min with the tips of the forceps. The ruptured spermatheca was removed and the resulting solution distributed between three haemocytometers and the number of stored sperm estimated. The number of sperm in the spermatheca of the remaining females was determined as above 48 h post-copulation. Elytra length was recorded for all beetles.
(e) Proportion of conspecific sperm in storage 3 and 48 h after a double mating
Given sperm lengths in C. subinnotatus (0.235±0.003 mm) and C. maculatus (0.177±0.003 mm) are significantly different (Rugman-Jones 2003), it is possible to make direct counts of the two sperm types in the spermatheca. Using the methodology described above, two treatments were established: females mated first to a conspecific and then a heterospecific male, and females mated first to a heterospecific and then a conspecific male, with a 48 h interval between inseminations to allow for oviposition. The proportions of conspecific and heterospecific sperm in the spermatheca of approximately half of the females in each treatment, was determined 3 h after the second insemination. Individual spermathecae were dissected free and ‘emptied’ as described previously. The sperm solution was mixed for 2 min using the tips of a pair of watchmakers’ forceps and a single drop was transferred to a glass microscope slide, covered with a glass cover-slip and examined under a Zeiss phase-contrast microscope. Starting from a random point, the first 50 sperm encountered were measured and classified as conspecific or heterospecific. The proportions of conspecific and heterospecific sperm in the spermatheca of the remaining females were determined 48 h post-copulation.
(f) The relationship between CSP and the relative proportions of conspecific and heterospecific sperm in storage
The present study revealed large variation in both the relative proportions of the two sperm types in storage and the extent of last-male sperm precedence (see §4). The following experiment was designed to examine the nature of the relationship between these two factors. P2 was measured as above except that C. subinnotatus males only were irradiated (99.7% sterility, n=14), again creating two treatments: C. subinnotatus females mated first to a conspecific and then a heterospecific male (n=7), and females mated first to a heterospecific and then a conspecific male (n=11), with a 48 h interval between inseminations. Following the second copulation, females were allowed to oviposit for 48 h, enabling the extent of last-male sperm precedence to be determined for this batch of eggs. Females were then euthanized and the proportions of conspecific and heterospecific sperm in the spermatheca of each double-mated female determined as described previously. This enabled us to assess the relationship between the relative proportions of each male's sperm in storage and its success in sperm competition.
3. Results
(a) Viability of the interspecific cross
A single conspecific-mated female produced only four eggs and was removed from the analysis as an outlier (Grubbs's test statistic=3.662, n=64, p<0.01; Sokal & Rohlf 1995). Following the removal of this outlier, and controlling for female body size (ANCOVA F1,60=21.72, p<0.001), C. subinnotatus females mated to heterospecific males were just as fecund as females mated to conspecific males: mean number of eggs ±s.e.=62.29±2.79 (n=29) and 63.37±2.23 (n=34), respectively (F1,60=0.06, p=0.813). The viability of eggs laid following heterospecific and conspecific copulations was also equivalent: 90.74±1.41% and 88.96±1.26%, respectively (t=0.94, d.f.=58, p=0.351). However, all hybrid offspring were sterile. In the culture box experiment, in which hybrid males and hybrid females were allowed to copulate and oviposit freely, 342 eggs were laid, but all failed to hatch. In the second experiment, 38 out of 40 hybrid females mated to a pure-strain C. subinnotatus or C. maculatus males failed to lay eggs. Thirty-seven out of 40 pure-strain C. subinnotatus females mated to hybrid males also failed to lay any eggs. Of the eggs laid by the five remaining females, all failed to hatch.
(b) Conspecific sperm precedence
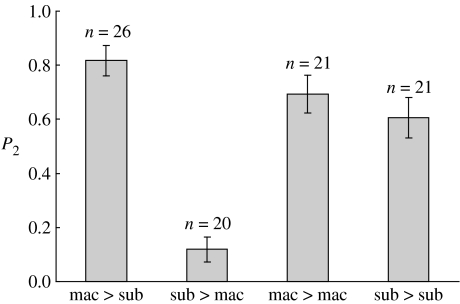
Logistic regression with a binomial error and logit link function was used to analyse P2 data with changes in deviance assessed using an F-test to correct for overdispersion (Crawley 2002). Treatment had a significant effect on the extent of P2, with conspecific sperm fertilizing the majority of eggs regardless of mating order (Δdev.=1211, d.f.=3, F=24, p<0.0001; figure 1, columns 1 & 2). Collapsing of factor levels (Crawley 2002) revealed the P2 of females mated to two conspecific males or two heterospecific males to be equivalent (Δdev.=12, d.f.=1, F=0.75, p=0.39; figure 1, columns 3 & 4). Further model simplification was not justified (Δdev.=135, d.f.=1, F=8.15, p=0.005) indicating that conspecific males were better able to pre-empt the sperm of rival heterospecific males in comparison to rival conspecific sperm (figure 1, columns 1 & 4).
Figure 1.
Conspecific sperm precedence (measured as mean P2±1 s.e.) in C. subinnotatus. In all the cases, the female was C. subinnotatus. (mac, C. maculatus male and sub, C. subinnotatus male).
(c) Number of sperm entering and remaining in the spermatheca
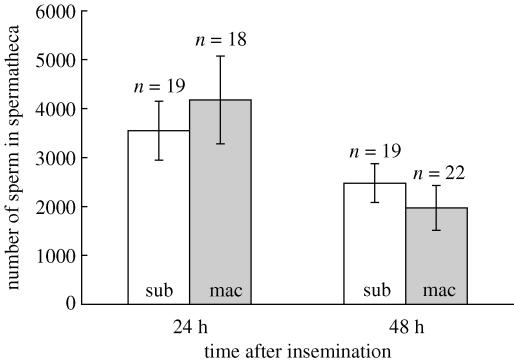
Two-way analysis of variance with time after insemination and species of male as fixed factors, revealed an overall temporal decline in the number of sperm in storage (F1,70=7.5, p=0.008) but no difference in the numbers of sperm entering or remaining in storage following copulation with a conspecific or heterospecific male overall (F1,70=0.01, p=0.93) nor the interaction between treatment and time (F1,70=0.88, p=0.35; figure 2). Neither male nor female size affected the number of sperm entering or remaining in storage (p=0.82 and 0.91, respectively).
Figure 2.
Temporal variation in the number of sperm in storage following conspecific or heterospecific insemination (mean±1 s.e.). Identity of the last-mating male given at base of each bar; sub, C. subinnotatus male; mac, C. maculatus male.
(d) Proportion of conspecific sperm in storage 3 and 48 h after a double mating
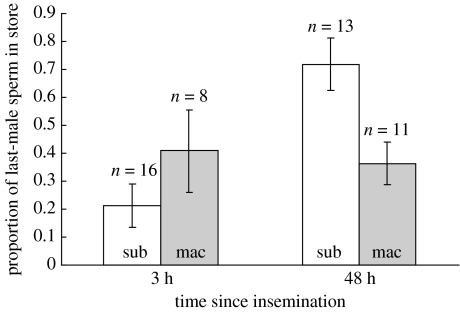
Logistic regression analysis with binomial error and logit link function (corrected for overdispersion) revealed treatment (whether the last male to mate was C. subinnotatus or C. maculatus) to have no overall effect on the proportion of last-male sperm in storage (Δdev.=22, d.f.=1, F=0.78, p=0.38). However, the relative proportions of last-male sperm in storage varied over time, with more last-male sperm in storage 48 h post-copulation compared with 3 h post-copulation (Δdev.=194, d.f.=1, F=6.9, p=0.01). This appears to be the result of a greater abundance of C. subinnotatus sperm in storage 48 h following copulation, as revealed by the significant interaction between treatment and time after copulation (Δdev.=181, d.f.=1, F=6.7, p=0.012; figure 3).
Figure 3.
Temporal variation in the proportion of last-male sperm in the spermatheca of C. subinnotatus females mated sequentially to a conspecific then heterospecific male, or vice versa (mean±1 s.e.). Identity of the last-mating male given at base of each bar.
(e) The relationship between conspecific sperm precedence and the relative proportions of conspecific and heterospecific sperm in storage
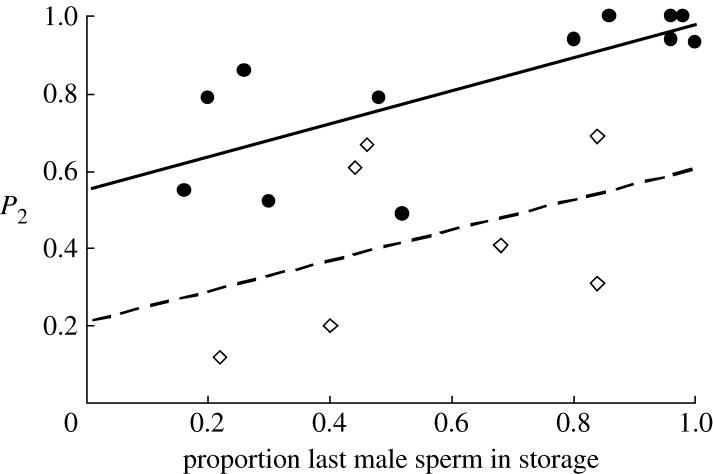
The maximal logistic regression model with binomial error and logit link function (corrected for overdispersion) contained last-male identity, the proportion of last-male sperm in storage and their interaction. Model simplification (Crawley 2002) revealed the extent of CSP to be positively related to the proportion of last-male sperm in storage (Δdev.=38, d.f.=1, F=10.97, p=0.005) and to whether the last male was conspecific or heterospecific (Δdev.=47, d.f.=1, F=13.54, p=0.002), with conspecific males achieving higher levels of sperm precedence than heterospecifics for a given proportion of sperm in storage (figure 4). The interaction was non-significant.
Figure 4.
Relationship between the last-male sperm precedence (measured as P2) and the relative number of last-male sperm in storage. Filled points and solid line represent C. subinnotatus males, unfilled points and dashed line C. maculatus males. Lines represent least squares regression on untransformed P2 data.
4. Discussion
Callosobruchus subinnotatus females that copulated with heterospecific males only, produced numerous, but sterile, hybrid offspring. Sperm from single conspecific and heterospecific copulations were stored in approximately equal amounts, and rates of sperm loss following copulation were equivalent. However, when females copulated with both a conspecific and heterospecific male, conspecific sperm fertilized the majority of the eggs, regardless of mating order.
Copulation with a heterospecific followed by a conspecific male, resulted in the relative proportion of conspecific sperm in the spermatheca rising from approximately 20%, 3 h post-copulation to approximately 70%, 48 h post-copulation. In contrast, when the heterospecific male was the second male to mate, the proportion of heterospecific sperm in storage remained constant at approximately 30% at both 3 and 48 h post-copulation. Thus, during sperm competition, there is an increased representation of conspecific relative to heterospecific sperm in the fertilization set, which may account for CSP in this system. Increased representation of conspecific sperm in storage may result from differences in egg production and resultant sperm utilization following conspecific or heterospecific copulation (Eady et al. 2004; Fricke et al. 2006). However, lifetime female fecundity was equivalent between the two groups as was the number of sperm in storage after 48 h of oviposition, following a single conspecific or heterospecific mating. Another possibility is that conspecific males are better able to displace heterospecific sperm via the transfer of more sperm or seminal fluid. Indeed, C. subinnotatus males copulate for longer than C. maculatus males (Rugman-Jones 2003), although they both transfer similar-sized ejaculates to C. subinnotatus females (Rugman-Jones 2003), thus numerical displacement seems unlikely to account for CSP. Price et al. (2000) have argued that components of the conspecific ejaculate displace or incapacitate rival heterospecific sperm. These ejaculatory components may act directly on rival ejaculates or they may interact with the female reproductive environment, providing conspecific sperm with an advantage over heterospecific sperm in the competition to remain in the fertilization set. Alternatively, cryptic female choice (Eberhard 1996) could result in the preferential storage of conspecific sperm, with females responding more strongly to the cryptic stimulation of conspecific males.
Conspecific sperm precedence in the present study also appears to result from a relatively greater fertilizing capacity of conspecific relative to heterospecific sperm. This conclusion stems from the observation that sperm are not used from the fertilization set in proportion to their abundance. Conspecific sperm fertilized a disproportionately higher number of available ova than predicted by their proportionate representation in the spermatheca, indicating the preferential use of conspecific sperm at fertilization (i.e. a loaded raffle; Parker et al. 1990). This may result from three (not mutually exclusive) phenomena.
First, components in the seminal fluid of conspecific males may incapacitate the rival, heterospecific sperm, in a manner similar to that reported in Drosophila (Price 1997; Price et al. 1999).
Second, conspecific sperm may be better able to move from storage to the site of fertilization. In several insect groups, comparative studies have shown strong correlations between sperm length and areas of the female reproductive tract, particularly the spermathecal duct, which may influence sperm movement to and from storage (e.g. Dybas & Dybas 1981; Pitnick et al. 1999; Presgraves et al. 1999; Morrow & Gage 2000). Longer sperm (Pitnick et al. 1999) or sperm of an equivalent length to the spermathecal duct (Morrow & Gage 2000) may generate greater progressive forces through the spermathecal duct, due to increased contact between the sperm tail and the duct wall, thereby enabling them to migrate more rapidly from storage to the site of fertilization. In the Bruchidae, spermathecal duct length is tightly correlated with sperm length (Rugman-Jones 2003); thus, the spermathecal duct may act as a filter favouring the passage of conspecific sperm from the spermatheca to the site of fertilization.
Finally, conspecific sperm may have an advantage over heterospecific sperm at fertilization in relation to their ability to attach to and penetrate an egg. Studies of taxa as diverse as sea urchins and humans highlight the importance of rapidly evolving gamete recognition systems (i.e. proteins that facilitate sperm–egg interactions) as isolating mechanisms (reviewed by Howard et al. 1998; Palumbi 1998; Howard 1999). One or more steps in the gamete recognition process may exhibit species specificity such that, conspecific sperm are better attracted to the egg, or reach/occupy the micropyle, bind faster and more tightly to the egg envelope, penetrate the egg envelope more quickly, or be more likely to fuse with the egg plasma membrane (Vacquier 1998; Howard 1999).
Such superior adaptation of ‘local’ males to the female reproductive environment may also account for patterns of intraspecific sperm precedence. In C. maculatus, significant interactions between male and female genotype have been shown to influence the outcome of sperm competition (Wilson et al. 1997), with sympatric males out-competing rival allopatric males (Brown & Eady 2001). Identification of the selection pressures that drive this coevolution promises to be a challenging field of investigation.
Acknowledgments
We are very grateful to Dr Graham Sandford for irradiating the beetles and to Drs Robert Smith and Peter Credland for supplying the original C. subinnotatus and C. maculatus populations. We also thank Drs Ciara Casey, Mark Curry, Charles Deeming and Daniel Mills for comments on earlier drafts of this MS.
References
- Arnold M.L, Hamrick J.L, Bennett B.D. Interspecific pollen competition and reproductive isolation in Iris. J. Hered. 1993;84:13–16. [Google Scholar]
- Bella J.L, Butlin R.K, Ferris C, Hewitt G.M. Asymetrical homogamy and unequal sex ratio from reciprocal mating-order crosses between Chorthippus parallelus subspecies. Heredity. 1992;68:345–352. [Google Scholar]
- Boorman E, Parker G.A. Sperm (ejaculate) competition in Drosophila melanogaster and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1976;1:145–155. [Google Scholar]
- Brown D.V, Eady P.E. Functional incompatibility between the fertilisation systems of two allopatric populations of Callosobruchus maculatus (Coleoptera: Bruchidae) Evolution. 2001;55:2257–2262. doi: 10.1111/j.0014-3820.2001.tb00740.x. doi:10.1554/0014-3820(2001)055[2257:FIBTFS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; London, UK: 2002. Statistical computing: an introduction to data analysis using S-plus. [Google Scholar]
- Dybas L.K, Dybas H.S. Coadaptation and taxonomic differentiation of sperm and spermathecae in featherwing beetles. Evolution. 1981;35:168–174. doi: 10.1111/j.1558-5646.1981.tb04869.x. doi:10.2307/2407950 [DOI] [PubMed] [Google Scholar]
- Eady P.E. Sperm competition in Callosobruchus maculatus (Coleoptera: Bruchidae): a comparison of two methods used to estimate paternity. Ecol. Entomol. 1991;16:45–53. [Google Scholar]
- Eady P.E. Intraspecific variation in sperm precedence in the bruchid beetle Callosobruchus maculatus. Ecol. Entomol. 1994a;19:11–16. [Google Scholar]
- Eady P.E. Sperm transfer and storage in relation to sperm competition in Callosobruchus maculatus. Behav. Ecol. Sociobiol. 1994b;35:123–129. doi:10.1007/s002650050078 [Google Scholar]
- Eady P.E. Why do male Callosobruchus maculatus beetles inseminate so many sperm? Behav. Ecol. Sociobiol. 1995;36:25–32. doi:10.1007/s002650050121 [Google Scholar]
- Eady P.E, Tubman S. Last-male sperm precedence does not breakdown when females mate with three males. Ecol. Entomol. 1996;21:303–304. [Google Scholar]
- Eady P.E, Rugman-Jones P.F, Brown D.V. Prior oviposition, female receptivity and last-male sperm precedence in the cosmopolitan pest Callosobruchus maculatus (Coleoptera: Bruchidae) Anim. Behav. 2004;67:559–565. doi:10.1016/j.anbehav.2003.07.003 [Google Scholar]
- Eberhard W.G. Princeton University Press; New Jersey, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Fricke C, Arnqvist G. Conspecific sperm precedence in flour beetles. Anim. Behav. 2004;67:729–732. doi:10.1016/j.anbehav.2003.08.014 [Google Scholar]
- Fricke C, Arnqvist G, Amaro N. Female modulation of reproductive rate and its role in postmating prezygotic isolation in Callosobruchus maculatus. Funct. Ecol. 2006;20:360–368. doi:10.1111/j.1365-2435.2006.01102.x [Google Scholar]
- Gregory P.G, Howard D.J. A postinsemination barrier to fertilization isolates two closely related ground crickets. Evolution. 1994;48:705–710. doi: 10.1111/j.1558-5646.1994.tb01355.x. doi:10.2307/2410480 [DOI] [PubMed] [Google Scholar]
- Haines C.P. Natural Resources Institute; Chatham, UK: 1991. Insects and arachnids of tropical stored products: their biology and identification. [Google Scholar]
- Howard D.J. Conspecific sperm and pollen precedence and speciation. Annu. Rev. Ecol. Syst. 1999;30:109–132. doi:10.1146/annurev.ecolsys.30.1.109 [Google Scholar]
- Howard D.J, Reece M, Gregory P.G, Chu J, Cain M.L. The evolution of barriers to fertilization between closely related organisms. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York, NY: 1998. pp. 279–288. [Google Scholar]
- Lale L.E.S, Vidal S. Intraspecific and interspecific competition in Callosobruchus maculatus (F.) and Callosobruchus subinnotatus (Pic) on stored bambara groundnut, Vigna subterranea (L.) Verdourt. J. Stored Prod. Res. 2001;37:329–338. doi: 10.1016/s0022-474x(00)00032-1. doi:10.1016/S0022-474X(00)00032-1 [DOI] [PubMed] [Google Scholar]
- Mbata G.N. Studies on the comparative susceptibility of varieties of bambara groundnut to infestation by Callosobruchus maculatus. Trop. Sci. 1992;32:47–51. [Google Scholar]
- Mbata G.N, Shu S, Ramaswamy S.B. Rhythmicity of mating and oviposition in Callosobruchus subinnotatus (Pic) (Coleoptera: Bruchidae) J. Insect. Behav. 1997;10:409–423. [Google Scholar]
- Mbata G.N, Shu S, Ramaswamy S.B. Sex pheromones of Callosobruchus subinnotatus and C. maculatus (Coleoptera: Bruchidae): congeneric responses and role of air movement. Bull. Entomol. Res. 2000;90:147–154. doi: 10.1017/s0007485300000250. [DOI] [PubMed] [Google Scholar]
- Morrow E.H, Gage M.J.G. The evolution of sperm length in moths. Proc. R. Soc. B. 2000;267:307–313. doi: 10.1098/rspb.2000.1001. doi:10.1098/rspb.2000.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S. Effect of interspecific mating on female fitness in two closely related ladybirds (Henosepilachna) Kontyû. 1985;53:112–119. [Google Scholar]
- Palumbi S.R. Species formation and the evolution of gamete recognition loci. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York, NY: 1998. pp. 271–278. [Google Scholar]
- Parker G.A, Simmons L.W, Kirk H. Analysing sperm competition data: simple models for predicting mechanisms. Behav. Ecol. Sociobiol. 1990;27:55–65. doi:10.1007/BF00183314 [Google Scholar]
- Pitnick S, Markow T.A, Spicer G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. doi:10.2307/2640442 [DOI] [PubMed] [Google Scholar]
- Presgraves D.C, Baker R.H, Wilkinson G.S. Coevolution of sperm and female reproductive tract morphology in stalk-eyed flies. Proc. R. Soc. B. 1999;266:1041–1047. doi:10.1098/rspb.1999.0741 [Google Scholar]
- Price C.S.C. Conspecific sperm precedence in Drosophila. Nature. 1997;388:663–666. doi: 10.1038/41753. doi:10.1038/41753 [DOI] [PubMed] [Google Scholar]
- Price C.S.C, Dyer K.A, Coyne J.A. Sperm competition between Drosophila males involves both displacement and incapaitation. Nature. 1999;400:449–452. doi: 10.1038/22755. doi:10.1038/22755 [DOI] [PubMed] [Google Scholar]
- Price C.S.C, Kim C.H, Posluszny J, Coyne J.A. Mechanisms of conspecific sperm precedence in Drosophila. Evolution. 2000;54:2028–2037. doi: 10.1111/j.0014-3820.2000.tb01246.x. doi:10.1554/0014-3820(2000)054[2028:MOCSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. doi:10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rieseberg L.H, Desrochers A.M, Youn S.J. Interspecific pollen competition as a reproductive barrier between sympatric species of Helianthus (Asteraceae) Am. J. Bot. 1995;82:515–519. doi:10.2307/2445699 [Google Scholar]
- Rugman-Jones, P. F. 2003 Mechanisms and consequences of post-copulatory sexual selection in the Bruchidae. Ph.D. thesis, University of Sunderland, UK.
- Simmons L.W. Princeton University Press; New Jersey, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W, Siva-Jothy M.T. Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 341–434. [Google Scholar]
- Sokal R.R, Rohlf F.J. edn. 3. W.H. Freeman & Co. Ltd; New York, NY: 1995. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Tuda M, Ronn J, Buranapanichpan S, Wasano N, Arnqvist G. Evolutionary diversification of the bean beetle genus Callosobruchus (Coleoptera: Bruchidae): traits associated with stored-product pest status. Mol. Ecol. 2006;15:3541–3551. doi: 10.1111/j.1365-294X.2006.03030.x. doi:10.1111/j.1365-294X.2006.03030.x [DOI] [PubMed] [Google Scholar]
- Vacquier V.D. Evolution of gamete recognition proteins. Science. 1998;281:1995–1998. doi: 10.1126/science.281.5385.1995. doi:10.1126/science.281.5385.1995 [DOI] [PubMed] [Google Scholar]
- Wade M.J, Patterson H, Chang N.W, Johnson N.A. Postcopulatory, prezygotic isolation in flour beetles. Heredity. 1994;72:163–167. doi: 10.1038/hdy.1994.23. [DOI] [PubMed] [Google Scholar]
- Wilson K, Hill L. Factors affecting egg maturation in the bean weevil Callosobruchus maculatus. Physiol. Entomol. 1989;14:115–126. [Google Scholar]
- Wilson N, Tubman S.C, Eady P.E, Robertson G. Female genotype affects male success in sperm competition. Proc. R. Soc. B. 1997;264:1491–1495. doi:10.1098/rspb.1997.0206 [Google Scholar]