Abstract
Adenosine A2A receptors localized in the dorsal striatum are considered as a new target for the development of antiparkinsonian drugs. Co-administration of A2A receptor antagonists has shown a significant improvement of the effects of L-DOPA. The present review emphasizes the possible application of A2A receptor antagonists in pathological conditions other than parkinsonism, including drug addiction, sleep disorders and pain. In addition to the dorsal striatum, the ventral striatum (nucleus accumbens) contains a high density of A2A receptors, which presynaptically and postsynaptically regulate glutamatergic transmission in the cortical glutamatergic projections to the nucleus accumbens. It is currently believed that molecular adaptations of the cortico-accumbens glutamatergic synapses are involved in compulsive drug seeking and relapse. Here we review recent experimental evidence suggesting that A2A antagonists could become new therapeutic agents for drug addiction. Morphological and functional studies have identified lower levels of A2A receptors in brain areas other than the striatum, such as the ventrolateral preoptic area of the hypothalamus, where adenosine plays an important role in sleep regulation. Although initially believed to be mostly dependent on A1 receptors, here we review recent studies that demonstrate that the somnogenic effects of adenosine are largely mediated by hypothalamic A2A receptors. A2A receptor antagonists could therefore be considered as a possible treatment for narcolepsy and other sleep-related disorders. Finally, nociception is another adenosine-regulated neural function previously thought to mostly involve A1 receptors. Although there is some conflicting literature on the effects of agonists and antagonists, which may partly be due to the lack of selectivity of available drugs, the studies in A2A receptor knockout mice suggest that A2A receptor antagonists might have some therapeutic potential in pain states, in particular where high intensity stimuli are prevalent.
Keywords: Adenosine A2A receptor, striatum, hypothalamus, peripheral nervous system, drug addiction, sleep, pain
1. Introduction
There is emerging evidence that A2A receptor antagonists might be useful clinically in treating Parkinson’s disease. A2A receptor antagonists show promising results as an adjuvant to L-DOPA therapy (see 2.3.). The dorsal striatum, which contains a high density of A2A receptors (Rosin et al., 1998), appears to be the site of action for the antiparkinsonian effects of A2A antagonists. The dorsal striatum receives its dopaminergic input from the substantia nigra (pars compacta), the predominant area of neurodegeneration in Parkinson’s disease (Hirsch et al., 1988; Rinne, 1993). The ventral striatum, which receives its dopaminergic input from the ventral tegmental area (VTA; see 2.1.), also contains the same density of A2A receptors (see Lillrank et al., 1999). The ventral striatum (nucleus accumbens) is implicated in drug addition. Here we review recent experimental evidence suggesting that A2A receptor antagonists could become new therapeutic agents for drug addiction.
It is commonly stated that A2A receptors are localized primarily in the striatum while A1 receptors are distributed much more widely (see Ferré et al., 1992, 1997). Although the striatum contains the highest density of A2A receptors in the brain (Rosin et al., 1998), morphological and functional studies have identified lower levels of A2A receptors in other brain areas. A2A receptors appear to be involved in some adenosine-regulated brain functions previously thought to be mediated solely by A1 receptors. Adenosine is an endogenous sleep-promoting substance and it is currently believed that A1 receptors in the cholinergic basal forebrain mediate sleep-inducing mechanisms (recently reviewed in Basheer et al., 2004). Here we review evidence demonstrating a key role of hypothalamic A2A receptors in sleep regulation.
Nociception is another adenosine-regulated neural function previously thought to mostly involve A1 receptors (recently reviewed in Liu and Salter, 2005). Here, we review recent evidence demonstrating that A2A receptors in peripheral nerve endings also regulate nociception. Taken together, this review emphasizes the possible application of A2A receptor ligands in pathological conditions other than Parkinson’s disease.
2. Adenosine A2A receptors in ventral striatum. Implications for drug addiction
2.1. The ventral striatum in drug addiction
The striatum is functionally subdivided into dorsal and ventral striatum. The dorsal striatum receives glutamatergic input from sensoriomotor and association cortical areas and dopaminergic input from the substantia nigra pars compacta (Parent and Hazrati, 1995; Gerfen, 2004). The ventral striatum, mostly represented by the nucleus accumbens (with its two compartments “shell” and “core”), receives glutamatergic input from limbic and paralimbic cortices and the amygdala and hipoccampus, as well as dopaminergic input from the VTA (Parent and Hazrati, 1995; Gerfen, 2004). Glutamatergic and dopaminergic inputs converge on the dendritic spines of the GABAergic striatal efferent neurons, also called medium-sized spiny neurons, which constitute more than 90% of the striatal neuronal population (Gerfen, 2004). Glutamatergic and dopaminergic inputs usually establish synaptic contact with the head and the neck of the dendritic spines, respectively (Gerfen, 2004), enabling dopaminergic neurotransmission to modulate glutamatergic neurotransmission (Fig. 1). Adenosine functions as a modulator of glutamatergic and dopaminergic neurotransmission on GABAergic striatal efferent neurons, in part by acting on heteromers of adenosine receptors with metabotropic glutamate and dopamine receptors (see below).
Figure 1. Scheme of a dendritic spine of the GABAergic enkephalinergic neuron and the A2A receptor-dependent mechanisms involved in drug addiction.
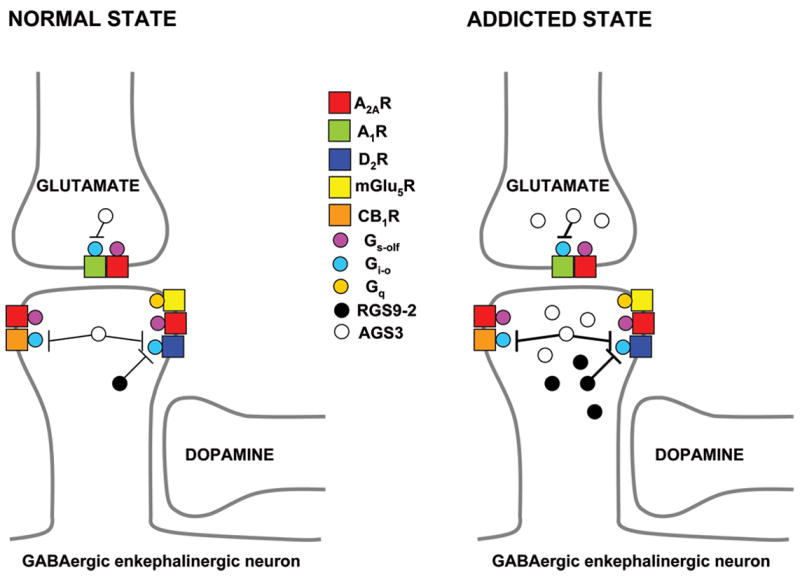
Glutamatergic and dopaminergic inputs usually establish synaptic contact with the head and the neck of the dendritic spines. In the dendritic spines, A2A receptors (A2AR) are localized in the perisynaptic ring adjacent to the postsynaptic density, where they form heteromers with D2. glutamate mGlu5 and cannabinoid CB1 receptors (D2R, mGlu5R and CB1R, respectively). In the glutamatergic terminals A2A receptors are found intrasynaptically, forming heteromers with adenosine A1 receptors (A1R). Involvement of A2A receptors in drug-seeking behavior seems to be related to its modulation of CB1 receptor signaling and its key role in both pre- and postsynaptic glutamatergic neurotransmission, including neuroadaptations such as upregulation of specific accessory proteins (RGS9-2 and AGS3) that dampen Gi/o protein-dependent signaling and promote A2A receptor signaling (see text).
The ventral striatum is a component of the brain circuitry involved in goal-directed behavior, in the conversion of motivation into action, i.e., in the selection of appropriate behavioral responses elicited by specific motivational stimuli (Mogenson et al., 1980). A current working model of the circuitry involved in goal-directed behaviour proposes that the glutamatergic input to the nucleus accumbens carries information about motivationally relevant stimuli to be selected for an appropriate behavioral response, which is implemented by parallel processing of cortico-striatal circuits involving both ventral and dorsal striatum. Dopaminergic input to the nucleus accumbens provides a mechanism to select the most appropriate stimuli according to predictive reward value. Furthermore, dopaminergic input modulates changes in excitatory synapses of the nucleus accumbens, thereby enhancing the probability that motivational stimuli elicit a goal-directed behaviour in the future (Horvitz, 2002; Schultz, 2002; Wolf, 2002; Kelley, 2004; Wise, 2004).
Dopamine release in the ventral striatum is a common characteristic of acute responses to addictive substances (opiates, ethanol, nicotine, amphetamine, cocaine, Δ9-tetrahydrocannabinol) (Pontieri et al., 1995; Di Chiara et al., 1999). It is generally assumed that addictive drugs take control of dopaminergic signals in the nucleus accumbens (and other dopamine-innervated limbic areas), and convert the normal elicitation and learning of goal-directed behaviors into “drug-seeking” and “drug-taking” behaviors (Wolf, 2002; Kelley, 2004). By contrast, advanced stages of addiction, characterized by an uncontrollable urge to obtain drugs and by relapse, seem to depend mostly on adaptations in cortical glutamatergic projections to the nucleus accumbens (Kalivas and Volkow, 2005).
Relapse can be modeled in laboratory animals as reinstatement of drug-seeking behavior after the behavior has been extinguished by drug deprivation. Reinstatement can be produced by administering one dose of the originally self-administered drug (“priming”), by drug-paired environmental cues, or by stress (for recent review, see Bossert et al., 2005). With this paradigm the animals reinstate operant responding (usually lever pressing) for drug in the absence of actual drug delivery. Reinstatement of drug-seeking behavior to different addictive drugs requires the release of glutamate and stimulation of AMPA glutamate receptors in cortico-accumbens synapses, particularly in the nucleus accumbens core (reviewed in Kalivas and Volkow, 2005). It has been hypothesized that pathophysiologic changes in cortico-accumbens glutamatergic synapses promote the compulsive character of drug seeking in addicts by decreasing the value of natural rewards, diminishing cognitive control (choice), and enhancing glutamatergic drive in response to drug-associated stimuli (Kalivas and Volkow, 2005).
2.2. Adenosine A2A receptors in the ventral striatum and the acute behavioral effects of addictive drugs
Both in the dorsal and ventral striatum, two classes of GABAergic efferent neurons, enkephalinergic and dynorphynergic, can be distinguished. GABAergic enkephalinergic neurons contain dopamine and adenosine receptors predominantly of the D2 and A2A subtype, respectively. On the other hand, GABAergic dynorphinergic neurons express A1 and D1 receptors predominantly (Robertson and Jian, 1995; Ferré, 1997; Svenningsson et al., 1997a; Lu et al., 1998). In the ventral striatum GABAergic enkephalinergic and dynorphinergic cells project to the ventral pallidum, which is considered to be an output structure of the basal ganglia (reviewed in Ferré, 1997). The nucleus accumbens also projects to the ventral mesencephalon, with the shell innervating the VTA and the core innervating the substantia nigra. These accumbens-mesencephalic projections are mostly represented by GABAergic dynorphinergic neurons (Robertson and Jian, 1995; Ferré, 1997; Svenningsson et al., 1997a; Lu et al., 1998).
In the brain, adenosine A2A receptors are mostly localized in the striatum (both dorsal and ventral), where they are found in two main locations: in the dendritic spines of GABAergic enkephalinergic neurons and in the glutamatergic terminals (Rosin et al., 2003). In the dendritic spines, A2A receptors seem to be mostly localized in the perisynaptic ring adjacent to the postsynaptic density, where they form heteromers with D2, glutamate mGlu5 and cannabinoid CB1 receptors (Agnati et al., 2003; Ferré et al., 2002, 2005; Ciruela et al., 2006; Carriba et al., 2007) (Fig. 1). In the glutamatergic terminals they are found intrasynaptically, forming heteromers with adenosine A1 receptors (Ciruela et al., 2006) (Fig. 1) and possibly with presynaptic D2, mGlu5 and CB1 receptors (Tanganelli et al., 2004; Rodrigues et al., 2005; Carriba et al., 2007). The different A2A receptor-containing heteromers provide distinct molecular entities with unique functional and pharmacological properties (as reviewed in Ferré et al., 2007). The cross-talk between the A1 and A2A receptors in the A1-A2A receptor heteromer modulates glutamate release. Thus, stimulation of the A2A receptor (at high intrasynaptic concentrations of adenosine) counteracts the inhibitory effect of A1 receptor stimulation (which inhibits glutamate release) and promotes glutamate release (Ciruela et al., 2006; Ferré et al., 2007). The receptor cross-talk in A2A-D2, A2A-mGlu5 and A2A-CB1 receptor heteromers modulates postsynaptic changes at the striatal glutamatergic synapses of the GABAergic enkephalinergic neuron (Ferré et al., 2007).
A2A-D2 receptor interactions have been intensively studied. A2A is a Gs-olf-coupled receptor and D2 is a Gi-o-coupled receptor. Depending on the conditions of study, the A2A-D2 receptors cross-talk implies antagonistic or synergistic interactions (Ferré et al., 1997, 2005, 2007; Agnati et al., 2003, Yao et al., 2002, 2003). The antagonistic A2A-D2 receptor interactions depend on the ability of A2A receptors to modulate the binding characteristics of D2 receptors in the A2A-D2 receptor heteromer (decrease in the affinity of D2 receptor for agonists upon A2A receptor activation) and on the ability of activated D2 receptors to counteract A2A receptor-mediated type V adenylyl cyclase (ACV) activation (Ferré et al., 1997, 2005, 2007; Agnati et al., 2003). Due to the existence of strong synergistic interactions between A2A and mGlu5 receptors, co-activation of mGlu5 receptor upon strong glutamatergic input allows A2A receptor to counteract D2 receptor antagonism (Ferré et al., 2002; 2005, 2007). The synergistic A2A-D2 receptor interactions depend on the presence of an activator of G protein signaling (AGS3), which facilitates a synergistic interaction between Gs- and Gi-coupled receptors on the activation of types II/IV adenylyl cyclase (ACII/IV; see below). Antagonistic A2A-D2 receptor interactions are however predominant in most conditions, since ACV is the most expressed type of adenylyl cyclase in the striatum (Chern, 2000). However, synergistic interactions can become important during conditions of upregulation of AGS3, such as during withdrawal from repeated treatment with cocaine (see below).
In rodents, systemic administration of A2A receptor antagonists counteracts most of the biochemical as well as the motor depressant and cataleptic effects produced by pharmacological or genetic inactivation of D2 receptors, which seems to be related to the predominant A2A receptor-mediated signaling, not opposed by the antagonistic interaction with the D2 receptor in the A2A-D2 receptor heteromer. This has been shown in several experimental models including rodents pretreated with D2 receptor antagonists, reserpine, 6-OH-dopamine or MPTP or after genetic inactivation of D2 receptors (Kanda et al., 1994; Pollack and Fink, 1995; Shiozaki et al., 1999; Ward and Dorsa, 1999; Chen et al., 2001, Ferré et al., 2001; Hauber et al., 2001; Wardas et al., 2001) or MPTP-treated monkeys (Kanda et al., 1998; Grondin et al., 1999). Reserpinized mice, rats with unilateral 6-OH-dopamine lesions and MPTP-treated monkeys are well-established validated models of Parkinson’s disease. The results obtained in animal models of Parkinson’s disease strongly support the hypothesis that A2A receptor antagonists could be useful for the symptomatic treatment of this disease (Ferré et al., 1992). Indeed, clinical trials show that A2A antagonists potentiate L-DOPA therapy in Parkinson’s disease (Bara-Jimenez et al., 2003; Hauser et al., 2003).
However, Parkinson’s disease is characterized by degeneration of dopaminergic neurons in the substantia nigra (pars compacta), reducing dopaminergic innervation of the dorsal striatum. Nevertheless, we have also demonstrated A2A-D2 receptor interactions in the ventral striatum that are similar to interactions in the dorsal striatum (reviewed in Ferré, 1997). At the behavioral level, by acting on the ventral striatum, A2A receptor agonists counteract and A2A antagonists potentiate the motor, discriminative and rewarding effects of psychostimulants (Heffner et al., 1989; Popoli et al., 1994; Rimondini et al., 1997; Shimazoe et al., 2000; Knapp et al., 2001; Poleszak and Malec, 2002; Justinova et al., 2003; Filip et al., 2006). The non-selective A1 and A2A antagonist caffeine also potentiates these responses to psychostimulants (Misra et al., 1986; Logan et al., 1989; Gauvin et al., 1990; Horger et al., 1991; Comer and Carroll, 1996; Gasior et al., 2000; Munzar et al., 2002). By contrast, different from the effects obtained with pharmacological blockade, A2A receptor knockout mice show a reduction in psychostimulant-induced motor responses (Chen et al., 2000, 2003; Soria et al., 2005; for discussion, see Filip et al., 2006). In summary, A2A receptor pharmacological blockade mostly potentiates the acute behavioral effects of psychostimulant addictive drugs, most probably involving the antagonistic interactions between A2A and D2 receptors in the ventral GABAergic enkephalinergic neurons. On the other hand, pharmacological blockade of A2A receptors reduced ethanol self-administration (Arolfo et al., 2004) and genetic and pharmacological experiments also indicate that A2A receptors are involved in the acute behavioral effects of cannabinoids and CB1 receptor agonists. Thus, Soria et al. (2004) have reported a decreased place-preference to Δ9-tetrahydrocannabinol (THC) in mice with genetic blockade of A2A receptors. Furthermore, genetic and also pharmacological blockade of A2A receptors significantly reduces cataleptogenic effects induced by systemic administration of the CB1 receptor agonist CP55,940 (Andersson et al., 2005). Finally, results obtained with A2A receptor knockout mice also suggest a requirement for A2A receptors in morphine and THC withdrawal syndromes (Berrendero et al., 2003; Bailey et al., 2003; Soria et al., 2004).
Some biochemical effects of CB1 receptor agonists had been reported to depend on A2A receptor function, although it was suggested that these effects might be due to indirect interactions involving D2 receptors (Yao et al., 2003, Andersson et al., 2005). We have recently demonstrated that A2A and CB1 receptors co-immunoprecipitate from extracts of rat striatum, where they co-localize in dendritic processes and possibly nerve terminals (Carriba et al., 2007). It was also shown that both receptors form direct physical interactions, i.e., A2A-CB1 receptor heteromers in co-transfected cells (Carriba et al., 2007). At a functional level, CB1 receptor function was found to be dependent on A2A receptor activation both in vitro and in vivo. Thus, activation of A2A receptors was necessary for CB1 receptor signaling in a human neuroblastoma cell line and blockade of A2A receptors significantly decreased the motor depressant effects of the central administration of a synthetic cannabinoid receptor agonist into the rat dorsal striatum (Carriba et al., 2007). This suggests that the rewarding effects of cannabinoids might also depend on striatal A2A-CB1 receptor heteromers, since CB1 receptors localized in the nucleus accumbens seem to be involved in those effects (Gardner, 2005). In fact, we have obtained preliminary evidence indicating that A2A receptor antagonists can counteract self-administration of THC or the endocannabinoid anandamide in squirrel monkeys (Ferré and Goldberg, unpublished). In summary, A2A receptor antagonists can either potentiate or attenuate the acute behavioral effects of addictive drugs, most likely depending on the receptor-receptor interactions and A2A receptor heteromers involved.
2.3. Adenosine A2A receptors in the ventral striatum and drug-seeking behavior
But, what about drug-seeking behavior? Although not yet demonstrated for psychostimulants, we have shown that A2A receptor antagonists, either administered systemically or locally into the nucleus accumbens, completely eliminate heroin-induced reinstatement in rats addicted to self-administered heroin (Yao et al., 2006). As mentioned above, cortico-accumbens glutamatergic neurotransmission seems to play a key role in drug seeking behaviour (Kalivas and Volkow, 2005) and the A2A receptors that are included in receptor heteromers localized pre- and postsynaptically at striatal glutamatergic synapses play an important facilitatory role of glutamatergic neurotransmission (Ferré et al., 2005, 2007). In fact, we have recently shown with in vivo experiments that an A2A receptor antagonist or the non-selective adenosine receptor antagonist caffeine, but not a selective A1 receptor antagonist, completely prevents striatal protein phosphorylation (ERK1/2 and AMPA receptor phosphorylation) induced by a strong cortical input (cortical electrical stimulation) (Quiroz et al., 2006). These experiments involved the dorsal striatum, but ongoing experiments will soon determine if A2A receptor antagonists can also impair cortico-accumbens glutamatergic neurotransmission and the involvement of pre- and presynaptic A2A receptors. Another mechanism by which A2A receptor blockade could prevent drug-seeking behavior is by impairing CB1 receptor function in the A2A-CB1 receptor heteromer (see above). The rational is that several experimental data suggest that CB1 receptor antagonists are particularly effective in reducing cue-induced reinstatement of drug seeking (reviewed in De Vries and Schoffelmeer, 2005).
In addition to blocking cortico-accumbens glutamatergic neurotransmission and interfering with striatal CB1 receptor signaling, A2A receptor antagonists could be beneficial in advanced stages of addiction because of the possible involvement of A2A receptors in the pre- and postsynaptic adaptations of the cortico-accumbens glutamatergic synapses associated with withdrawal from chronic exposure to addictive drugs (Fig. 1). A consistent biochemical finding associated with exposure to different addictive drugs is a reduced signaling through Gi/o protein-coupled receptors (Nestler et al., 1990; Terwilliger et al., 1991; Striplin and Kalivas, 1993; Self et al., 1994; Zhang et al., 2000; Hummel and Unterwald, 2003; Rahman et al., 2003; Bowers et al., 2004). Recent studies suggest that these drug-induced biochemical responses involve specific accessory proteins that regulate Gi/o protein-coupled receptors, such as RGS9-2 and AGS3 (Rahman et al., 2003; Bowers et al., 2004). Regulators of G protein signaling (RGS) constitute a large family of proteins that potently modulate G proteins by stimulating the GTPase activity of the Gα subunits, which can thus dampen G protein-mediated signaling (De Vries et al., 2000; Ross and Wilkie, 2000). RGS9-2 is an RGS family member highly enriched in the GABAergic enkephalinergic and GABAergic dynorphinergic neurons with very low expression in the rest of the brain or peripheral tissues (Thomas et al., 1998; Rahman et al., 2003). RGS9-2 is up-regulated in the dorsal and ventral striatum during chronic cocaine administration, which decreases D2 receptor-mediated signaling in the GABAergic enkephalinergic neurons (Rahman et al., 2003) (Fig. 1).
The activator of G protein signaling (AGS) family consists of three functionally distinct groups (Blumer et al., 2005). AGS3 is particularly enriched in neurons and belongs to Group II, which has the ability to bind preferentially to Giα and stabilize the GDP-bound conformation of Giα, thereby dampening the signaling of the receptor through Giα-GTP, while simultaneously increasing the activity of Gβγ-regulated effectors (Blumer et al., 2005). In primary cultures of nucleus accumbens neurons, D2, μ-opioid and cannabinoid CB1 receptors associate with Giα3 (Yao et al., 2005, 2006) and AGS3 binds preferentially to Giα3, preventing reassociation with released βγ subunits. Upon activation of Giα3-coupled receptors, unbound βγ subunits released in the presence of AGS3 are free to transiently stimulate ACII/IV which can lead to increased cAMP production and therefore to a paradoxical activation of PKA and the cAMP-dependent gene expression (Yao et al. 2002, 2003, 2005, 2006). This βγ-mediated activation of adenylyl cyclase depends on co-stimulation of a Gs-coupled receptor, such as A2A. Thus, activation of D2, μ-opioid or cannabinoid CB1 receptors synergistically stimulates adenylyl cyclase II and IV via released Gβγ subunits. Furthermore, synergy between these receptors requires tonic activation of A2A receptors (Yao et al., 2003, 2006).
It has recently been shown that withdrawal from repeated treatment with cocaine (self- or non-self-administered) up-regulates AGS3 in the prefrontal cortex and in the core region of the nucleus accumbens (Bowers et al., 2004). In rats, knocking down AGS3 expression in the prefrontal cortex or the nucleus accumbens core (with antisense oligonucleotides) counteracts reinstatement of cocaine- or heroin-seeking behaviour, respectively (Bowers et al., 2004; Yao et al., 2005). Therefore, upregulation of AGS3, with the consequent dampening of Giα3 signaling while simultaneously promoting βγ-dependent signaling of Gs-coupled receptors, such as D1 in the prefrontal cortex or in the nucleus accumbens (as suggested by Bowers et al., 2004), or A2A in the nucleus accumbens (as suggested in the present review), can be an important mechanism responsible for the pathophysiologic changes in cortico-accumbens glutamatergic function associated with different addictive drugs (Fig. 1). Upregulation of AGS3 associated with the withdrawal of addictive drugs most probably leads to a predominant signaling of A2A receptors over D2 receptors in the A2A-D2 receptor heteromer and to a paradoxic βγ-mediated synergistic A2A-D2 receptor interaction in the ventral GABAergic enkephalinergic neuron. At the presynaptic level upregulation of AGS3 probably leads to a predominant signaling of A2A receptors over A1 receptors in the A1-A2A receptor heteromer (or over other Gi-coupled receptors, such as group II and III metabotropic glutamate receptors, as suggested by Kalivas and Volkow, 2005) in the cortico-accumbens glutamatergic terminals.
Thus, A2A receptor antagonists, although they can potentiate the acute effects of psychostimulants, could be useful in the treatment of drug addiction and relapse during drug withdrawal. This hypothesis might seem at odds with the results of several reports that indicate that caffeine can reinstate or prevent the extinction of cocaine-seeking behaviour (Worley et al., 1994; Schenk et al., 1996; Self et al., 1996; Kuzmin et al., 1999; Green and Schenk, 2002; Weerts and Griffiths, 2003). However, these effects of caffeine appear to be due to A1 rather than A2A receptor blockade (Kuzmin et al., 1999; Green and Schenk, 2002, Solinas et al, 2002, 2005; Karcz-Kubicha et al., 2003; Quarta et al., 2004 Quarta et al., 2005; Antoniou et al., 2005). Thus, caffeine and selective A1 receptor antagonists have many behavioral, subjective and biochemical similarities to other psychostimulants, including their ability to induce dopamine release in the nucleus accumbens (Solinas et al, 2002, 2005; Karcz-Kubicha et al., 2003; Quarta et al., 2004a, 2004b; Antoniou et al., 2005). Nevertheless, we should distinguish between acute and chronic caffeine treatment. We recently demonstrated that A1 receptor antagonism plays a key role in the acute motor-activating, discriminative stimulus and dopamine-releasing effects of systemically administered caffeine in rats (Solinas et al, 2002, 2005; Karcz-Kubicha et al., 2003; Quarta et al., 2004a, 2004b; Antoniou et al., 2005). However, the A1 receptor antagonistic effects disappear with chronic treatment with caffeine, while A2A receptor antagonistic effects remain (Karcz-Kubicha et al., 2003; Quarta et al., 2004a). Thus, although acute caffeine use can enhance the effects of addictive drugs, chronic caffeine use could be beneficial to avoid relapse. Although more preclinical work needs to be done, particularly using different reinstatement paradigms to study different addictive drugs, the current literature suggests that A2A receptor antagonists could be clinically useful to treat relapse whereas A1 antagonists may be contraindicated.
3. Adenosine A2A receptors in hypothalamus. Implications for sleep regulation
3.1. Adenosine and its role in sleep
The concept of humoral, rather than neural, regulation of sleep dates as far back as almost 100 years ago when Kuniomi Ishimori and Henri Piéron demonstrated the presence of some endogenous sleep-promoting substance(s) that accumulated in the cerebrospinal fluid (CSF) of sleep-deprived dogs (Kubota, 1989). More than 30 so-called endogenous sleep substances in the brain, CSF, urine, and other organs and tissues of animals have been reported since then by numerous investigators. For example, delta-sleep-inducing peptide, muramyl peptides, uridine, oxidized glutathione, vitamin B12, prostaglandin (PG) D2 and adenosine have been identified as endogenous somnogenic substances (Inoué, 1989; Hayaishi, 1991; Basheer et al., 2004). Among these compounds, PGD2 and adenosine are the most plausible candidates for endogenous sleep-promoting substances.
PGD2 is the most potent endogenous sleep substance and adenosine has been proposed to be involved in the somnogenic effect of PGD2 (Hayaishi et al., 2004; Hayaishi and Urade, 2007). PGD2 is produced as a major PG in the brain of various mammals including humans by lipocalin-type PGD synthase (L-PGDS; Qu et al, 2006), which is dominantly present in the arachnoid membrane surrounding the brain, the choroid plexus in the ventricles, and oligodendrocytes in the brain parenchyma (Beuckmann et al., 2000). From its sites of synthesis, it is secreted into the CSF and bound to DP receptors (Qu et al, 2006), which are also present in the arachnoid membrane yet limited to that of the rostral basal forebrain (Mizoguchi et al., 2001). The binding of PGD2 to DP receptors on the meninges is followed by an increase in the extracellular concentration of adenosine (Mizoguchi et al., 2001), which transfers the somnogenic signal from the meningeal membrane into the brain parenchyma including the sleep and wake centers in the hypothalamus (Scammell et al., 1998; 2001).
Adenosine has been considered to be an endogenous sleep substance based on experimental evidence obtained from a variety of pharmacological and behavioral studies. For example, adenosine and its stable analogues are known to induce sleep when administered to rats, cats, and other experimental animals (Basheer et al., 2004). The concentration of extracellular adenosine increases in the cortex and basal forebrain during sleep deprivation of cats and decreases during the recovery period after sleep deprivation (Pokka-Heiskanen et al., 2000). Because energy restoration is one of the functions of sleep, adenosine is proposed to be produced as a terminal product of energy metabolism and to act as a homeostatic regulator of energy in the brain during sleep. Caffeine inhibits sleep by acting as an adenosine antagonist (Fredholm et al., 1999), as described later in detail.
3.2. Adenosine A1 and A2A receptors in sleep regulation
Increased evidences indicate that A2A receptors play a crucial role in the sleep promoting effect of both PGD2 and adenosine (Huang et al., 2007). PGD2-induced sleep is inhibited by pretreatment of rats with the A2A receptor antagonist, KF17837 (Satoh et al., 1996). A2A receptor agonists, such as CGS 21680 and APEC, induce non-rapid eye movement (NREM) sleep when infused into the PGD2-sensitive zone of the basal forebrain of rats during the night (Satoh et al., 1998). On the other hand, A1 receptor agonists, such as CPA, are ineffective. However, previous results suggested that A1 receptors are involved in sleep regulation by inhibiting ascending cholinergic neurons of the basal forebrain (Porkka-Heiskanen et al., 2000).
When CGS 21680 or CPA are infused into the lateral ventricle of wild-type mice, CGS 21680 induces both NREM and REM sleep in a dose- and time-dependent manner, while CPA does not affect their sleep-wake patterns (Urade et al., 2003). A high concentration of CPA slightly increases NREM sleep of wild-type mice. However, CPA-induced sleep was not observed in A2A receptor knockout mice, suggesting that the effect of high concentration of CPA is due to its loss of selectivity for A1 receptors. Thus, although the subtype of adenosine receptor responsible for sleep regulation is still a matter of debate (Basheer et al., 2004), these results strongly suggest a predominant role of A2A receptors in sleep regulation.
3.3. Activation of hypothalamic sleep center in adenosine A2A agonist-induced sleep
There are two important nuclei related to sleep-wake regulation in the hypothalamus, one is ventrolateral preoptic (VLPO) area, a sleep center, and the other is the histaminergic tuberomammillary nucleus (TMN), a wake center. To determine which neuronal groups respond to adenosine, especially to A2A receptor agonists, Fos-immunoreactivity was examined (Satoh et al., 1999; Scammell et al., 2001). When the A2A receptor agonist CGS 21680 was infused for 2 h into the subarachnoid space of the ventral surface of the rat basal forebrain, a marked increase in the number of Fos-positive cells was observed in the VLPO of the anterior hypothalamusr, concomitant with the induction of NREM sleep. In contrast, the number of Fos-positive neurons decreased markedly in the histaminergic TMN of the posterior hypothalamus. Using Fos-immunoreactivity, Sherin et al. (1996) showed a discrete cluster of neurons in the VLPO play a critical role in the generation of sleep. The VLPO is known to send specific inhibitory GABAergic and galaninergic efferents to the TMN, which nucleus contains the ascending histaminergic arousal system. These VLPO neurons may directly induce NREM sleep or send inhibitory signals to the TMN to down-regulate the wake neurons. Thus, the sleep-wake cycle is proposed to be regulated by a flip-flop mechanism involving the interaction between these two centers (Hayaishi and Huang, 2004; Saper et al., 2005).
CGS 21680 was recently demonstrated to inhibit histamine release in both the frontal cortex and medial preoptic area in a dose-dependent manner, and to increase GABA release specifically in the TMN but not in the frontal cortex (Hong et al., 2005). Furthermore, CGS 21680-induced inhibition of histamine release was antagonized by perfusion of the TMN with a GABAA antagonist, picrotoxin, suggesting that the A2A receptor agonist induces sleep by increasing GABA release in the TMN and, therefore, inhibiting the histaminergic arousal system. These results provide further evidence to support the original idea of a flip-flop mechanism by which sleep is promoted by up-regulating the activity of the sleep neurons in the VLPO and, at the same time, down-regulating the activity of the wake neurons in the TMN (Saper et al., 2001, Hayaishi et al., 2004).
Disinhibition of VLPO sleep-active neurons through presynaptic reduction of GABA release by adenosine was suggested by the intracellular recording of VLPO neurons in vitro (Morairty et al., 2004). More recent experiments using intracellular recording of VLPO neurons in rat brain slices demonstrated the existence of two distinct types of VLPO neurons, type-1 and type-2, in terms of their responses to serotonin and adenosine (Gallopin et al., 2005). VLPO neurons are inhibited uniformly by two arousal neurotransmitters, noradrenaline and acetylcholine, and mostly by the A1 receptor agonist CPA. Serotonin inhibits type-1 neurons but excites type-2 neurons. The A2A receptor agonist CGS 21680 excites postsynaptically type-2, but not type-1, neurons. These results suggest that type-2 neurons are involved in the initiation of sleep and that type-1 neurons contribute to sleep consolidation, since they are activated only when released from inhibition by arousal systems.
3.4. Sleep-wake regulation in adenosine A1 and A2A receptor knockout mice
The sleep-wake patterns of A1 receptor knockout mice of the inbred C57BL/6 strain generated by Dr. Fredholm and collaborators (Johansson et al., 2001) and of A2A receptor knockout mice generated by Dr. Chen and collaborators (Chen et al., 2001) were compared with those of wild-type mice. Both knockout mice showed clear circadian variations of sleep-stage distribution during basal conditions, similar to WT mice (Fig. 2). However, A2A receptor knockout mice showed REM sleep rebound after sleep deprivation but not NREM sleep rebound at all, similar to L-PGDS knockout mice and DP receptor knockout mice, indicating that the L-PGDS-DP receptor-A2A receptor system plays a crucial role in the homeostatic regulation of NREM sleep (Hayaishi et al., 2004). On the other hand, A1 receptor knockout mice showed almost the same amounts of NREM and REM sleep rebound after sleep deprivation as those of wild-type mice, suggesting that A1 receptors are not essential for the homeostatic regulation of NREM sleep.
Figure 2. Time course of changes in NREM and REM sleep after caffeine (15 mg/kg) treatment in adenosine A2A receptor wild-type (A2AR WT, a) and knockout (A2AR KO, b), and A1 receptor wild-type (A1R WT, c) and knockout (A1R KO, d) mice.
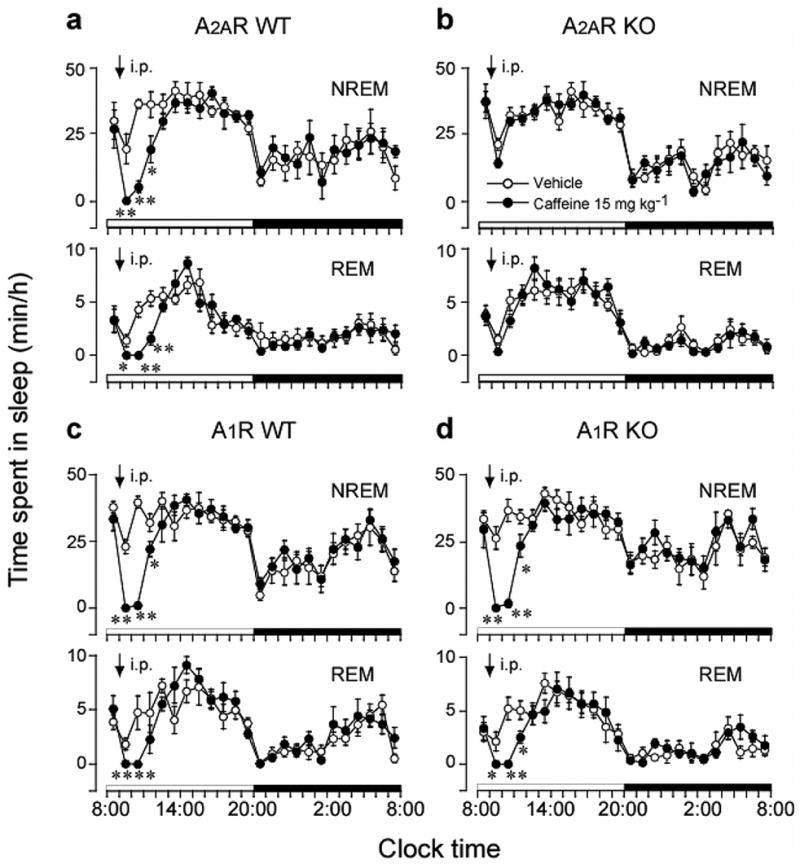
Each circle represents the hourly mean ± s.e.m. (n = 5-7). Open and closed circles stand for the baseline and experimental day profiles, respectively. The arrows indicate the injection time (9 a.m.). *, P < 0.05; **. P < 0.01, significantly different from the vehicle, by the paired t-test. Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Huang et al., 2005).
Caffeine binds to A1 and A2A receptors and acts as a non-selective antagonist and induces insomnia and wakefulness (Fredholm et al., 1999). We have recently obtained evidence for a predominant role of A2A receptor in caffeine-induced wakefulness using A1 and A2A receptor knockout mice. Thus, caffeine increased wakefulness in both wild-type and A1 receptor knockout mice, but not in A2A receptor knockout mice (Huang et al., 2005). When caffeine is administered by the intraperitoneal route to wild-type mice (Fig. 2a and 2c) and A1 receptor knockout mice (Fig. 2d) at a dose of 15 mg/kg, it suppresses both NREM and REM sleep almost completely for 2 hr after the administration. NREM and REM sleep returned to the normal levels by 4 hr after the caffeine injection. In contrast, caffeine given to A2A receptor knockout mice does not change the profiles of NREM and REM sleep (Fig. 2b). Thus, insomnia should be considered as a possible side effect when considering the application of A2A receptor antagonists in Parkinson’s disease, drug addiction or pain, which could probably be counteracted by avoiding evening administration. Also, A2A receptor antagonists could be considered as a possible treatment for narcolepsy and other sleep-related disorders.
4. Adenosine A2A receptors in nociceptive circuitry. Implications for pain
4.1. Adenosine and its role in pain
In considering the role of the A2A receptor in pain it should first be stressed that the evidence comes predominantly from experimental animal studies, and thus strictly speaking we are talking about the role of the A2A receptor in nociception (i.e. a response to a noxious stimulus). The description of pain requires verbalization and is thus exclusively a human phenomenon. Nevertheless, there has been clinical use of adenosine in pain and thus some aspects of adenosine receptor modulation can truly be described as pain modulation. Throughout this review there will be some synonymous use of the terms pain and nociception and also of analgesia and antinociception, recognizing that animal models of nociception have led to the development and clinical use of analgesics for pain conditions.
The endogenous mediator adenosine has complex effects in pain, and can be either pronociceptive or antinociceptive depending on the site of administration and the receptor subtype activated (see Sawynok, 1998; Sawynok and Liu, 2003). In addition, as adenosine has similar nanomolar affinities for both A1 and A2A receptors (see Fredholm et al., 2001b) their relative contribution to the endogenous role of adenosine in pain processing may be finely balanced. Also, inevitably ascribing a role to each receptor in the action of adenosine is dependent on the use of highly subtype-selective receptor ligands or receptor knockout mouse models.
Most attention has been focused on the effects on pain pathways of adenosine acting via the A1 receptor. There is ample evidence that activation of spinal A1 receptors results in antinociceptive effects in a wide range of animal models, including both acute nociceptive tests and models of neuropathic and inflammatory pain (see Dickenson et al., 2000; Sawynok, 1998). There is debate as to whether A1 receptors on peripheral sensory nerves are pro- or antinociceptive, as inhibition of PGE2-induced nociception (Aley et al., 1995; Aley and Levine, 1997) and stimulation of sensory afferents (Dowd et al., 1998; Kirkup et al., 1998) has been reported after A1 receptor activation. Overall, however, the antinociceptive effects of central A1 receptors dominate and this is confirmed in A1 receptor gene knockout mice, which have an enhanced response to nociceptive stimuli (Johansson et al., 2001).
In humans, systemic infusion or intrathecal injection of adenosine has been demonstrated to have long-lasting clinical benefit in patients with neuropathic pain (Belfrage et al., 1995, 1999) and also after major surgery (Segerdahl, 1997; Segerdahl et al., 1995; Segerdahl and Sollevi, 1998). It is believed that this is largely A1 receptor mediated. Accordingly most of the interest in adenosine and pain has related to its A1 effects and the importance of the A2A receptor and the endogenous role of adenosine at this site has been relatively neglected.
4.2. Pain circuitry and localization of adenosine A2A receptors
The production of pain and its amelioration involves sites in the periphery, the spinal cord and via ascending and descending pathways to and from the brain. Clearly the localization of any receptor potentially involved in pain circuitry has a bearing on where any pain modulation resulting from receptor agonism or antagonism might occur. Both ascending and descending pain pathways have been well characterized (see Millan, 1999; 2002) and several mid and hindbrain regions, such as the thalamus, hypothalamus, parabrachial nucleus, pons, superior colliculus, raphe, periaqueductal grey and locus coreleus, are well established as key relay structures in pain circuitry. In addition, integration of pain signals involves the somatosensory cortex and other frontal cortical regions. It might be easy to be dismissive of a central role for A2A receptors in pain processing as autoradiographic mapping of the receptor with highly selective ligands in both rat (Johansson et al., 1997; Svenningsson et al., 1999) and mouse (Bailey et al., 2002; Bailey et al., 2004; Kelly et al., 2004) as well as in humans (Svenningsson et al., 1997b; Ishiwata et al., 2005) shows predominantly a localization to the dorsal and ventral striatum, structures with no major recognized role in pain circuitry. However, there is some evidence that both the dorsal and ventral striatum are directly accessed by nociceptive neurones (Newman et al., 1996) and that the ventral striatum, like many parts of the limbic system, modulates pain processing (see Millan, 1999). Using immunohistochemical techniques, most of the receptor expression is also seen in the basal ganglia (Rosin et al., 1998). However, it should be stressed that A2A receptors are found at relatively low levels in somatosensory cortex (Johansson et al., 1997; Kelly et al., 2004) and their presence in the cortex has been confirmed by western blotting (Lopes et al., 2004), and thus a potential role of A2A receptors in the central integration of pain cannot be completely ignored.
Early studies suggested that A2 as well as A1 receptors were present in the dorsal spinal cord of the rat (Choca et al., 1987). However, the A2A receptor gene does not appear to be expressed in the spinal cord (Kaelin-Lang et al., 1998) and autoradiographic techniques with selective A2A receptor ligands, at concentrations well above KD values, confirm the absence of spinal cord receptor binding (Bailey et al., 2002). Despite this evidence, recent immunohistochemistry studies coupled with functional electrophysiology have shown responses to the A2A selective agonist CGS 21680 in spinal cord (Brooke et al., 2004) and it has been proposed that these receptors are located on presynaptic inhibitory terminals of descending fibers from higher centers (Brooke et al., 2004). Thus, as for the central nervous system, the possibility of spinal effects of A2A receptor activation cannot be completely excluded.
The A2A receptor gene is expressed in the dorsal root ganglion (Kaelin-Lang et al., 1998) and this had led to the suggestion that there is retrograde transport of the receptor to the peripheral terminals in sensory nerve fibres. Added to this the effects of pharmacological modulation (see 4.3) at sensory nerve sites, the primary site of A2A receptor modulation of pain is most likely to be at peripheral nerve terminals (Sawynok, 1998).
4.3. Pharmacological studies with adenosine A2A receptor agonists and antagonists (Table 1)
Table 1.
Pain responses to modulation of the adenosine A2A receptor
| A2A modulation | Species, dose and route | Nociceptive test | Effect | Reference |
|---|---|---|---|---|
| A2A agonists | ||||
| CGS 21680 | Mouse, 10 μg i.th. | Tail flick | Analgesia | Suh et al., 1997 |
| Rat, 0.1–10 nmol i.th. | Carrageenan (2 mg/100 μl) | Analgesia | Poon and Sawynok, 1998 | |
| Rat, 2–40 nmol i.th. | Neuropathic spinal ligation | Analgesia | Lee and Yaksh, 1996 | |
| Mouse, 0.015–0.3 mg/kg i.p. | Formalin (5%) | Analgesia (early phase) | Borghi et al., 2002 | |
| Mouse, 0.01–0.1 mg/kg i.p. | Writhing (0.6% acetic acid) | Analgesia (not dose related) | Bastia et al., 2002 | |
| Mouse, 1–10 mg/kg i.p | Hot plate 52.5°C | Hyperalgesia (0.01 mg/kg only) | Bastia et al., 2002 | |
| Rat, 1.5–50 nmol i.pl. | Formalin (0.5%) | Hyperalgesia (1.5 nmol) Analgesia (50 nmol) | Doak and Sawynok, 1995 | |
| DPMA | Mouse, 0.1–1 mg/kg i.p. | Writhing (1% acetic acid) | Analgesia (not dose-related) | Pechlivanova and Georgiev, 2002 |
| APEC | Mouse, 0.1 μM i.pl. | Formalin | Hyperalgesia | Karlsten et al., 1992 |
| A2A antagonists | ||||
| SCH 58261 | Mouse, 1–10 mg/kg i.p | Writhing (0.6% acetic acid) | Analgesia | Bastia et al., 2002 |
| Mouse, 1–10 mg/kg i.p | Hot plate52.5°C | No effect | Bastia et al., 2002 | |
| Mouse 3 mg/kg i.p. | Hot plate 55°C | Analgesia | Godfrey et al, 2006 | |
| Mouse 3 mg/kg i.p. | Tail immersion 53°C | Analgesia | Godfrey et al., 2006 | |
| DMPX | Mouse, 0.05–1 mg/kg i.p. | Writhing (1% acetic acid) | No effect | Pechlivanova and Georgiev, 2002 |
| Rat, 50 nmol i.pl. | Formalin (2.5%) | Analgesia | Doak and Sawynok, 1995 | |
| Mouse, 1–40 μg i.th. | Tail flick | No effect | Suh et al, 1997 | |
| MSX-3 | Mouse 30–100 mg/kg i.p. | Hot plate 52°C | No effect | Abo-Salem et al., 2004 |
| A2A gene knockout | Mouse | Hot plate 55°C | Hypoalgesia | Ledent et al., 1997 |
| Tail flick | Hypoalgesia | Ledent et al., 1997 | ||
| Tail immersion 55°C | Hypoalgesia | Bailey et al., 2002 | ||
| Tail immersion 53°C | Hypoalgesia | Godfrey et al., 2006 | ||
| Tail immersion 52°C | No effect | Bailey et al., 2002 | ||
| Tail pressure | No effect | Bailey et al., 2002 | ||
| Formalin (5%) | Analgesia (flinches and bites, early phase; flinches only late phase) | Hussey et al., 2007 |
i.p. intraperitoneal, i.pl. intraplantar, i.th. intrathecal. CGS 21680, DMPA, APEC, SCH 58261, DMPX, MSX-3
Much of the pharmacological evidence for A2 receptor involvement in nociception comes from studies using pharmacological tools with limited selectivity over the A1 receptor. Because the A1 receptor has potent effects on nociception at peripheral, spinal and supraspinal sites, it is highly likely that many of these studies are confounded by lack of selectivity for the A2A receptor of the compounds used. Nonetheless, studies with A2A receptor agonists have shown antinociceptive effects in some, but not all, nociceptive tests. For example, CGS 21680 is antinociceptive in the writhing test, a visceral pain model (Bastia et al., 2002), an effect reversed by the A2A receptor antagonist, SCH 58261. Antinociception in the writhing test is also observed with the A2A receptor agonist DPMA (Pechlivanova and Georgiev, 2002). CGS 21680 has also been shown to induce antinociception in thermal tests after intrathecal administration (Suh et al., 1997) and also in inflammatory (Poon and Sawynok, 1998) and neuropathic models (Lee and Yaksh, 1996). Some of these studies represent high dose pharmacology and raise the issue of possible activation of the A1 receptor, and several did not benefit from reversal by selective A2A receptor antagonists that are available now.
Local administration of formalin into the hindpaw activates both C- and Aδ fibers, results in peripheral release of adenosine (Liu et al., 2001) and produces licking, biting and flinching behaviours indicative of pain. In the formalin test CGS 21680 has been shown to be both antinociceptive (Borghi et al., 2002) and pronociceptive in rats (Doak and Sawynok, 1995) as is the A2 receptor agonist APEC in mice (Karlsten et al., 1992). A2 receptor agonists are also pronociceptive in a mechanical paw pressure test in the rat (Taiwo and Levine, 1990), though these early studies used very poorly selective drugs. Added to this, the recent development of highly selective A2B antagonists shows that these compounds have antinociceptive activity in the hot-plate test (Abo-Salem et al., 2004) and thus the A2A-A2B selectivity of the pharmacological tools used in nociceptive studies also needs to be considered alongside their A1 activity. The literature on A2A receptor agonists is therefore contradictory although microdialysis studies of the release of adenosine in the hind paw in response to formalin has led to the proposal that adenosine may act at pronociceptive A2A receptors. (Liu et al., 2000)
Studies with A2A receptor antagonists have been rather more consistent showing predominantly antinociception, which would accord with a pronociceptive effect of A2A receptor agonism and also with the hypoalgesia observed in A2A receptor gene knockout mice (see 4.4). SCH 58261 is antinociceptive in both the writing test (Bastia et al., 2002) and in the tail flick and hot plate test, two acute thermal pain tests (Godfrey et al., 2006). A less selective A2A receptor antagonist, DMPX, is not antinociceptive in the writhing test in mice (Pechlivanova and Georgiev, 2002), but does reverse formalin-induced flinching responses in the rat (Doak and Sawynok, 1995), again indicative of pronociceptive A2A receptors on peripheral nerves.
4.4. Adenosine A2A receptor gene knockouts and nociception (Table 1)
Deletion of the A2A receptor gene was first achieved in 1997 and the original behavioural phenotyping of the A2A receptor knockout mice showed a hypoalgesia in thermal tests of pain (Ledent et al., 1997). These observations have been extended, and although hypoalgesia is observed in the tail immersion test and hot-plate test at 55°C (Bailey et al., 2002; Godfrey et al., 2006) and in the tail immersion test at 53°C (Godfrey et al., 2006) in A2A receptor knockout mice, at the lower temperature of 52°C nociceptive responses are not altered (Bailey et al., 2002). In addition, there are no significant differences in nociceptive latencies in the knockout mice in the tail pressure test (Bailey et al., 2002). Thus, alteration in pain sensitivity from deletion of the A2A receptor gene is stimulus dependent, both in terms of intensity and modality, but under some circumstances loss of the A2A receptor leads to reduced pain, suggesting a role for the receptor in activating pain processing. Other observations support this concept of a tonic role for the receptor in pain transmission from peripheral sensory nerves to the spinal cord. For example, A2A receptor knockout mice have greatly reduced NMDA receptor binding in all laminae and regions of the spinal cord, perhaps because of altered glutamate signaling in C-fibres (Hussey et al., 2007). In addition, A2A receptor knockout mice have reduced biting and flinching responses to intraplantar formalin injections in the first phase (0–15min) of the formalin test and reduced flinching only in the second phase (15–60min) of the test (Hussey et al., 2007). As the first phase of the formalin test is recognized to be due to direct stimulation of peripheral sensory nerves whilst the second phase results from an inflammatory component and involves more complex pain circuitry (Dickenson and Sullivan, 1987), this data further implicates A2A receptors on peripheral sensory nerves in pain modulation. The effect on the second phase also suggests that A2A receptors on inflammatory cells might be of importance in pain modulation and a well defined regulatory role and a potential therapeutic target has been suggested (Lappas et al., 2005). In addition, a potential for A2A receptor antagonists in inflammatory pain is further supported by blockade of both phases of the formalin response by SCH 58261 (Hussey et al, 2007).
4.5. Adenosine A2A receptors and interactions with opioid nociception
Important interactions between adenosine systems and opioid (μ, δ and κ) receptors in the central nervous system and spinal cord have long been recognized. In relation to pain, in the spinal cord there is evidence that a component of the action of morphine is due to the release of adenosine (Sweeney et al., 1987; Sweeney et al., 1989) and there is also evidence of analgesic synergy between adenosine and opioid agonists active at the δ and κ but not the μ opioid receptors (De Lander and Keil II, 1994). Others have shown that the relatively selective A2A receptor antagonist DMPX administered intrathecally antagonises morphine analgesia after intracerebroventricular administration, suggesting a spinal A2A receptor involvement in the supraspinal action of morphine (Suh et al., 1997). The adenosine receptor subtype involved in the adenosine released by morphine has not been defined though at the spinal level much of the pharmacology points to a key role for A1 receptors (Montegazza et al., 1984; Reeve and Dickenson, 1995; Zarrindast and Nikfar, 1994). Several studies have also reported morphine-induced or enhanced release of adenosine in the brain (Fredholm and Vernet, 1978; Phillis and Jiang, 1980; Stone, 1981) but much of the behavioural interactions have been focused to addiction rather than pain. Although central administration of theophylline does not alter morphine antinociception (De Lander et al., 1992), the adenosine analogue and A1 receptor agonist L-PIA can potentiate tolerance to the analgesic effect of morphine (Ahlijanian and Takemori, 1985). There are also a number of studies that show plasticity in both A1 and A2A receptors in the brain after chronic administration of opioids (Kaplan and Leite-Morris, 1997; Kaplan et al., 1994), and A2A receptor knockout mice show altered expression of the opioid peptide precursors proenkephalin, prodynorphin (Ledent et al., 1997) and pro-opiomelanocortin (Jegou et al., 2003) though not opioid receptors (Bailey et al., 2002). Although A1 receptor knockout mice are hyperalgesic, antinociceptive responses to morphine after systemic injection are unaffected in the A1 receptor knockout mice which would point to a lack of involvement of the A1 receptor in morphine’s effect on pain (Johansson et al., 2001). However, if injected intrathecally, the morphine response is reduced in the A1 receptor knockout mice implying a spinal interaction (Wu et al., 2005). In addition, it should be noted that changes in μ opioid receptor expression in the spinal cord in response to formalin injections in the paw can be counteracted by treatment with either A1 or A2A receptor agonists and thus both receptors may play a role in spinal responses to opioids (Borghi et al., 2002).
Studies on peripheral mechanisms of pain have pointed to important interactions of adenosine and opioid systems at sensory nerves. Intradermal co-injections of μ opioid and A1 receptor agonists with the inflammatory mediator PGE2 show a bi-directional cross-tolerance to peripheral antinociception, suggesting a common cellular role for the μ opioid and A1 receptors on primary afferent nociceptors (Aley et al., 1995). Further cross-tolerance and cross-withdrawal studies have led to the proposal that a μ opioid, α2 adrenergic, A1 receptor complex mediates antinociception in the periphery (Aley and Levine, 1997). A role for the A2A receptor on peripheral nerve terminals altering opioid mediated responses has also been suggested (Bailey et al., 2002). Whilst antinociceptive responses to the μ opioid receptor agonist morphine are unaffected in A2A receptor knockout mice, δ opioid receptor-mediated antinocieption is decreased and κ opioid antinociception is increased. These behavioural effects can be correlated with parallel changes in spinal opioid receptor expression and suggest that lack of the A2A receptor at peripheral terminals alters pain processing to the spinal cord resulting in altered opioid modulation (Bailey et al., 2002).
In conclusion, the evidence points to opioid-adenosine interactions at all levels of pain processing. At peripheral sites, A1 receptors synergise with MOP receptors, and this is also the predominant response in the spinal cord. Spinal interactions of the A2A receptor are also evident for the δ and κ opioid receptors, but not the μ opioid receptor. In the brain, although opioid-adenosine interactions have been reported for other behaviors their potential co-operative role in supraspinal nociception is less clear.
4.6. Therapeutic potential of adenosine A2A receptor modulation and pain
Overall the evidence suggests that A1 receptors exert inhibitory effects on nociception at peripheral and spinal sites whilst the A2A receptor has an opposing role. Our current understanding suggests that the A2A receptor plays a role in pain processing and that, although spinal and supraspinal sites cannot be completely ignored, peripheral A2A receptors on sensory nerves are probably the most important in this regard. Although there is some conflicting literature on the effects of agonists and antagonists, which may be partly due to the lack of selectivity of available drugs, the studies in A2A receptor knockout mice and the analgesic effects of SCH 58261 in inflammatory models, suggest that blockade of the A2A receptor might have some therapeutic potential in pain states. As there appear to be some differences in the involvement of the A2A receptor dependent on the intensity and modality of the stimulus, more detailed studies are needed to determine whether A2A receptor antagonists might have clinical utility in certain types of pain conditions, in particular where high intensity stimuli are prevalent.
Acknowledgments
This work has been supported by the National Institute on Drug Abuse Intramural Research Funds, by EC (LSHM-CT-2004-005166) and Glaxo Smith Kline.
Abbreviations
- AGS
activator of G protein signaling
- AMPA
α-amino-3-hydroxy-5-methyl-isoxazole-4-proprionate
- APEC
2-[(2-aminoethylamino)carbonylethyl phenylethylamino]-5’-N-ethylcarboxamido adenosine
- CGS 21680
2-[p-(2-carboxyethyl)phenethylamino]-5’-N-ethylcarboxamido- adenosine
- CSF
cerebrospinal fluid
- DMPX
3,7-dimethyl-1-propargylxanthine
- CPA
N(6)-cyclopentyladenosine
- GABA
γ-aminobutyric acid
- DPMA
N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine. KF17837, (E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine
- L-PIA
[L(−)N(6)-(2-phenylisopropyl)adenosine]
- L-PGDS
lipocalin-type prostaglandin D synthase
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NMDA
N-methyl-D-aspartate
- NREM
non-rapid eye movement
- PG
prostaglandin
- PKA
protein kinase A
- REM
rapid eye movement
- RGS
regulator of G protein signaling
- SCH 58261
[5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-epsilon]-1,2,4-triazolo[1,5-c]-pyrimidine
- TMN
tuberomammillary nucleus
- VLPO
ventrolateral preoptic
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Muller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther. 2004;308:358–366. doi: 10.1124/jpet.103.056036. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Ahlijanian MK, Takemori AE. Effects of (−)-N6-(-phenylisopropyl)-adenosine (PIA) and caffeine on nociception and morphine-induced analgesia, tolerance and dependence in mice. Eur J Pharmacol. 1985;112:171–179. doi: 10.1016/0014-2999(85)90493-5. [DOI] [PubMed] [Google Scholar]
- Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8031. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Multiple receptors involved in peripheral alpha2, mu and A1 antinociception, tolerance and withdrawal. J Neurol Sci. 1997;17:735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Usiello A, Borgkvist A, Pozzi L, Dominguez C, Fienberg AA, Svenningsson P, Fredholm BB, Borrelli E, Greengard P, Fisone G. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25:8432–8438. doi: 10.1523/JNEUROSCI.1289-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Muller CE, Goldberg SR, Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Bailey A, Ledent C, Kelly M, Hourani SM, Kitchen I. Changes in spinal delta and kappa opioid systems in mice deficient in the A2A receptor gene. J Neurosci. 2002;22:9210–9220. doi: 10.1523/JNEUROSCI.22-21-09210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Davis L, Lesscher HM, Kelly MD, Ledent C, Hourani SM, Kitchen I. Enhanced morphine withdrawal and mu-opioid receptor G-protein coupling in A2A adenosine receptor knockout mice. J Neurochem. 2003;88:827–834. doi: 10.1046/j.1471-4159.2003.02214.x. [DOI] [PubMed] [Google Scholar]
- Bailey A, Weber D, Zimmer A, Zimmer AM, Hourani SM, Kitchen I. Quantitative autoradiography of adenosine receptors and NBTI-sensitive adenosine transporters in the brains of mice deficient in the preproenkephalin gene. Brain Res. 2004;1025:1–9. doi: 10.1016/j.brainres.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, Morris MJ, Mouradian MM, Chase TN. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bastia E, Varani K, Monopoli A, Bertorelli R. Effects of A1 and A2A adenosine receptor ligands in mouse acute models of pain. Neurosci Lett. 2002;328:241–244. doi: 10.1016/s0304-3940(02)00524-4. [DOI] [PubMed] [Google Scholar]
- Belfrage M, Sollevi A, Segerdahl M, Sjolund KF, Hansson P. Systemic adenosine infusion alleviates spontaneous and stimulus-evoked pain in patients with peripheral neuropathic pain. Anesthesia and Analgesia. 1995;81:713–717. doi: 10.1097/00000539-199510000-00010. [DOI] [PubMed] [Google Scholar]
- Belfrage M, Segerdahl M, Arner S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesthesia and Analgesia. 1999;89:136–142. doi: 10.1097/00000539-199907000-00023. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. Increase of morphine withdrawal in mice lacking A2a receptors and no changes in CB1/A2a double knockout mice. Eur J Neurosci. 2003;17:315–324. doi: 10.1046/j.1460-9568.2003.02439.x. [DOI] [PubMed] [Google Scholar]
- Beuckmann CT, Lazarus M, Gerashchenko D, Mizoguchi A, Nomura S, Mohri I, Uesugi A, Kaneko T, Mizuno N, Hayaishi O, Urade Y. Cellular localization of lipocalin-type prostaglandin D synthase (beta-trace) in the central nervous system of the adult rat. J Comp Neurol. 2000;428:62–78. doi: 10.1002/1096-9861(20001204)428:1<62::aid-cne6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Cismowski MJ, Sato M, Lanier SM. AGS proteins: receptor-independent activators of G-protein signaling. Trends Pharmacol Sci. 2005;26:470–476. doi: 10.1016/j.tips.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Borghi V, Przewlocka B, Labuz D, Maj M, Ilona O, Pavone F. Formalin-induced pain and mu-opioid receptor density in brain and spinal cord are modulated by A1 and A2a adenosine agonists in mice. Brain Res. 2002;956:339–348. doi: 10.1016/s0006-8993(02)03568-0. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke RE, Deuchars J, Deuchars SA. Input-specific modulation of neurotransmitter release in the lateral horn of the spinal cord via adenosine receptors. J Neurosci. 2004;24:127–137. doi: 10.1523/JNEUROSCI.4591-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Yu L, Martin AB, Xu K, Bastia E, Hackett E, Alberti I, Schwarzschild MA. Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology. 2003;28:1086–1095. doi: 10.1038/sj.npp.1300152. [DOI] [PubMed] [Google Scholar]
- Chern Y. Regulation of adenylyl cyclase in the central nervous system. Cell Signal. 2000;12:195–204. doi: 10.1016/s0898-6568(99)00084-4. [DOI] [PubMed] [Google Scholar]
- Choca JI, Proudfit HK, Green RD. Identification of A1 and A2 adenosine receptors in the rat spinal cord. J Pharmacol Exp Ther. 1987;242:905–910. [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2005;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Carroll ME. Oral caffeine pretreatment produced modest increases in smoked cocaine self-administration in rhesus monkeys. Psychopharmacology. 1996;126:281–285. doi: 10.1007/BF02247378. [DOI] [PubMed] [Google Scholar]
- De Lander GE, Mosberg HI, Porreca F. Involvement of adenosine in antinociception produced by spinal or supraspinal receptor-selective opioid agonists: dissociation from gastrointestinal effects in mice. J Pharmacol Exp Ther. 1992;263:1097–104. [PubMed] [Google Scholar]
- De Lander GE, Keil GJ., II Antinociception induced by intrathecal coadministration of selective adenosine receptor and selective opioid receptor agonists in mice. J Pharmacol Exp Ther. 1994;268:943–951. [PubMed] [Google Scholar]
- De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurons. Neurosci Lett. 1987;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Suzuki R, Reeve AJ. Adenosine as a potential analgesic target in inflammatory and neuropathic pains. CNS Drugs. 2000;13:77–85. [Google Scholar]
- Doak GJ, Sawynok J. Complex role of peripheral adenosine in the genesis of the response to subcutaneous formalin in the rat. Eur J Pharmacol. 1995;281:311–318. doi: 10.1016/0014-2999(95)00257-l. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. Adenosine A1 receptor-mediated excitation of nociceptive afferents innervating the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:1267–1271. doi: 10.1038/sj.bjp.0702185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Gimenez-Llort L, Rimondini R, Muller CE, Stromberg I, Ogren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Borycz J, Goldberg SR, Hope BT, Morales M, Lluis C, Franco R, Ciruela F, Cunha R. Role of adenosine in the control of homosynaptic plasticity in striatal excitatory synapses. J Integr Neurosci. 2005;4:445–464. doi: 10.1142/s0219635205000987. [DOI] [PubMed] [Google Scholar]
- Ferré S, Agnati LF, Ciruela F, Lluis C, Woods AS, Fuxe K, Franco R. Neurotransmitter receptor heteromers and their integrative role in “local modules”. The striatal spine module. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegalinski E, Muller CE, Agnati L, Franco R, Roberts DC, Fuxe K. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Vernet L. Morphine increases depolarization induced purine release from rat cortical slices. Acta Physiol Scan. 1978;104:502–504. doi: 10.1111/j.1748-1716.1978.tb06308.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001a;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001b;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 295:1101–1111. [PubMed] [Google Scholar]
- Gauvin DV, Criado JR, Moore KR, Holloway FA. Potentiation of cocaine’s discriminative effects by caffeine: a time-effect analysis. Pharmacol Biochem Behav. 1990;36:195–197. doi: 10.1016/0091-3057(90)90149-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; Amsterdam: 2004. pp. 445–508. [Google Scholar]
- Godfrey L, Yan L, Clarke GD, Ledent C, Kitchen I, Hourani SMO. Modulation of paracetamol antinoiception by caffeine and by selective adenosine A2 receptor antagonists in mice. Eur J Pharmacol. 2006;531:80–86. doi: 10.1016/j.ejphar.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Grondin R, Bedard PJ, Hadj Tahar A, Gregoire L, Mori A, Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/wnl.52.8.1673. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Muller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Hubble JP. Truong, D.D., Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Molecular mechanisms of sleep-wake regulation: roles of prostaglandins D2 and E2. FASEB J. 1991;5:2575–2581. [PubMed] [Google Scholar]
- Hayaishi O, Urade Y, Eguchi N, Huang ZL. Genes for prostaglandin D synthase and receptor as well as adenosine A2A receptor are involved in the homeostatic regulation of nrem sleep. Arch Ital Biol. 2004;142:533–539. [PubMed] [Google Scholar]
- Hayaishi O, Huang ZL. Role of orexin and prostaglandin E2 in activating histaminergic neurotransmission. Drug News Perspect. 2004;17:105–109. doi: 10.1358/dnp.2004.17.2.829043. [DOI] [PubMed] [Google Scholar]
- Hayaishi O, Urade Y. Prostaglandins and sleep/wake regulation. The Neurochemistry of Sleep and Wakefulness. 2007 in press. [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology. 1989;98:31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Agatsuma S. Genetic susceptibility to substance dependence. Mol Psychiatry. 2005;10:336–344. doi: 10.1038/sj.mp.4001622. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S. Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport. 1991;2:53–56. doi: 10.1097/00001756-199101000-00013. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. Intra-accumbens pertussis toxin sensitizes rats to the locomotor activating effects of a single cocaine challenge. Brain Res. 2003;965:100–107. doi: 10.1016/s0006-8993(02)04142-2. [DOI] [PubMed] [Google Scholar]
- Hussey M, Clarke GD, Ledent C, Hourani SMO, Kitchen I. Reduced response to the formalin test and lowered spinal NMDA glutamate receptor binding in adenosine A2A receptor knockout mice. Pain. 2007 doi: 10.1016/j.pain.2006.10.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Inoué S. Biology of Sleep Substances. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- Ishiwata K, Mishina M, Kimura Y, Oda K, Sasaki T, Ishii K. First visualization of adenosine A(2A) receptors in the human brain by positron emission tomography with [11C]TMSX. Synapse. 2005;55:133–136. doi: 10.1002/syn.20099. [DOI] [PubMed] [Google Scholar]
- Jegou S, Yacoubi ME, Mounien L, Ledent C, Parmentier M, Costentin J, Vaugeois JM, Vaudry H. Adenosine A2A receptor gene disruption provokes marked changes in melanocortin content and pro-opiomelanocortin gene expression. J Neuroendocrinol. 2003;15:1171–1177. doi: 10.1111/j.1365-2826.2003.01116.x. [DOI] [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Fredholm BB. Distribution and postnatal ontogeny of adenosine A2A receptors in rat brain: comparison with dopamine receptors. Neuroscience. 1997;80:1187–1207. doi: 10.1016/s0306-4522(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Lauterburg T, Burgunder JM. Expression of adenosine A2a receptor gene in rat dorsal root and autonomic ganglia. Neurosci Lett. 1998;246:21–24. doi: 10.1016/s0304-3940(98)00216-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kanda T, Shiozaki S, Shimada J, Suzuki F, Nakamura J. KF17837: a novel selective adenosine A2A receptor antagonist with anticataleptic activity. Eur J Pharmacol. 1994;256:263–268. doi: 10.1016/0014-2999(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H, Kuwana Y, Jenner P. Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA. Up-regulation of adenosine transporter-binding sites in striatum and hypothalamus of opiate tolerant mice. Brain Res. 1997;763:215–220. doi: 10.1016/s0006-8993(97)00413-7. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Sears MT. Alterations of adenosine A1 receptors in morphine dependence. Brain Res. 1994;657:347–350. doi: 10.1016/0006-8993(94)90990-3. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Karlsten R, Gordh T, Post C. Local antinociceptive and hyperalgesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacol Toxicol. 1992;70:434–438. doi: 10.1111/j.1600-0773.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;30:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelly M, Bailey A, Ledent C, Kitchen I, Hourani S. Characterization of [3H]ZM 241385 binding in wild-type and adenosine A2A receptor knockout mice. Eur J Pharmacol. 2004;504:55–59. doi: 10.1016/j.ejphar.2004.09.052. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Eastwood C, Grundy D, Chessell IP, Humphrey PPA. Characterisaion of adenosine receptors evoking excitation of mesenteric afferents in the rat. Br J Pharmacol. 1998;125:1352–1360. doi: 10.1038/sj.bjp.0702202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Kubota K. Kuniomi Ishimori and the first discovery of sleep-inducing substances in the brain. Neurosci Res. 1989;6:497–518. doi: 10.1016/0168-0102(89)90041-2. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Zvartau EE, Fredholm BB. Caffeine, acting on adenosine A(1) receptors, prevents the extinction of cocaine-seeking behavior in mice. J Pharmacol Exp Ther. 1999;290:535–542. [PubMed] [Google Scholar]
- Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Exp Opin Invest Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois J, Schiffman SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen J, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- Lillrank SM, Lipska BK, Weinberger DR, Fredholm BB, Fuxe K, Ferré S. Adenosine and dopamine receptor antagonist binding in the rat ventral and dorsal striatum: lack of changes after a neonatal bilateral lesion of the ventral hippocampus. Neurochem Int. 1999;34:235–244. doi: 10.1016/s0197-0186(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Liu XJ, White TD, Sawynok J. Potentiation of formalin-evoked adenosine release by an adenosine kinase inhibitor and an adenosine deaminase inhibitor in the rat hind paw: a microdialysis study. Eur J Pharmacol. 2000;408:143–152. doi: 10.1016/s0014-2999(00)00742-1. [DOI] [PubMed] [Google Scholar]
- Liu XJ, White TD, Sawynok J. Involvement of primary sensory afferents, postganglionic sympathetic nerves and mast cells in the formalin-evoked peripheral release of adenosine. Eur J Pharmacol. 2001;429:147–155. doi: 10.1016/s0014-2999(01)01316-4. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Salter MW. Purines and pain mechanisms: recent developments. Curr Opin Investig Drugs. 2005;6:65–75. [PubMed] [Google Scholar]
- Logan L, Carney JM, Holloway FA, Seale TW. Effects of caffeine, cocaine and their combination on fixed-interval behavior in rats. Pharmacol Biochem Behav. 1989;33:99–104. doi: 10.1016/0091-3057(89)90436-x. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br J Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha SS, Kasahara K, Hayaishi O. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci USA. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Misra AL, Vadlamani NL, Pontani RB. Effect of caffeine on cocaine locomotor stimulant activity in rats. Pharmacol Biochem Behav. 1986;24:761–764. doi: 10.1016/0091-3057(86)90587-3. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Eguchi N, Kimura K, Kiyohara Y, Qu WM, Huang ZL, Mochizuki T, Lazarus M, Kobayashi T, Kaneko T, Narumiya S, Urade Y, Hayaishi O. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Montegazza P, Tammiso R, Zambotti F, Zecca L, Zonta S. Purine involvement in morphine antinociception. Br J Pharmacol. 1984;83:883–888. doi: 10.1111/j.1476-5381.1984.tb16527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateralpreoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Ferré S, Goldberg SR. Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2002;161:348–355. doi: 10.1007/s00213-002-1075-5. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. JComp Neurol. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Pechlivanova DM, Georgiev VP. Interaction of angiotensin II and adenosine A1 and A2A receptor ligands on the writhing test in mice. Pharmacol Biochem Behav. 2002;72:23–38. doi: 10.1016/s0091-3057(01)00707-9. [DOI] [PubMed] [Google Scholar]
- Phillis JWC, Jiang ZGW. The effect of morphine on purine and acetylcholine release from rat cerebral cortex: Evidence for a purinergic component in morphinès action. Pharmacol Biochem Behav. 1980;13:421–427. doi: 10.1016/0091-3057(80)90249-x. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Cocaine-induced hyperactivity is more influenced by adenosine receptor agonists than amphetamine-induced hyperactivity. Pol J Pharmacol. 54:359–366. [PubMed] [Google Scholar]
- Pollack AE, Fink JS. Adenosine antagonists potentiate D2 dopamine-dependent activation of Fos in the striatopallidal pathway. Neuroscience. 1995;68:721–728. doi: 10.1016/0306-4522(95)00168-i. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon A, Sawynok J. Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain. 1998;74:235–245. doi: 10.1016/s0304-3959(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, de Carolis AS. Modulation of striatal adenosine A1 and A2 receptors induces rotational behaviour in response to dopaminergic stimulation in intact rats. Eur J Pharmacol. 1994;12(257):21–25. doi: 10.1016/0014-2999(94)90689-0. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi O, Nambu F, Narumiya S, Urade Y, Hayaishi O. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci USA. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004a;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, Lluis C, Franco R, Woods AS, Goldberg SR, Ferré S. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004b;91:873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Gomes C, Pak AC, Ribeiro JA, Goldberg SR, Hope BT, Ferré S. Blockade of adenosine A2A receptors prevents protein phosphorylation in the striatum induced by cortical stimulation. J Neurosci. 2006;26:10808–108012. doi: 10.1523/JNEUROSCI.1661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Dickenson AH. Electrophysiological study on spinal antinociceptive interactions between adenosine and morphine in the dorsal horn of the rat. Neurosci Lett. 1995;194:81–84. doi: 10.1016/0304-3940(95)11732-c. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Ferré S, Ogren SO, Fuxe K. Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- Rinne JO. Nigral degeneration in Parkinson’s disease. Mov Disord 8 Suppl. 1993;1:S31–5. doi: 10.1002/mds.870080507. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. JComp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Hayaishi O. Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol. 1998;351:155–162. doi: 10.1016/s0014-2999(98)00302-1. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–40. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Scammell T, Geraschchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Worley CM, McNamara C, Valadez A. Acute and repeated exposure to caffeine: effects on reinstatement of extinguished cocaine-taking behavior in rats. Psychopharmacology. 1996;126:17–23. doi: 10.1007/BF02246406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Segerdahl M. Antinociceptive effects of adenosine infusion in man. Assessments in experimentally induced pain and in surgery. Acta Anaesthesiologica Scandinavica. 1997;41:90–90. doi: 10.1111/j.1399-6576.1997.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Segerdahl M, Ekblom A, Sandelin K, Wickman M, Sollevi A. Peroperative adenosine infusion reduces the requirements for isoflurane and postoperative analgesics. Anesthesia and Analgesia. 1995;80:1145–1149. doi: 10.1097/00000539-199506000-00013. [DOI] [PubMed] [Google Scholar]
- Segerdahl M, Sollevi A. Adenosine and pain relief: a clinical overview. Drug Dev Res. 1998;45:151–158. [Google Scholar]
- Self DW, Terwilliger RZ, Nestler EJ, Stein L. Inactivation of Gi and G(o) proteins in nucleus accumbens reduces both cocaine and heroin reinforcement. J Neurosci. 1994;14:6239–6247. doi: 10.1523/JNEUROSCI.14-10-06239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shimazoe T, Yoshimatsu A, Kawashimo A, Watanabe S. Roles of adenosine A(1) and A(2A) receptors in the expression and development of methamphetamine-induced sensitization. Eur J Pharmacol. 2000;388:249–254. doi: 10.1016/s0014-2999(99)00899-7. [DOI] [PubMed] [Google Scholar]
- Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology. 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22:6321–6324. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Ferré S, Antoniou K, Quarta D, Justinova Z, Hockemeyer J, Pappas LA, Segal PN, Wertheim C, Muller CE, Goldberg SR. Involvement of adenosine A1 receptors in the discriminative-stimulus effects of caffeine in rats. Psychopharmacology. 2005;179:576–586. doi: 10.1007/s00213-004-2081-6. [DOI] [PubMed] [Google Scholar]
- Soria G, Castane A, Berrendero F, Ledent C, Parmentier M, Maldonado R, Valverde O. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci. 2004;20:2203–2213. doi: 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2005;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Stone TW. The effects of morphine and methionine enkephalin on the release of purines from cerebral cortex slices of rats and mice. Br J Pharmacol. 1981;74:171–176. doi: 10.1111/j.1476-5381.1981.tb09970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striplin CD, Kalivas PW. Robustness of G protein changes in cocaine sensitization shown with immunoblotting. Synapse. 1993;14:10–15. doi: 10.1002/syn.890140103. [DOI] [PubMed] [Google Scholar]
- Suh HW, Song DK, Kim YH. Differential effects of adenosine receptor antagonists injected intrathecally on antinociception induced by morphine and beta-endorphin administered intracerebroventricularly in the mouse. Neuropeptides. 1997;31:339–444. doi: 10.1016/s0143-4179(97)90069-x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997a;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997b;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, White TD, Jhamandas KH, Sawynok J. Morphine releases endogenous adenosine from the spinal cord in vivo. Eur J Pharmacol. 1987;141:169–170. doi: 10.1016/0014-2999(87)90428-6. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, White TD, Sawynok J. Morphine, capsaicin and K+ release purines from capsaicin-sensitive primary afferent nerve terminals in the spinal cord. J Pharmacol Exp Ther. 1989;248:447–454. [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neurosci. 38:757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Sandager Nielsen K, Ferraro L, Antonelli T, Kehr J, Franco R, Ferré S, Agnati LF, Fuxe K, Scheel-Kruger J. Striatal plasticity at the network level. Focus on adenosine A2A and D2 interactions in models of Parkinson’s Disease Parkinsonism. Relat Disord. 2004;10:273–280. doi: 10.1016/j.parkreldis.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Danielson PE, Sutcliffe JG. RGS9: a regulator of G-protein signalling with specific expression in rat and mouse striatum. J Neurosci Res. 1998;52:118–124. doi: 10.1002/(SICI)1097-4547(19980401)52:1<118::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink JS, Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61:S94–96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. Molecular and behavioral effects mediated by Gs-coupled adenosine A2a, but not serotonin 5-Ht4 or 5-Ht6 receptors following antipsychotic administration. Neuroscience. 1999;89:927–938. doi: 10.1016/s0306-4522(98)00364-9. [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology. 2003;168:155–163. doi: 10.1007/s00213-003-1410-5. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48:217–421. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc Natl Acad Sci USA. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci USA. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc Natl Acad Sci USA. 2006;103:7877–1882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Nikfar S. Different influences of adenosine receptor agonists and antagonists on morphine antinociception in mice. Gen Pharmacol. 1994;25:139–142. doi: 10.1016/0306-3623(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Zhang K, Tarazi FI, Campbell A, Baldessarini RJ. GABA(B) receptors: altered coupling to G-proteins in rats sensitized to amphetamine. Neuroscience. 2000;101:5–10. doi: 10.1016/s0306-4522(00)00344-4. [DOI] [PubMed] [Google Scholar]


