Abstract
Previous in vitro studies in our laboratory have shown that mancozeb (MZ) and maneb (MB), both widely used EBDC fungicides, are equipotent neurotoxicants that produce cell loss in mesencephalic dopaminergic and GABAergic cells after an acute 24 h exposure. Mitochondrial uncoupling and inhibition were associated with fungicide exposure. Inhibition of mitochondrial respiration is known to increase free radical production. Here the mechanism(s) of neuronal damage associated with MZ exposure was further explored by determining the role that reactive oxygen species (ROS) played in toxicity. Damage to mesencephalic dopamine and GABA cell populations were significantly attenuated when carried out in the presence of ascorbate or SOD indicative of a free radical mediated contribution to toxicity. ROS generation monitored by H2O2 production using Amplex Red increased in a dose-dependent manner in response to MZ. Inhibition of intracellular catalase with aminotriazole had little effect on H2O2 generation, whereas exogenously added catalase significantly reduced H2O2 production demonstrating a large extracellular contribution to ROS generation. Conversely, cells preloaded with the ROS indicator dye DCF showed significant MZ-induced ROS production, demonstrating an increase in intracellular ROS. Both the organic backbone of MZ as well as its associated Mn ion, but not Zn ion were responsible and required for H2O2 generation. The functionally diverse NADPH oxidase inhibitors, diphenylene iodonium chloride, apocynin, and 4-(2-aminoethyl)benzene- sulfonyl fluoride hydrochloride significantly attenuated H2O2 production by MZ. In growth medium lacking cells, MZ produced little H2O2, but enhanced H2O2 generation when added with xanthine plus xanthine oxidase whereas, in cultured cells, allopurinol partially attenuated H2O2 production by MZ. Minocycline, an inhibitor of microglial activation, modestly reduced H2O2 formation in mesencephalic cells. In contrast, neuronal enriched cultures or cultures treated with MAC-1-SAP to kill microglia, did not show an attenuation of ROS production. These findings demonstrate that Mn-containing EBDC fungicides such as MZ and MB can produce robust ROS generation that likely occurs via redox cycling with extracellular and intracellular oxidases. The findings further show that microglia may contribute to but are not required for ROS production by MZ.
Keywords: fungicide, oxidative stress, Parkinson’s disease, manganese, NADPH oxidase
INTRODUCTION
The development and progression of several neurodegenerative diseases have been linked to factors other than genetic predisposition. Environmental exposure to pesticides, including the ethylene-bis-dithiocarbamate (EBDC) fungicides, may contribute to neuronal toxicity and subsequent pathologies. Mancozeb (MZ), a Mn/Zn-containing EBDC fungicide, is widely utilized on golf courses, residential lawns, and agricultural lands throughout the United States. The adverse effects of MZ in humans and other living organisms have not been widely studied and there are few studies to evaluate the neurotoxic action of MZ in experimental models (Soleo et al., 1996; Vaccari et al., 1999; Domico et al., 2006a). However, other dithiocarbamate fungicides, including maneb (MB), have been implicated in selective dopaminergic neurotoxicity and mitochondrial dysfunction in rodents and humans, resulting in motor deficits, and ultimately, parkinsonism (Morato et al., 1989; Meco et al., 1994; Soleo et al., 1996; Zhang et al., 2003).
Reactive oxygen species and other free radicals have been implicated in the pathogenesis of neurodegenerative diseases, like Parkinson’s disease (PD) (Rao and Balachandran, 2002). The substantia nigra (SN) region of the brain is vulnerable to oxidative stress because of its local environment. The auto-oxidation of dopamine (Hastings, 1995), the enzymatic deamination of DA by monoamine oxidases (Halliwell, 1992), and the high iron content which catalyzes Fenton reactions (Dexter et al., 1989) make the SN vulnerable to oxidative stress and cellular injury. Pesticide exposure in experimental rodent and cell culture models has been linked to ROS generation and/or an inflammatory response that potentiates ROS production. Dopaminergic toxicity by rotenone, a naturally occurring complex I inhibitor and a common herbicide, is caused by oxidative stress and is mediated, in part, by the activation of microglia (Gao et al., 2002, 2003b; Sherer et al., 2002; Sherer et al., 2003; Zeevalk and Bernard, 2005). The neurotoxicity of paraquat (PQ), a bipyridial herbicide, mimics PD in experimental models and results in microglia activation and redox cycling via microglial NADPH oxidase (McCormack et al., 2002; Bonneh-Barkay et al., 2005). EBDCs, including MB, have enhanced the toxic effects of the prooxidants MPTP (Takahashi et al., 1989; Bachurin et al., 1996) and PQ (Thiruchelvam et al., 2000a,b, 2005) in rodent models, contributing to the PD-like phenotype.
Previous studies have implicated MZ as neurotoxic to mesencephalic DA and GABA neuronal cell populations following acute exposure (Soleo et al., 1996; Domico et al., 2006a). In addition, MZ and other similar EBDCs, like MB, have been reported to be inhibitors and/or uncouplers of the mitochondrial electron transport chain (Zhang et al., 2003; Domico et al., 2006a). Since mitochondrial dysfunction is often associated with ROS generation, the purpose of the present study was to assess the role of ROS in the neurotoxic action of MZ and other EBDCs in mesencephalic cell culture. The studies reported within determined the contribution of ROS production to EBDC toxicity, measured ROS generation in the extracellular and intracellular environment of mesencephalic neurons, and evaluated potential contributors to MZ-induced ROS production, including the organic EBDC backbone, metal ions, metabolites, microglia and redox cycling with oxidases.
METHODS
Materials
Timed-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). MZ, MB, and Nabam (NB) were purchased from ChemService (West Chester, PA). The purity of MB and NB are reported to be >95%. MZ, a polymeric complex, contains approximately 20% Mn and 2.55% Zn and does not have a reported purity. MnCl2 and ZnCl2 were purchased from Sigma-Aldrich (St. Louis, MO). ETU was purchased from Reidel-de Haan (Seelze, Germany). All other chemicals were purchased from Sigma Aldrich, unless otherwise noted.
Primary mixed mesencephalic cell cultures
Mesencephalic cells were isolated from embryonic day 15 Sprague-Dawley rats, cultured, and maintained exactly as described in Domico et al., 2006a with few exceptions. Cells were plated at 1.0–2.0 x 105 cells/cm2 on polyornithine- and serum-coated wells.
Neuronal-rich cultures
Two approaches were used to establish neuronal-rich cultures. To eliminate microglia from the mixed mesencephalic culture, cells were treated with MAC-1-SAP (final concentration 2 ug/ml) (Advanced Targeting Systems, San Diego, CA), a microglial (CD11b)-specific antibody conjugated with saporin, a ribosome inactivating protein, for approximately 72 h. Thus, only cells containing the CD11b marker, namely microglia, were labeled and targeted by the saporin toxin. The result was a culture rich in neurons and deficient in microglia. Neuronal cells were then rinsed 3 times with HEPES buffer (25 mM HEPES, 5.6 mM glucose, 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.3 mM CaCl2, and 1.2 mM MgCl2) and treated with toxicants. To verify the elimination of microglia cells, MAC-1-SAP treated cells were immunostained using mouse monoclonal unconjugated MAC-1 antibody (1:150) (Advanced Targeting Systems, San Diego, CA) that recognizes rat MAC-1 (CD11b). Cells were fixed and stained as described in Zeevalk et al., 1998. The Elite Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) was used to detect antigen signal. The final product was visualized using 3,3’-diaminobenzidine tetrachloride.
In a second approach to reduce microglial and glial growth in mesencephalic culture, cells were isolated from E15 rat as described above. Approximately 4 h after cells were plated, serum-containing DMEM was replaced with serum-free N2 supplemented (Invitrogen, Eugene, OR) media, as described in Wood et al. (2003). N2 supplement (8.6 uM insulin, 1 mM human transferrin, 2 uM progesterone, 10 mM putrescine, 3 uM selenite) is a serum-free supplement that promotes the growth of post-mitotic neurons (Invitrogen, Eugene, OR).
Preparation of toxicants and culture treatment
MZ, MB, and ethylene thiourea (ETU) were prepared exactly as described in Domico et al., 2006a. Briefly, MZ and MB were solubilized in 100% DMSO, sonicated in a water bath for 10 min, and then diluted with HPLC grade water to yield 10% DMSO plus MZ or MB stock solutions. Cells treated with 30 uM MZ, MB, or ETU contained no more than 0.1% DMSO. NB, MnCl2, and ZnCl2, were solubilized in 100% HPLC grade water.
Dopamine and GABA high-affinity uptake
Toxicity in cell cultures was determined with a functional assay of high-affinity transporter activity via the measurement of high-affinity uptake of [3H]DA and [14C]GABA exactly as described in Domico et al., 2006a. Briefly, cells were exposed to MZ for 24 h and allowed to recover for 72 h to ensure that results were due to irreversible damage and were not an effect of toxicant treatment. Cells were then incubated with [3H]DA (specific activity 240 mCi/mmol; New England Nuclear, final concentration 20 nM) and [14C]GABA (specific activity 40 Ci/mmol; Amersham, final concentration 5 uM) using 0.05 μCi/assay for 15 min at 37ºC in 0.5 ml of HEPES buffer containing 1 mM ascorbate, 100 uM pargyline, 10 uM aminoxyacetic acid, and 1 mM β–alanine. Radioactive material was removed from cells with 95% ethanol and was measured using a scintillation counter. Non-energy dependent uptake was determined by carrying out the uptake assay on ice and was subtracted from radioactivity determined at 37C. (Ehrhart and Zeevalk, 2003).
Extracellular ROS measurement
The Amplex® Red (AR) Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, Eugene, OR) was used to detect ROS generation in mesencephalic cell culture (Zhou et al., 1997). The AR reagent (10-acetyl-3,7-dihydroxyphenoxazine), in the presence of horseradish peroxidase (HP), reacts with peroxides released into the extracellular environment by cells and generates a fluorescent product. The assay was carried out according to the manufacturer’s protocol, with some exceptions. The AR reagent from the kit was reconstituted with 60 ul DMSO, while the HP from the kit was reconstituted with 1 ml of HEPES buffer. To make the reaction mixture, 50 ul of the AR solution and 100 ul of the HP solution were added to 5 ml of pre-warmed HEPES buffer and protected from light. Cell media was removed and replaced with 125 ul of reaction mixture. Antioxidants catalase (CAT) or superoxide dismutase (SOD) or selected inhibitors aminotriazole (ATZ), apocycnin (APO), diphenylene iodonium chloride (DPI), 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), minocycline (MINO), or allopurinol (ALLO) were added to cells, followed by the desired concentration of toxicants. Hydrogen peroxide generation was monitored every 2.5 min for 20 min at 37ºC in a CytoFluor multiwell plate reader (Series 4000, PerSeptive Biosystems) at 530 nm excitation and 580 nm emission. The average rate of hydrogen peroxide generation over 20 min was recorded.
Intracellular ROS measurement
Dichlorofluorescin diacetate (DCF) (Molecular Probes, Eugene, OR) is readily converted to its fluorescent product in the presence of ROS in cells. We used this fluorescent-based assay to detect intracellular ROS generation in cells treated with toxicants, as previously described (Moy et al., 2000). Cells were loaded with 5 uM DCF for 30 min and then washed extensively with Krebs-Ringer buffer (119 mM NaCl, 4.8 mM KCl, 25 mM 3-[N-morpholino]propane sulfonic acid, 1.7 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, 23.8 mM sodium bicarbonate, pH 7.4) to remove extracellular DCF. Cells were then treated with toxicants and monitored for ROS generation every 2.5 min for 30 min at 37ºC in a CytoFluor multiwell plate reader (Series 4000, PerSeptive Biosystems) at 485 nm excitation and 530 nm emission.
Statistical analyses
Data were analyzed for statistical significance by ANOVA with Bonferonni’s and/or Student-Newman-Keuls post-hoc tests using SigmaStat statistical software version 1.0 (Jandel Corporation). If data failed the normality test, they were analyzed by Kruskal-Wallis ANOVA on ranks with Dunn’s post-hoc test and/or Mann Whitney rank sum test. A p value of <0.05 was considered statistically significant.
RESULTS
Oxidative stress contributes to MZ-induced neuronal toxicity
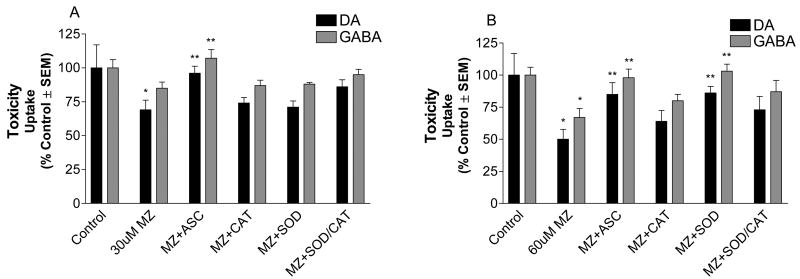
Since MZ can inhibit the mitochondrial electron transport chain (Domico et al., 2006a), we hypothesized that ROS generation was associated, in part, with the neurotoxic effects observed in mesencephalic cells after MZ exposure. High-affinity uptake for [3H]DA and [14C]GABA was assessed as a measure of cell viability 72 h after mesencephalic cells were pretreated with antioxidants for 3 h followed by exposure to MZ for 24 h (Fig. 1). This functional assay of toxicity when assessed several days after toxin treatment correlates well with cell viability (Zeevalk and Bernard, 2005; Domico et al., 2006a) and has the added advantage of monitoring toxicity in two different neurotransmitter populations. MZ at 30 and 60 uM significantly decreased the uptake of DA (31% ± 7.15 and 50% ± 7.67, reduction ± SEM, respectively). GABA uptake was also significantly affected in cells exposed to 60 uM MZ (33% ± 6.96, reduction ± SEM). However, cells pretreated with ascorbate, an antioxidant that directly scavenges superoxide anion and hydroxyl radicals, were nearly completely protected against MZ toxicity. In addition, SOD, an antioxidant enzyme that catalyzes the conversion of superoxide anion to hydrogen peroxide, significantly protected cells from the toxic effects observed after acute exposure to 60 uM MZ. Catalase, an antioxidant enzyme that converts hydrogen peroxide to water, alone or in combination with SOD showed a trend towards protection but results were not significantly different. These data suggest that ROS are in part responsible for the toxic effects observed in mesencephalic cells acutely exposed to MZ.
Fig. 1.
Antioxidant effects on acute MZ toxicity in mesencephalic DAergic and GABAergic neurons. Mesencephalic cells were pre-treated for 3h with 400 uM ascorbate (Asc), 25 U/ml catalase (CAT), 25 U/ml SOD, or catalase plus SOD, exposed to (A) 30 uM or (B) 60 uM MZ for 24 h, and allowed to recover for 72 h. After recovery, toxicity was determined by measuring the high-affinity uptake for [3H]DA and [14C]GABA in treated cells. The data are from three to five separate experiments run in duplicate. *Significant difference from control. ** Significant difference from MZ alone.
Location of ROS generation by Mancozeb
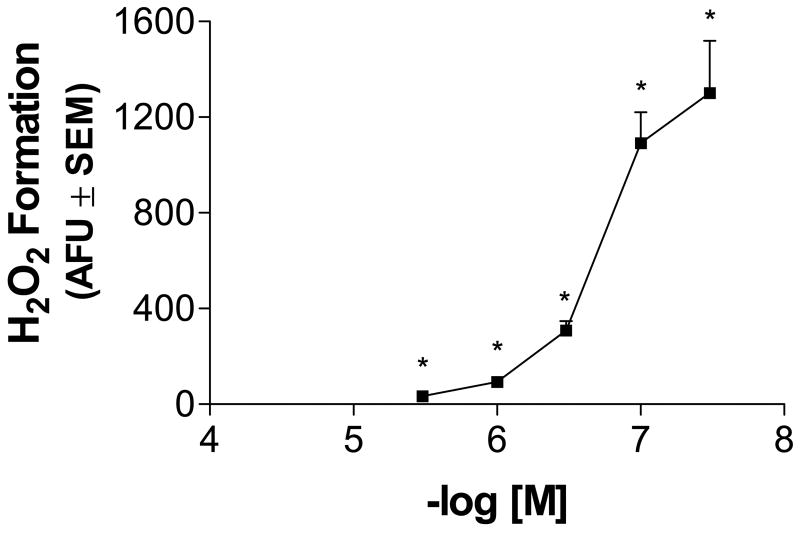
Ongoing studies in the laboratory indicate that approximately 8% of exogenously applied MZ enters the neuron while 92% remains outside the cell membrane (Domico et al., 2007). To investigate the events that potentially lead to MZ-induced neuronal toxicity, we assessed ROS generation both extracellularly and intracellularly. The Amplex® Red (AR) Hydrogen Peroxide/Peroxidase Assay Kit was used to detect hydrogen peroxide generation by treated cells. ROS, as measured by H2O2 in MZ-treated cells, was measured over a period of 20 min. MZ exposure (0.3–30 uM) resulted in a clear dose-dependent increase of peroxide generation, with significance at all doses (Fig. 2). The lowest MZ dose tested, 0.3 uM generated approximately 3.5 times more hydrogen peroxide than controls, while the highest MZ dose (30 uM) generated approximately 134 times more hydrogen peroxide than controls. It is evident that MZ exposure results in the robust generation of hydrogen peroxide. Based on a mean rate of 1000 AFU for 30 uM MZ generated from multiple standard curves, the rate of H2O2 formed was 40 pmol/min/2.0 x 105 cells.
Fig. 2.
Dose-response effect of MZ exposure on H2O2 produced by mesencephalic DAergic and GABAergic neurons. Mesencephalic cells were exposed to 0–30 uM MZ and immediately assessed for extracellular H2O2 by the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. Note that H2O2 levels increased as the dose of MZ increased. The data are from three separate experiments run in duplicate. *Significant difference from control.
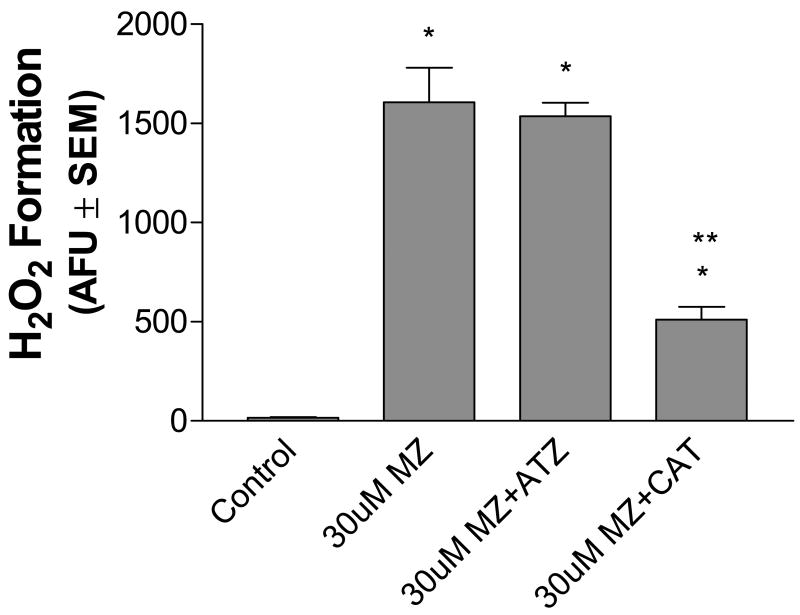
To help determine the location of H2O2 generation in MZ treated cultures, cells were pretreated with aminotriazole (ATZ), an inhibitor of endogenous intracellular catalase. Previous work has shown that pretreatment of cultures for 3 h with 5 mM ATZ decreases catalase activity by 90% (Ehrhart and Zeevalk, 2001). MZ-exposed cells pretreated with ATZ did not show a significant augmentation in peroxide generation, suggesting that endogenous intracellular catalase does not have access to the compartment in which the bulk of H2O2 is generated (Fig. 3). On the other hand, when cells were pretreated with exogenous catalase, which does not cross the cellular membrane, hydrogen peroxide levels significantly decreased by 68%. These data suggest that a large component of the H2O2 formed is generated extracellularly.
Fig. 3.
Effects of aminotriazole (ATZ) and exogenous catalase (CAT) on MZ-induced H2O2 production by mesencephalic cells. Cells were pre-treated for 3 h with 5 mM ATZ, an endogenous CAT inhibitor, or 25 U/ml CAT added just prior to the assay run. MZ (30uM) was then added and the cells immediately assessed for extracellular H2O2 by the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. These data indicate that most of the H2O2 detected is from extracellular sources. The data are from three to seven separate experiments run in duplicate. *Significant difference from control. ** Significant difference from MZ alone.
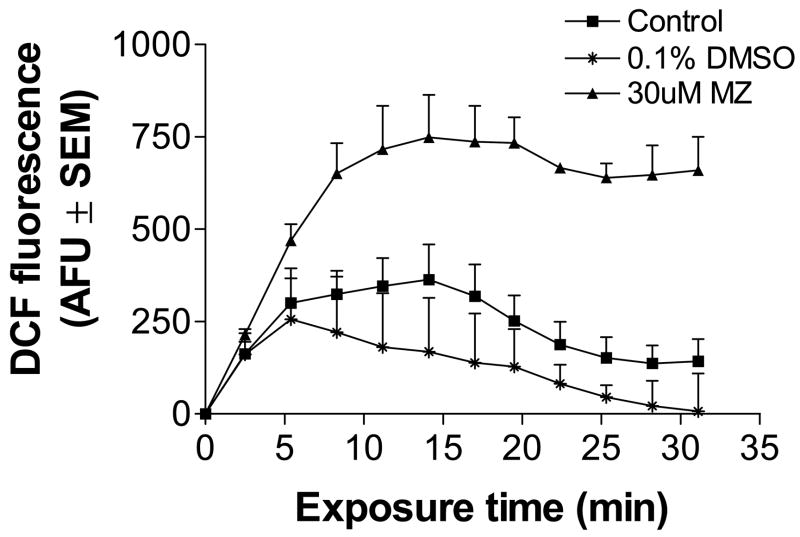
To provide insight into whether there were increases in intracellular ROS, cells were loaded with the ROS sensing dye, DCF and extracellular DCF thoroughly removed by washing. Loaded cells were then treated with 30 uM MZ and the DCF fluorescence monitored over 30 min. Under control conditions, cells generated very low levels of ROS, as indicated in Fig. 4. However, when cells were exposed to MZ (30 uM), fluorescence increased, indicative of increased intracellular ROS. Between 14–17 min, ROS levels reached a threshold in cells exposed to MZ. The dose of MZ used in this assay (30 uM) contained 0.1% DMSO as vehicle. DMSO has known antioxidant properties (Nicolaides et al., 2004). In DMSO-control cultures, ROS generation was minimal and after 5 min was less than that observed in controls lacking DMSO. In separate experiments, catalase (25 U/ml) was added to cells after DCF loading and prior to MZ addition to determine if removal of extracellular peroxide influenced the intracellular generation of DCF oxidation. Under these conditions, catalase had no effect on MZ-induced DCF fluorescence: AFU for control, 30 uM MZ and catalase plus 30 uM MZ at the end of 30 min were 41 ± 20, 152 ± 30 and 154 ± 30, mean AFU ± SEM, respectively, n=3.
Fig. 4.
Intracellular ROS levels detected in mesencephalic cells treated with MZ. Cells were treated with 30 uM MZ or 0.1% DMSO, used as a vehicle control. Intracellular ROS levels were detected by DCF fluorescence over a 30 min period as described in Methods. The data are from three separate experiments run in duplicate.
ROS generation by MZ may involve redox cycling with cellular oxidases
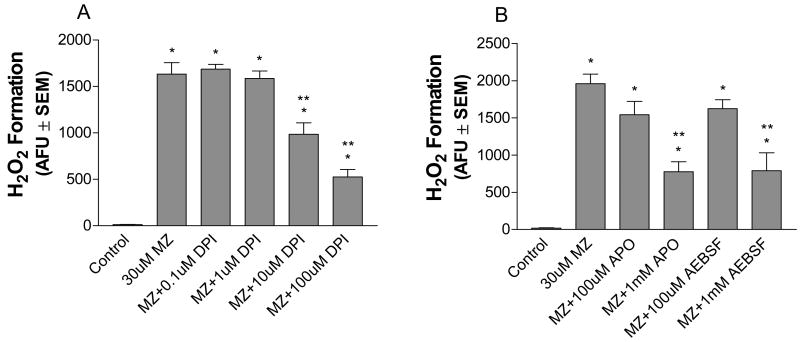
Other pesticides, like paraquat (PQ), have been implicated in neurotoxicity. PQ can generate ROS outside the cell via redox cycling with NADPH oxidase (Bonneh-Barkay et al., 2005a,b). NADPH oxidase is a multi-subunit enzyme predominantly expressed in microglia in the brain (Babior et al., 1999; Wu et al., 2003), but is also present on neurons (Serrano et al., 2003; Tejada-Simon et al., 2005). When NADPH oxidase subunits assemble on the cell membrane they can catalyze the electron transfer from NADPH to intermediate ligands, like PQ, which then redox cycle and generate ROS (Wu et al., 2003; Zhang et al., 2004; Bonneh-Barkay, 2005b). To investigate the potential involvement of cellular oxidases, like NADPH oxidase, in the extracellular ROS generation by MZ, cultures were pretreated with several different NADPH oxidase inhibitors with differing mechanisms of action (O’Donnell et al., 1993; Stolk et al., 1994; Megyeri et al., 1995; Diatchuk et al., 1997; Li et al., 1998; Doussiere et al., 1999). DPI, apocynin and AEBSF all significantly attenuated H2O2 generation in cultures treated with 30 uM MZ (Figs. 5A and B). DPI was effective at 10 and 100 uM (40% reduction, 68% reduction, respectively) (Fig. 5A). Cells treated with APO (1 mM) or AEBSF (1 mM) also significantly reduced extracellular peroxide levels (APO, 61% reduction; AEBSF, 60% reduction) (Fig. 5B). These data suggest that NADPH oxidase, a cellular membrane oxidase, is involved, in part, in the generation of ROS outside the cell.
Fig. 5.
Effects of NADPH oxidase inhibitors on extracellular H2O2 formation in MZ treated mesencephalic cells. Cells were pre-treated with (A) DPI (0.1–100 uM), (B) apocynin (APO, 100 uM or 1 mM), and AEBSF (100 uM or 1 mM) for 1 h, exposed to 30 uM MZ, and assayed for extracellular H2O2 by the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. Note that the NADPH oxidase inhibitors used here have different modes of action (see Discussion), yet all resulted in significant attenuation of H2O2 formation. The data are from three separate experiments run in duplicate. *Significant difference from control. ** Significant difference from MZ alone.
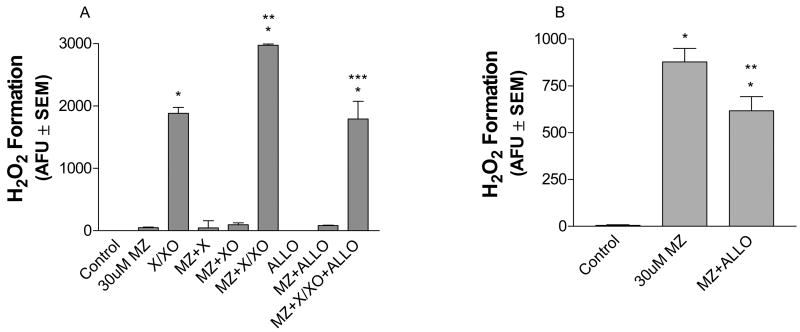
Given that ROS generation by MZ could also occur intracellularly, the potential for intracellular oxidases to redox cycle with MZ to generate ROS was evaluated. Xanthine (X), in the presence of xanthine oxidase (XO) in the cell, readily generates intracellular superoxide in addition to uric acid during normal metabolism. Thus, in this study we used the X/XO system to mimic intracellular oxidase activity. Using a cell free system, ROS generation was measured in the presence of 30 uM MZ, X (0.3 mM) and/or XO (0.02U/ml), MZ plus X and/or XO, allopurinol (5 mM, XO inhibitor), MZ plus allopurinol, and MZ plus X/XO and allopurinol (Fig. 6A). In the absence of cells, MZ alone generated ROS, but the amount was minimal when compared with ROS generation in the presence of cells (i.e., approximately 2.5% of the total AFU/min generated by MZ in the presence of cells (Fig 6A and B). As expected, X/XO in the absence of cells robustly increased ROS production to levels approximately 1600-fold greater than control. When MZ was added with X/XO, there was an even greater increase in the rate of ROS production. Statistically significant, the rate of ROS production with MZ plus X/XO increased by 37% as compared with X/XO alone. When allopurinol was added to the MZ plus X/XO reaction mixture, ROS production decreased approximately 40%. Likewise, when allopurinol was preincubated with cells for 24 h followed by treatment with 30 uM MZ, MZ-induced ROS generation significantly decreased by an approximate 30% (Fig 6B). In addition, fixed cells no longer redox cycled with MZ to generate H2O2. When 30 uM MZ was added to cells previously fixed with 10% formalin and thoroughly rinsed, H2O2 generation reported as AFU/min ± SD was 203 ± 97.5 as compared with 15.2 ± 7.5, and 1553 ± 391, for control, and 30 uM MZ (live cells), respectively. These findings demonstrate that MZ-induced generation of ROS requires the presence of cells and further suggests the potential for MZ to redox cycle with intracellular oxidases like xanthine oxidase.
Fig. 6.
MZ-induced extracellular ROS generation requires the presence of live cells and is attenuated by allopurinol. H2O2 levels were measured by the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit in a cell free system (A) and in mesencephalic neuronal cultures (B). 30 uM MZ alone or in combination with either xanthine (X, 0.3 mM), xanthine oxidase (XO, 0.02 U/ml) and/or allopurinol (ALLO, 5 mM) were added to the Amplex Red reaction mixture without mesencephalic cells (A). Mesencephalic cells were pre-incubated with ALLO 24 h prior to 30 uM MZ exposure (B). H2O2 levels were measured immediately after MZ treatment. The data are from three to five separate experiments run in duplicate. *Significant difference from control. **Significant difference from X/XO alone (A) or MZ alone (B). ***Significant difference from MZ plus X/XO.
The organic portion of the EBDC compound in combination with the associated Mn metal contribute to ROS generation and subsequent toxicity
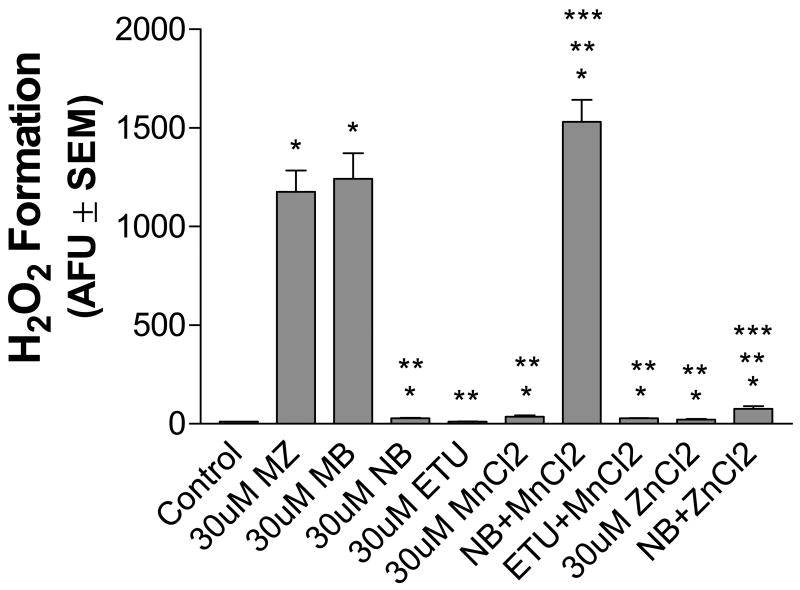
Previous studies in our laboratory have demonstrated that the Mn-metal component and the organic portion of the EBDC fungicide contribute to cellular toxicity (Domico et al., 2006a). We found that the EBDC fungicide, Nabam (NB), which contains Na+ instead of Mn ion, was less toxic than the EBDC fungicides, MZ and MB, that contain a Mn moiety. Moreover, we found that addition of MnCl2 along with NB increased toxicity to the same extent as MZ or MB. The active metabolite of MZ and MB, ETU, was not toxic to DA or GABA neuronal cell populations. To determine whether Mn present in both MZ and MB or Zn, present only in MZ contributed to ROS generation in the mixed neuronal cultures H2O2 levels were measured in cells exposed to MZ, MB, NB, ETU, MnCl2, ZnCl2, as well as a combination of NB/MnCl2, NB/ZnCl2, or ETU/MnCl2.
Cells exposed to 30uM of MZ or MB generated more than a 100-fold increase in peroxide as compared with controls, whereas NB, MnCl2, or ZnCl2 alone showed only a 2 to 4- fold increase (Fig. 7). However, when NB was combined with MnCl2 to mimic the structure of MB, cells generated peroxide levels that were similar to MZ and MB and were significantly greater than H2O2 produced by NB alone. Because NB and MnCl2 alone generate only small but significant levels of peroxide, it is evident that the addition of Mn to the organic portion of these fungicides has a potentiating effect on ROS production. NB in combination with ZnCl2 resulted in an approximate 8-fold increase in peroxide generation, suggesting that Zn2+ does not have the same potentiating effect as Mn 2+. Thus, ZnCl2, although a pro-oxidant in these studies, is not a significant contributing factor in MZ-induced ROS generation. Cells exposed to ETU showed no significant generation of ROS either alone or in combination with MnCl2.
Fig. 7.
Extracellular peroxide formation in mesencephalic neurons exposed to various EBDCs and their major metabolite, ETU. Cells were treated with 30 uM of MZ, MB, NB, ETU, MnCl2, ZnCl2, ETU plus MnCl2, or NB plus MnCl2 or ZnCl2 and immediately assessed for extracellular H2O2 by the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. The data are from three to seven separate experiments run in duplicate. *Significant difference from control. ** Significant difference from MZ alone. *** Significant difference from NB alone.
Microglia contribute but are not required for extracellular peroxide generation induced by MZ exposure
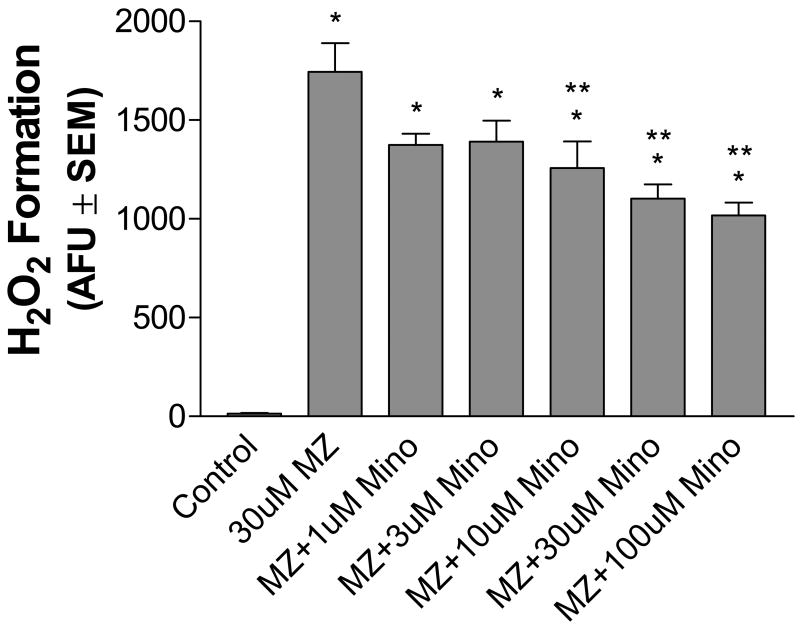
Microglia, the resident immune cells of the brain, have been implicated in neurotoxicity and neurodegeneration via ROS generation (Gao et al., 2002, 2003b,c; Liu and Hong, 2003; Wu et al., 2003; Zhang et al., 2004; Wu et al., 2005). Microglia are also a major source of NADPH oxidase in the nervous system (Babior et al., 1999; Wu et al., 2003). To determine whether or not microglia contributed to MZ-induced ROS generation and neurotoxicity, H2O2 levels were measured with the Amplex® Red assay in cells pretreated with 1–100 uM minocycline, an antibacterial agent that inhibits microglial activity (Yrjanheikki et al., 1998; Fan et al., 2005) (Fig. 8). Pretreatment with minocycline resulted in a dose-dependent decrease of H2O2 levels from cells treated with 30 uM MZ (10 uM minocycline, 28% decrease; 30 uM minocycline, 37% decrease; 100 uM minocycline, 42% decrease). Although minocycline significantly decreased ROS generation in MZ-treated cells, H2O2 levels were still significantly greater than controls.
Fig. 8.
Dose-response of minocycline, a non-specific microglia inhibitor, on extracellular ROS production by MZ in mesencephalic cells. Cells were pre-treated with 1–100 uM minocycline for 3 h and then exposed to 30 uM MZ. H2O2 levels were measured via the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. The data are from four separate experiments run in duplicate. *Significant difference from control. ** Significant difference from MZ alone.
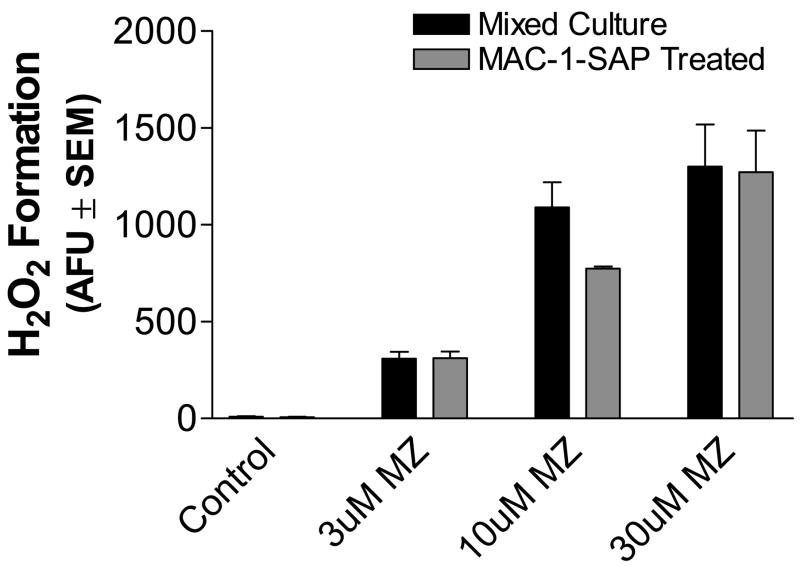
To further assess the involvement of microglia in MZ-induced ROS generation, microglia were reduced in mixed mesencephalic cell culture with MAC-1-SAP, a microglial-specific antibody that is conjugated with the toxin saporin, which targets microglial ribosomes thereby eliminating them (Kanai et al., 2001; Dommergues et al., 2003). As shown in Fig. 9, microglia were decreased in MAC-1-SAP treated cultures as compared with controls. Despite the reduction in microglia and contrary with the 40% decrease in H2O2 generation observed in cultures pretreated with 100 uM minocycline, MAC-1-SAP treated cultures did not show an attenuation of H2O2 generation when challenged with MZ (Fig. 10). In addition, cultures enriched in neurons by growing in serum-free N2 medium (Ricart and Fiszman, 2001) to greatly reduce glial growth produced a large increase in H2O2 generation in the presence of MZ (15.5 ± 5.0 and 658.9 ± 59.5, AFU ± SEM in control and 3 uM MZ treated cultures, respectively, n=4). These findings indicate that while microglia may contribute to MZ-induced ROS generation, their presence is not required.
Fig. 9.
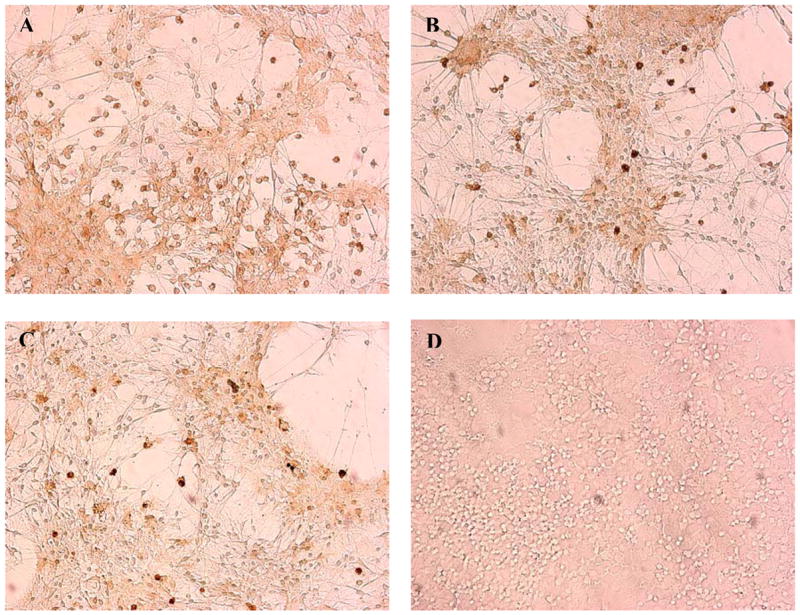
Micrographs of MAC-1 (CD11b)-immunostained mesencephalic cells. Mesencephalic cells were treated with (A) control, (B) 0.5 ug/ml, (C) 1.0 ug/ml, and (D) 2.0 ug/ml MAC-1-SAP, a microglia-specific Ab conjugated with the ribosomal toxin, saporin, for 72 h. Cells were fixed and immunostained for MAC-1 (1:150) as described in Methods. Note the loss of microglia, represented by dark circular cells, with increasing doses of MAC-1-SAP.
Fig. 10.
Comparison of extracellular peroxide formation in mixed and microglial-free mesencephalic cultures. Primary mixed and microglial-free cultures treated with MAC-1-SAP were prepared as described in Methods. All cells were exposed to 3, 10, and 30 uM MZ and assayed for H2O2 via the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit as described in Methods. The data are from three separate experiments run in duplicate. There were no significant differences in H2O2 levels between mixed and microglial-free cultures.
DISCUSSION
This research is an extension of previous work that evaluated the mechanism of MZ neurotoxicity. Previous studies have shown that MZ and MB compromise mitochondrial respiration (Zhang et al., 2003; Domico et al., 2006a). Research from our laboratory has shown that 15 uM MZ or MB uncouple the mitochondrial electron transport chain (ETC), while higher doses (30 uM) inhibit respiration (Domico et al., 2006a). Moreover, Zhang et al. (2003) found that MB selectively inhibits complex III of the ETC. During normal respiration, small amounts of ROS are produced as byproducts of the ETC process. However, perturbations in mitochondrial respiration can lead to excessive ROS generation and inundate cellular antioxidant capacity, leading to DNA damage, lipid peroxidation, protein modification, and eventually cell death (Rao and Balachandran, 2002; Kumar et al., 2005). The present study was done to investigate the potential involvement of ROS in MZ-induced neurotoxicity.
Major findings from this study indicate that MZ-treated neuronal cells produce large amounts of ROS and that ROS generation induced by MZ exposure contributes to MZ neurotoxicity. Our findings were based on data obtained from two common approaches that independently assess ROS generation. The Amplex® Red Hydrogen Peroxide/Peroxidase Assay and DCF fluorescence showed that ROS levels were significantly higher in MZ-treated neuronal cultures than in controls. Moreover, ascorbate and SOD attenuated MZ-induced toxicity, as assessed by DA and GABA uptake. Ascorbate, an antioxidant water-soluble vitamin, directly scavenges superoxide anion and hydroxyl radical while SOD, an antioxidant mitochondrial and/or cytosolic enzyme, catalyses the conversion of superoxide anion to hydrogen peroxide and water (Rao and Balachandran, 2002). These data are consistent with findings from previous studies that evaluated other EBDCs and their role in oxidative stress and neuronal toxicity. Fitsanakis et al. (2002) found that Mn-EBDCs catalyze a one electron transfer from dopamine or norepinephrine to molecular oxygen, resulting in ROS products like superoxide and semiquinone species. Barlow et al. (2005) reported that MB, a Mn-EBDC very similar to MZ, at doses not affecting cell viability induced significant changes in the GSH antioxidant system in PC12 cells (catecholaminergic cell line) and in mesencephalic cells. More specifically, low doses (0.0376–3.76 uM) of MB diminished GSH levels and prevented the recovery of GSH levels in cell culture systems. Zhou et al. (2004) measured oxidative stress in MES cells (dopaminergic cell line) exposed to Mn-EBDC via protein carbonyl quantification and reported that total cellular carbonyl content was 160% and 167% greater in cells treated for 3 and 7 d respectively with 6 uM Mn-EBDC than in controls. Moreover, Mn-EBDC exposure also resulted in α-synuclein aggregation and proteasomal inhibition. In that same study, pretreatment with the antioxidant N-acetyl-L-cysteine (NAC) resulted in a decrease of protein carbonyls and prevented proteasomal inhibition. In the PQ + MB model of Parkinson’s disease, overexpression of SOD or glutathione peroxidase in mice led to protection against lipid peroxidation and subsequent DAergic neurodegeneration (Thiruchelvam et al., 2005).
Findings from this study and previous studies (Domico et al., 2006a) show that acute exposure to MZ produces toxicity to midbrain DAergic neurons. Neuronal damage due to MZ exposure, however, was not restricted to the DA population and was also observed in another major midbrain neuronal population, i.e. GABAergic neurons. In contrast, other neurotoxicants like MPTP/MPP+ (Forno et al., 1993; Gao et al., 2003c) or rotenone (Gao et al., 2003a,b; Zeevalk and Bernard, 2005) show selectivity in damage to dopaminergic neurons. Although PD is not associated with GABAergic neurodegeneration until much latter stages of disease progression, other neurodegenerative conditions like Huntington’s disease, an inherited neurodegenerative disorder, are demarcated by the loss of GABAergic neurons in the striatum (Fix, 2005). The DAergic neuronal cell population has been hypothesized to be vulnerable to oxidative stress because of the auto-oxidation of DA itself (Hastings, 1995). Mesencephalic GABAergic neurons are not at risk of such intrinsic oxidative stress, but are equally as vulnerable as DA neurons to an exogenous oxidative stress such as H2O2 exposure (Zeevalk et al., 2003). Given the large extracellular generation of ROS by MZ in the in vitro mesencephalic culture system, it is, therefore, not surprising that similar susceptibilities were found for DA and GABA neurons upon exposure to MZ.
Nevertheless, MZ-induced toxicity to some degree mimics the pathologies particular to idiopathic PD. Brown et al. (2006) proposed that agents of potential concern in the development of parkinsonism meet the following criteria; (1) cause changes in the striatal DAergic system, including a decrease in DA and/or an increase in DA turnover, (2) produce effects in the SN, which is predominantly DAergic, that may or may not be specific to DAergic neurons, and (3) mimic cellular and molecular mechanisms as seen in PD, like mitochondrial dysfunction, oxidative stress, and/or alpha-synuclein aggregation. MZ meets most of these criteria. It should be noted, however, that the findings regarding selectivity of damage as presented here were done in vitro and may differ from in vivo exposure. Thiruchelvam et al., (2000a,b), found little damage in vivo to the nigrostriatal system with MB alone, but an enhancement of damage to the DAergic system when combined with PQ. In addition, Zeevalk and Bernard (2005) reported that acute high dose exposure to rotenone produced non-selective toxicity in mesencephalic neurons, whereas chronic low dose exposure resulted in selective DAergic neurotoxicity. These data suggest that MZ when administered acutely and in toxic doses may non-selectively affect various neuronal populations. In contrast, when administered chronically or at lower doses in combination with other pesticides, MZ may elicit more selective and/or profound effects to the DAergic system.
A large component of ROS generation by MZ exposure occurs extracellularly. Treating cells with catalase, which does not permeate the cell membrane, attenuated H2O2 levels in MZ- treated cells. However, when endogenous catalase was inhibited by ATZ, H2O2 levels in MZ- treated cells were unaffected, suggesting that the production of peroxides in mesencephalic neurons mainly occurs in the extracellular environment. One potential source of MZ-induced extracellular ROS is redox cycling of MZ with oxidases that reside on the membrane of mesencephalic cells. NADPH oxidase is a multimeric enzyme with catalytic flavin- and/or heme-binding subunits (gp91phox, p22phox, p47phox, p67phox, and p40phox, and RAC) that exist in the cytosolic portion of the cell and on the cellular membrane (Baboir, 1999; Wu et al., 2003; Guzik and Harrison 2006; Bedard and Krause, 2007). Upon activation, cytosolic subunits of the enzyme (p47phox, p67phox, and p40phox, RAC) relocate to the cell membrane and assemble with the transmembrane subunits (gp91phox, p22phox) (Wu et al., 2003). NADPH oxidase, when activated, has been implicated as a mediator of oxidative stress because of its ability to transfer one electron from NADPH to molecular oxygen, generating superoxide anion and other secondary reactive species (Gao et al., 2003b,c; Wu et al., 2003; Zhang et al., 2004; Bonneh-Barkay et al., 2005b; Wu et al., 2005). During this electron transfer, NADPH oxidase can interact with exogenous ligands like MZ, resulting in redox cycling. Other toxicants have been reported to redox cycle via NADPH oxidase. PQ, a bipyridyl herbicide that is structurally similar to the MPTP metabolite MPP+, redox cycles with microglial NADPH oxidase and/or nitric oxide synthase (NOS), resulting in significant levels of superoxide anion that are attenuated with apocynin (NADPH oxidase inhibitor) or Nω-nitro-L-arginine methyl ester (L-NAME, NOS inhibitor) (Bonney-Barkay et al., 2005b). Consistent with this, we showed that the inhibition of NADPH oxidase via three distinctly different inhibitors resulted in an attenuation of extracellular peroxide levels in MZ-treated cells. DPI, a non-specific NADPH oxidase inhibitor, prevents electron transport within the NADPH oxidase multi-subunit complex (O’Donnell et al., 1993; Li and Trush, 1998; Doussiere et al., 1999). Because of its action, however, DPI can also inhibit other electron transporters, like nitric oxide synthase (Stuehr et al., 1991), xanthine oxidase (Doussiere and Vignais, 1992), and mitochondrial complex I (Li and Trush, 1998). APO, which needs activation by peroxidases, inhibits the association of the NADPH oxidase cytosolic subunits (Stolk et al., 1994). It is the most commonly used NADPH oxidase inhibitor and has been reported to decrease NADPH oxidase-mediated ROS in previous studies (Gao et al., 2002; Casarejos et al., 2006; Wang et al., 2006; Abramov et al., 2007). AEBSF, a non-specific serine protease inhibitor, deters NADPH oxidase activation by inhibiting the subunit p47phox (Megyeri et al., 1995; Diatchuk et al., 1997). All three inhibitors attenuated extracellular peroxide levels in MZ-treated mesencephalic cells. Other studies have utilized these NADPH oxidase inhibitors as tools to evaluate the role of NADPH oxidase in redox cycling and/ or ROS generation. Gao et al. (2002) reported a decrease in superoxide levels in rotenone-treated neutrophils preloaded with 5 uM DPI or 0.5 mM APO. Similarly, Hwang et al. (2002) reported attenuation in brain-derived neurotrophic factor-induced ROS generation in cortical cultures by 50 uM AEBSF. Although NADPH oxidase inhibitors are relatively nonspecific, the use of 3 different inhibitors with differing mechanisms of action and at concentrations consistent with their selective action on NADPH oxidase support a contribution of the extracellular oxidase in MZ-induced ROS generation. On the other hand, significant ROS production with MZ even in the presence of high concentrations of the NADPH oxidase inhibitors remained suggesting that additional factors may contribute to MZ-mediated ROS generation.
Since microglia are a major source of NADPH oxidase (Babior et al., 1999; Wu et al., 2003), we assessed the role of microglia in MZ-induced ROS generation. We found that inhibition of microglia with minocycline modestly, but significantly decreased ROS generation in MZ-treated cells, whereas elimination of microglia with MAC-1-SAP did not significantly attenuate ROS levels. Moreover, neuronal-enriched cultures generated ROS after MZ treatment at levels similar to mixed (neurons and glia/microglia) cell cultures. These findings indicate that microglial NADPH oxidase plays only a modest role in ROS generation. Although microglia have been implicated in the neurotoxicity of other pesticides, like PQ (Bonneh-Barkay et al., 2005; Wu et al., 2005), they do not appear to be essential to MZ-induced ROS generation, as our data indicate. Despite the involvement or lack thereof of microglia in MZ neurotoxicity, NADPH oxidase may still be involved in MZ-induced ROS generation. Nonphagocytic NADPH oxidase is important in the central nervous system where it is involved in cell signaling (Infanger et al., 2006), differentiation (Suzukawa et al., 2000), potentiation (Thiels et al., 2000; Knapp and Klann, 2002), and memory (Thiels et al., 2000). Functional subunits of NADPH oxidase have been detected in the neurons of various regions of the mouse brain, including hippocampus, cortex, amygdala, striatum, and thalamus (Serrano et al., 2003; Tejada-Simon et al., 2005). Astrocytic NADPH oxidase has been implicated in the neurotoxicity of amyloid beta peptides (Abramov and Duchen, 2005). MZ may interact with nonphagocytic NADPH oxidase generating ROS independent of microglia involvement. Our data suggest that microglia may contribute to MZ toxicity but are not required for significant levels of extracellular ROS production. Furthermore, because NADPH oxidase inhibitors do not fully eradicate extracellular ROS in MZ-treated cells, other cellular constituents in addition to NADPH oxidase may be involved in MZ redox cycling and ROS generation.
Based on the data presented here, it is possible that MZ may also induce intracellular ROS generation. Intracellular ROS levels in MZ-treated cells were assessed by DCF fluorescence, a common monitor of ROS generation (Rosenkranz et al., 1992; Moy et al., 2000; Zeevalk and Bernard, 2005). DCF, an oxidant-sensing probe, is readily taken up by the cell and is oxidized to a fluorescent product by peroxides and other ROS inside the cell (Carter et al., 1994). Although DCF fluorescence indicates an increase in ROS inside the cell, it is not necessarily indicative of intracellular generation. Peroxides can readily diffuse across the plasma membrane from an extracellular source where they can elicit toxic effects. Catalase greatly attenuated extracellular H2O2 generation, but did not attenuate MZ-induced DCF fluorescence. If oxidation of DCF occurred due to diffusion of extracellularly generated ROS some attenuation would have been expected. These findings support production of ROS by MZ both intracellularly and extracellularly.
There are many potential sources of intracellular ROS in cells exposed to MZ. Intracellular oxidases, including xanthine oxidase, monoamine oxidase, and cyclooxygenase-2 (Teismann et al., 2003), are available to transfer electrons to exogenous ligands like MZ, resulting in ROS generation. In this study, allopurinol attenuated MZ-induced ROS levels by an approximate 30%, indicating that intracellular xanthine oxidase, in part, plays a role in ROS generated as a result of MZ exposure. Other studies involving ROS-generating pesticides have reported similar results. Sakai et al. (1995) found that superoxide levels and cytotoxicity in bovine endothelial cells exposed to PQ were reduced in cells treated with allopurinol. In cerebellar granule cells treated with PQ, allopurinol partially prevented the release of cytochrome C from mitochondria and thus, inhibited PQ-induced apoptosis (Gonzalez-Polo et al., 2004).
Moreover, it has been postulated that DA neurons are more susceptible to the damage caused by ROS because of the potential neurotoxicity of DA itself (Halliwell, 1992; Hastings et al., 1996; Hastings and Zigmond, 1997). That is, DA is metabolized to 3,4-dihydroxyphenyl acetic acid via monamine oxidase, producing H2O2 (Halliwell, 1992). In addition, the oxidation of dopamine produces dopamine quinones, reactive species that can cause damage to lipids, proteins, and DNA (Hastings, 1995). Mn-EBDCs, like MB and MZ, can catalyze the oxidation of catechols (Fitsanakis et al., 2002). If DA becomes available to MZ or MB in the cytosol or extracellularly, the EBDC-catalyzed oxidation of catecholamines denotes another potential source of highly reactive free radicals and ROS.
Ongoing studies in our laboratory have determined that the parent compound MZ or a MZ ion fragment permeates neuronal cells (Domico et al., 2006b, 2007). We found that approximately 8% of the load of extracellular MZ crosses the neuronal membrane to access the intracellular compartment. Maximum intracellular levels are reached at 3 h post-treatment and remain detectable for up to 14 h. While only a small percentage of the extracellular load of MZ permeates the cell, its presence in the limited volume of the intracellular compartment likely results in substantial levels sufficient to generate intracellular ROS.
Previous studies in our laboratory have shown that MZ and MB inhibit mitochondrial respiration (Domico et al., 2006a). MZ-induced perturbations in the electron transport chain may result from distinct mechanisms; either MZ can directly interact with mitochondrial complexes in the respiratory chain to result in mitochondrial inhibition, or MZ-induced ROS can interact with mitochondrial proteins and lead to mitochondrial dysfunction. Either mechanism could further augment ROS production in DA neurons, potentially leading to dopaminergic neurodegeneration and subsequent pathologies. Further studies are needed to better clarify the intracellular generation of ROS and the mechanism underlying mitochondrial perturbation by MZ.
The role of Mn in the neurotoxic action of MZ has been debated in previous studies. Soleo et al. (1996) suggest that the organic portion of the EBDC fungicide is responsible for toxicity. Data from our laboratory indicate that both the metal component, specifically Mn, and the organic backbone result in significant toxicity (Domico et al., 2006a). Consistent with these data, extracellular ROS levels assessed in this study were significantly higher in cells exposed to the Mn-free, Na+-containing EDBC, NB in combination with MnCl2 (153-fold increase) as opposed to NB (3-fold increase) or MnCl2 (4-fold increase) alone. Adding MnCl2 to NB mimics the components of the metal-containing EBDC, MB. It is evident that, although exposure to NB plus ZnCl2 results in a significant increase in ROS (7.5-fold increase), it is not of the same magnitude as NB plus MnCl2 or MZ, both of which result in similar levels of H2O2 (153-fold increase and 118-fold increase, respectively). This lends support to a role for both the Mn-metal component and the organic backbone in MZ neurotoxicity. ETU exposure does not result in significant levels of H2O2, consistent with our previous observation that ETU did not produce toxicity in the mesencephalic cells (Domico et al., 2006a). Interestingly, while addition of MnCl2 to NB greatly potentiated ROS production (this study) and toxicity (Domico et al., 2006a), it did not mimic the inhibitory effects of MZ or MB on mitochondrial function (Domico et al., 2006a). These coupled findings argue that the primary mechanism of neurotoxic action after acute exposure to MZ or MB is oxidative stress. Perturbations in mitochondrial respiration are secondary. This mode of toxic action is common to other neurotoxins, like rotenone (Greenamyre et al., 1999; Gao et al., 2002; Gao et al., 2003a,b; Zeevalk and Bernard, 2005), and neurotoxicants like MPTP (Gao et al., 2003c; Wu et al., 2003; Zhang et al., 2004).
This study identifies MZ as a pro-oxidant neurotoxicant. Based on these data, it is postulated that EBDC fungicides that contain the Mn moiety can redox cycle outside and inside of the cell via cellular oxidases and result in the generation of ROS. Although NADPH oxidase does, in part, contribute to peroxide generation, ROS generation is likely also mediated by other cellular oxidase systems. ROS generation by MZ appears to be the primary mechanism of toxicity and is contributed to by both the organic backbone and associated Mn-ion. Findings also show that while microglia are involved in MZ-induced ROS generation, their involvement is not essential to the process. The mechanistic data from this study will be useful in assessing the effect of pesticide exposure and its involvement in neurodegeneration.
Acknowledgments
The authors wish to thank Mrs. Cindy Song for her technical assistance in the laboratory and Dr. Nikolay Filipov for his assistance with the immunological methodology. This research was supported by the NIEHS training grant ES07148 and a Cook College (Rutgers University) New Jersey Agricultural Experimental Station grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Phil Trans R Soc B. 2005;360:2309–14. doi: 10.1098/rstb.2005.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–38. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- Barlow BK, Lee DW, Cory-Slechta DA, Opanashuk LA. Modulation of antioxidant defense systems by the environmental pesticide maneb in dopaminergic cells. Neurotoxicology. 2005;26:63–75. doi: 10.1016/j.neuro.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bachurin SO, Shevtzova EP, Lermontova NN, Serkova TP, Ramsay RR. The effect of dithiocarbamates on neurotoxic action of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and on mitochondrial respiration chain. Neurotoxicology. 1996;17:897–903. [PubMed] [Google Scholar]
- Bedard K, Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Langston WJ, Di Monte DA. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid Redox Signal. 2005a;7:649–53. doi: 10.1089/ars.2005.7.649. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Mol Brain Res. 2005b;134:52–6. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capelton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease – is there a link? Environ Health Perspec. 2006;114:156–64. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–8. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem. 2006;97:934–6. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. Oxidative stress, mitochondrial respiration, and Parkinson’s disease. Annal NY Acad Sci. 2000;899:112–120. doi: 10.1111/j.1749-6632.2000.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem. 1989;52:1830–6. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin P, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Domico LM, Yang I, Buckley B, Zeevalk GD, Cooper KR. Measurement of the Mn/Zn-ethylene-bis-dithiocarbamate mancozeb in biological matrixes and demonstration of uptake by neuronal cells. Tox Sci. 2007 manuscript submitted. [Google Scholar]
- Domico LM, Zeevalk GD, Bernard LP, Cooper KR. Acute neurotoxic effects of mancozeb and maneb in mesencephalic cells are associated with mitochondrial dysfunction. Neurotoxicology. 2006a;27:816–25. doi: 10.1016/j.neuro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Domico LM, Zeevalk GD, Yang I, Buckley B, Johnson W, Bernard LP, Thiruchelvam M, Cooper KR. The role of oxidative stress in the neurotoxicity of mancozeb and maneb. Annual Meeting of the Society of Toxicology; San Diego, CA. 2006b. Abstract and poster presentation #1484. [Google Scholar]
- Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neurosci. 2003;121:619–28. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- Doussiere J, Gaillard J, Vignais PV. The heme component of the neutrophil NADPH oxidase complex is target for aryliodonium compounds. Biochem. 1999;38:3694–703. doi: 10.1021/bi9823481. [DOI] [PubMed] [Google Scholar]
- Doussiere J, Vignais PV. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Eur J Biochem. 1992;208:61–71. doi: 10.1111/j.1432-1033.1992.tb17159.x. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Zeevalk GD. Cooperative interaction between ascorbate and glutathione during mitochondrial impairment in mesencephalic cultures. J Neurochem. 2003;86:1487–97. doi: 10.1046/j.1471-4159.2003.01954.x. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Zeevalk GD. Hydrogen peroxide removal and glutathione mixed disulfide formation during metabolic inhibition in mesencephalic cultures. J Neurochem. 2001;77:1496–507. doi: 10.1046/j.1471-4159.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neurosci. 2005;133:159–68. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Amarnath V, Moore JT, Montine KS, Zhang J, Montine TJ. Catalysis of catechol oxidation by metal-dithiocarbamate complexes in pesticides. Free Radic Biol Med. 2002;33:1714–23. doi: 10.1016/s0891-5849(02)01169-3. [DOI] [PubMed] [Google Scholar]
- Fix JD. High-Yield Neuroanatomy. Vol. 3. New York: Lippincott Williams and Wilkins; 2005. pp. 142–8. [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonism and Parkinson’s disease: neuropathologic considerations. Adv Neurol. 1993;60:600–8. [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–90. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Liu B, Hong J. Critical role for microglial NADPH oxidase in rotenone-induced neurodegeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–7. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Rodriguez-Martin A, Moran JM, Niso M, Soler G, Fuentes JM. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Res. 2004;1011:170–6. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie GM, Peng TI, Stephans SE. Mitochondrial dysfunction in Parkinson’s disease. Biochem Soc Symp. 1999;66:85–97. doi: 10.1042/bss0660085. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discovery Today. 2006;11:524–33. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in the central nervous system. J Neurochem. 1992;59:1609–23. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem. 1995;64:919–24. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Pro Nat Acad Sci. 1996;3:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings TG, Zigmond MJ. Loss of dopaminergic neurons in parkinsonism: possible role of reactive dopamine metabolites. J Neural Trans Supp. 1997;49:103–10. doi: 10.1007/978-3-7091-6844-8_11. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi S, Koh J. The role of NADPH oxidase, neuronal nitric oxide synthase and poly(ADP ribose) polymerase in oxidative neuronal death induced in cortical cultures by brain-derived neurotrophic factor and neurotrophin-4/5. J Neurochem. 2002;82:894–902. doi: 10.1046/j.1471-4159.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–96. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Tosuka T, Iiyama R, Okamoto R, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn’s disease. Gastroenterol. 2001;121:875–88. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. Phila: Elsevier Saunders; 2005. pp. 15–19. [Google Scholar]
- Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–9. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;48:1583–8. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate) Scand J Work Environ Health. 1994;20:301–5. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Megyeri P, Pabst KM, Pabst MJ. Serine protease inhibitors block priming of monocytes for enhance release of superoxide. Immunol. 1995;86:629–35. [PMC free article] [PubMed] [Google Scholar]
- Morato GS, Lemos T, Takahashi RN. Acute exposure to maneb alters some behavioral function in the mouse. Neurotox Teratol. 1989;11:421–5. doi: 10.1016/0892-0362(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Moy LY, Zeevalk GD, Sonsalla PK. Role for dopamine in malonate-induced damage in vivo in striatum and in vitro in mesencephalic cultures. J Neurochem. 2000;74:1656–65. doi: 10.1046/j.1471-4159.2000.0741656.x. [DOI] [PubMed] [Google Scholar]
- Nicolaides DN, Gautam DR, Litinas KE, Hadjipavlou-Litinas DJ, Fylaktakidou KC. Synthesis and evaluation of the antioxidant and antiinflammatory activities of some benzo[1]khellactone derivatives and analogues. Eur J Med Chem. 2004;39:323–32. doi: 10.1016/j.ejmech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- O’Donnell VB, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–9. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Schapira AHV. Mitochondrial involvement in Parkinson’s disease. Neurochem Internat. 2002;40:533–41. doi: 10.1016/s0197-0186(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J Immunol Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Sakai M, Yamagami K, Kitazawa Y, Takeyama N, Tanaka T. Xanthine oxidase mediates paraquat-induced toxicity on cultured endothelial cells. Pharmacol Toxicol. 1995;77:36–40. doi: 10.1111/j.1600-0773.1995.tb01911.x. [DOI] [PubMed] [Google Scholar]
- Santos MS, Moreno AJ, Carvalho AP. Relationships between ATP depletion, membrane potential, and the release of neurotransmitters in rat nerve terminals. Stroke. 1996;27:941–950. doi: 10.1161/01.str.27.5.941. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–8. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–64. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleo L, Defazio G, Scarselli R, Zefferino R, Livrea P, Foa V. Toxicity of fungicides containing ethylene-bis-dithiocarbamate in serumless dissociated mesencephalic-striatal primary coculture. Arch Toxicol. 1996;70:678–82. doi: 10.1007/s002040050328. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–8. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Sarna T, Zecca L. Modulation by neuromelanin of the availability and reactivity of metal ions. Annals Neuro. 1992;32:S69–S75. doi: 10.1002/ana.410320712. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Rogerio R, Zanin M. Maneb enhances MPTP neurotoxicity in mice. Res Commun Chem Pathol Pharmacol. 1989;66:167–70. [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci. 2003;100:5473–8. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Kiann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–9. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain Res. 2000a;873:225–34. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000b;20:9207–14. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Richfield EK, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson’s disease phenotype. J Bio Chem. 2005;280:22530–9. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Vaccari A, Saba P, Mocci I, Ruiu S. Dithiocarbamate pesticides affect glutamate transport in brain synaptic vesicles. J Pharmacol Exp Ther. 1999;288:1–5. [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Wood TK, Sullivan AM, McDermott KW. Viability of dopaminergic neurons is influenced by serum and astroglial cells in vitro. J Neurocyto. 2003;32:97–103. doi: 10.1023/a:1027384416811. [DOI] [PubMed] [Google Scholar]
- Wu D, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulus H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. PNAS. 2003;100:6145–50. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Block ML, Zhang W, Qin L, Wilson B, Zhang W, Veronesi B, Hong J. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–61. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Ehrhart J. Glutathione and ascorbate: Their role in protein glutathione mixed disulfide formation during oxidative stress and potential relevance to Parkinson’s disease. Ann NY Acad Sci. 2003;991:342–5. [Google Scholar]
- Zeevalk GD, Bernard LP. Energy status, ubiquitin proteasomal function, and oxidative stress during chonic and acute complex I inhibition with rotenone in mesencephalic cells. Antioxid Redox Signal. 2005;7:662–72. doi: 10.1089/ars.2005.7.662. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Nicklas WJ. Role of oxidative stress and the glutathione system in loss of dopamine neurons due to impairment of energy metabolism. J Neurochem. 1998;70:1421–30. doi: 10.1046/j.1471-4159.1998.70041421.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, Montine TJ. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link though mitochondrial dysfunction. J Neurochem. 2003;84:336–46. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Qin L, Gao H, Wilson B, Ali SF, Zhang W, Hong J, Liu B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J. 2004;18:589–91. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shei FS, Piccardo P, Montine TJ, Zhang J. Proteosomal inhibition induced by manganese ethylene-bis-dithiocarbamate: relevance to Parkinson’s disease. Neurosci. 2004;128:281–91. doi: 10.1016/j.neuroscience.2004.06.048. [DOI] [PubMed] [Google Scholar]