Abstract
Magnetic resonance images were collected in 76 chimpanzees and the sylvian fissure was examined for the presence of a posterior bifurcation. A bilateral bifurcation of the sylvian fissure into an ascending and descending ramus was identified in 58 of the subjects. The posterior ascending ramus was measured in both hemispheres in order to evaluate the presence, magnitude, and direction of a planum parietale asymmetry. Statistical analysis revealed a main effect for sex. Specifically, females showed a significant rightward bias, whereas males did not. Moreover, an examination of posterior bifurcation patterns of the sylvian fissure revealed differences between the left and right hemispheres. In humans, subject handedness and sex have been found to have an effect on planum parietale asymmetry. To determine if this was also the case in our chimpanzee subjects, we evaluated whether or not planum parietale asymmetry was related to subject handedness. Although subject handedness was not directly related to planum parietale asymmetry quotients, significant differences in the pattern of posterior bifurcation of the sylvian fissure were found between males and females. These results support the view that asymmetries in the peri-sylvian language areas were present in the common ancestor of humans and chimpanzees.
Keywords: planum parietale, chimpanzee, brain asymmetry, lateralization, handedness, language
Introduction
Ever since the early observations of Broca [2] and Wernicke [37], there has been considerable interest in neuroanatomical asymmetries in the perisylvian regions of the human brain [6]. The majority of attention has focused on the measurement of asymmetries in the posterior region of the temporal lobe demarking the planum temporale [see 1]. There have also been a number of studies concerning variability in the posterior region of the sylvian fissure, particularly as it relates to the occurrence of a bifurcation into a posterior ascending and descending branch. For example, at least 4 different patterns of bifurcation of the posterior sylvian fissure have been identified in human cadaver brains [21, 38]. Ide and colleagues [21] identified these 4 variants as Superior (acending ramus longer than descending), Inferior (descending ramus longer than ascending), Symmetric (both rami are of approximately equal length), and Inverted (rami are usually symmetric, but acending ramus is oriented anteriorly instead of posteriorly). Moreover, variations in the patterns of ascending and descending limbs are associated with the sex and handedness of subjects. Ide et al. [21] reported that a larger ascending compared to descending limb of the sylvian fissure (their Superior type) was more prevalent in males and more frequently seen in the right compared to the left hemisphere. In contrast, females showed more symmetrical ascending and descending limbs of the sylvian fissure but the branches differed in orientation with the ascending branch oriented rostrally whereas the descending limb was oriented more caudally.
Recently, MRI imaging technology has provided an alternative means of assessing asymmetries in the persylvian fissure. Specifically, the posterior wall of the posterior ascending ramus (PAR) of the sylvian fissure demarks the planum parietale (PP). As observed in cadaver brains, analysis of MRI scans reveal a rightward asymmetry (PAR longer in the right compared to the left hemisphere) of the PP in humans [5, 9, 23, 32]. Also consistent with the cadaver results, it has been demonstrated that handedness and gender have an influence on PP asymmetry such that right-handed men and left-handed women show pronounced rightward asymmetries [23]. In addition, a larger PP in the right hemisphere is more common in individuals who utilize their right hand to write compared to those who use their left hand [5].
Historically, there has been interest in the evolutionary origins of sylvian fissure asymmetry because it presumably reflects expansion of the posterior temporal cortex or Wernicke’s area. Early work with endocasts and cadaver brains reported evidence of asymmetries in the length and height of the sylvian fissure in primates [26, 39]. The sylvian fissure was reported to be higher in the right compared to the left hemisphere, particularly among chimpanzees, gorillas and orangutans, presumably reflecting an asymmetry of the planum parietale [25]. However, in two species of macaques, no significant differences were found in either the height or the length of the ascending limb of the sylvian fissure [10]. With respect to sylvian fissure length, Yeni-Komshian and Benson [39] reported that human and chimpanzee cadaver specimens showed a leftward asymmetry whereas rhesus monkeys did not. Subsequent MRI studies have revealed similar leftward asymmetries in the sylvian fissure in a sample comprised of all four species of great apes, replicating the findings from cadaver specimens [15]. In monkeys, the results have been less consistent. Leftward asymmetries have been reported in cadaver specimens of two species of macaques (M. fascicularis and M. mulatta) as well as in cotton-top tamarins and common marmosets [10]. However, leftward asymmetries were not observed in rhesus monkey (M. mulatta) endocasts [4]. Studies in other species have also reported mixed results. In cats, no overall asymmetry is evident but a significant sex difference exists in sylvian fissure length [35] with males showing a rightward bias and females a leftward bias. In terms of the height of the sylvian fissure, cats do show an overall rightward bias [36]. However, In dogs, no population-level asymmetry is found for length, but the sylvian fissure is reported to be higher in the left compared to the right hemisphere [34], a pattern that is opposite to that seen in apes and humans.
More direct evidence of asymmetries in the posterior temporal lobe have recently been documented with the reports of population-level leftward asymmetries in the planum temporale of great apes, the tissue that lies posterior to Heschl’s gyrus and whose terminal point is the posterior end of the sylvian fissure [3, 7, 13]. Notwithstanding, there still remains little investigation of sulci variation in the sylvian fissure in the comparative literature on brain asymmetry. For example, in a number of studies on sylvian fissure length in cadaver brains of monkeys and apes, no mention or specification is provided regarding bifurcation patterns in the specimens [e.g., 10, 39].
Recently, it has been reported that a rightward asymmetry in the planum parietale (PP) is present in two species of great apes, chimpanzees (Pan) and orangutans (Pongo) [8]. The purpose of this study was to further evaluate the evidence of an asymmetrical PP in chimpanzees. Specifically, Gannon and colleagues [8] report a rightward asymmetry in the PP, but the sex of the subjects was not provided in this report. In light of the findings of sex effects on PP asymmetry in humans (see above), the first aim of this study was to evaluate the potential influence of this factor on PP asymmetry in chimpanzees. The second purpose of this study was to assess asymmetries in the PP in chimpanzees using in vivo magnetic resonance imaging. Gannon et al. [8] measured asymmetries in the PP from surface sulci in cadaver specimens. MRI offers an alternative approach to assessing brain asymmetries and therefore we sought to determine whether similar patterns of asymmetry in the PP would be evident using this assessment technique. In addition, the relatively small sample size, (23 chimpanzee brains), is a limitation of the Gannon et al. [8] study, and therefore we sought to evaluate asymmetries in the PP in a larger sample of great apes consisting of only a single species, chimpanzees. Lastly, in humans, handedness is associated with variation in PP asymmetry. In the previous study by Gannon et al. [8], no handedness data were available in their sample of apes. Therefore, PP asymmetries were correlated with hand use to determine whether or not handedness is similarly associated with variation in PP asymmetries in chimpanzees.
Materials and Methods
Magnetic Resonance Image Collection Procedures
Magnetic resonance images (MRI) of the brain were collected in a sample of 78 chimpanzees (Pan troglodytes) including 31 males and 47 females. All of the subjects were housed at the Yerkes National Primate Research Center (YNPRC). Twenty of the MRI scans were performed on cadaver chimpanzee brains, whereas the remaining subjects were alive and healthy at the time of data collection.
The cadaver specimens were stored in 10% buffered formalin solution for intervals ranging from 1 week to 2 years. For the in vivo scans, subjects were first immobilized by ketamine injection (10 mg/kg) and subsequently anaesthetized with propofol (10 mg/kg/h)) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. The subjects remained anaesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ~ 2 h). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min as a function of brain size. After completing MRI procedures, the subjects were returned to the YNPRC and temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage.
The majority (n = 63) of the subjects were scanned using one of two 1.5 Tesla scanners (Phillips, Model 51). The remaining 15 subjects were scanned using a 3.0 Tesla scanner (Siemens Trio) at the YNPRC. The scanning methods have been described in detail elsewhere [17]. For all subjects scanned in vivo using the 1.5 T machine (n = 43; 15 males, 28 females), T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, and a 256 × 256 matrix). For the 20 post mortem scans using the 1.5 T scanner (10 males, 10 females), T2- weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 3000 ms, echo time = 40.0 ms, number of signals averaged = 4, and a 256 × 256 matrix). These scan parameters were developed in previous studies [13], and provided excellent resolution of the brain areas of interest to this study. The remaining 15 subjects (6 males, 9 females) were scanned using a 3.0 Tesla scanner (Siemens Trio), T1-weighted images were collected using a 3D gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320). The archived MRI data were transferred to a PC running Analyze 6.0 (Mayo Clinic) software for post-image processing.
Quantification of the Planum Parietale and Posterior Descending Ramus
The planum parietale (PP) was selected as the brain region of interest (ROI) because it has been implicated in language-related behaviors in humans, and morphological asymmetries have been identified previously in chimpanzees [3, 7, 8, 13, 15]. For the PP, the length of the posterior ascending ramus (PAR) of the sylvian fissure (SF) was used. Both the PAR and posterior descending ramus (PDR) of the SF are shown in Figure 1.
Figure 1.
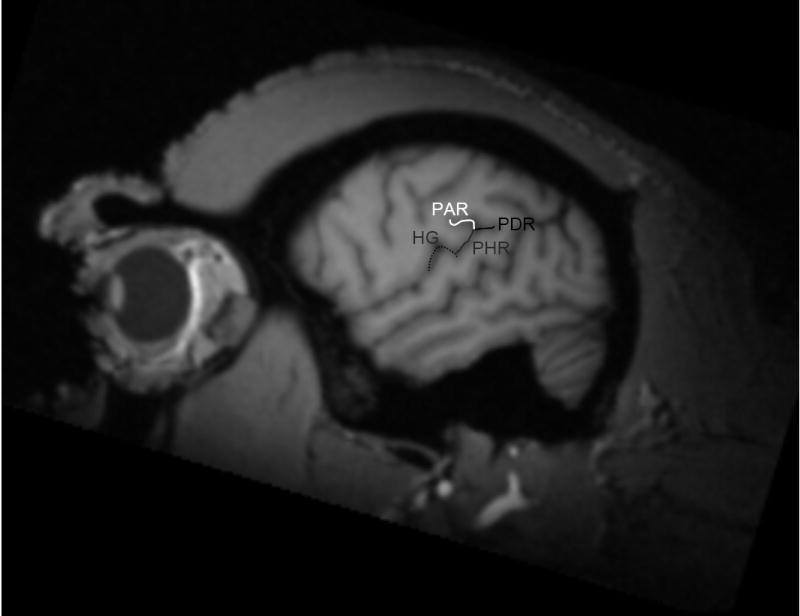
Planum parietale and planum temporale morphology. MR image of a chimpanzee brain in the sagittal plane. The sylvian fissure bifurcates into a posterior ascending ramus (PAR) and a posterior descending ramus (PDR). The posterior wall of the posterior ascending ramus demarks the PP. The caudal extent of the posterior horizontal ramus (PHR) of the sylvian fissure demarks the posterior border of the PT. Heschl’s gyrus (HG) is labeled in white.
An observer blind to the sex and handedness of the subjects, traced both rami using a mouse-driven onscreen cursor (Analyze 6.0) (Figure 1). A free-hand line tool with no anchors was used to measure the lengths of the PAR and PDR in the left and right hemispheres. To do this, consecutive 1 mm sagittal slices were traced from the most medial slice immediately lateral to the insula until both rami of the SF were no longer completely visible.
Inter-rater reliabilities were established for the PAR and PDR based on 7 (~ 9 %) randomly selected subjects in the sample. Two raters independently measured both the PAR and PDR in the left and right hemispheres. Asymmetry quotients were derived as described below, and the intraclass correlation coefficients for PAR and PDR were .78 and .93, respectively. Both of these coefficients are significant at p < .05.
In addition to the quantification of the PAR and PDR, we sought to further evaluate the bifurcation patterns of the sylvian fissure for all subjects in which a posterior bifurcation was present. To accomplish this, an observer blind to the sex and handedness of the subjects examined the most lateral slice in each hemisphere in which both the PAR and PDR were visible, and classified the bifurcation pattern into one of the following types, Superior, Inferior, Symmetric, and Inverted as defined by Ide et al., [21]. Examples of each bifurcation type are shown in Figure 2.
Figure 2.
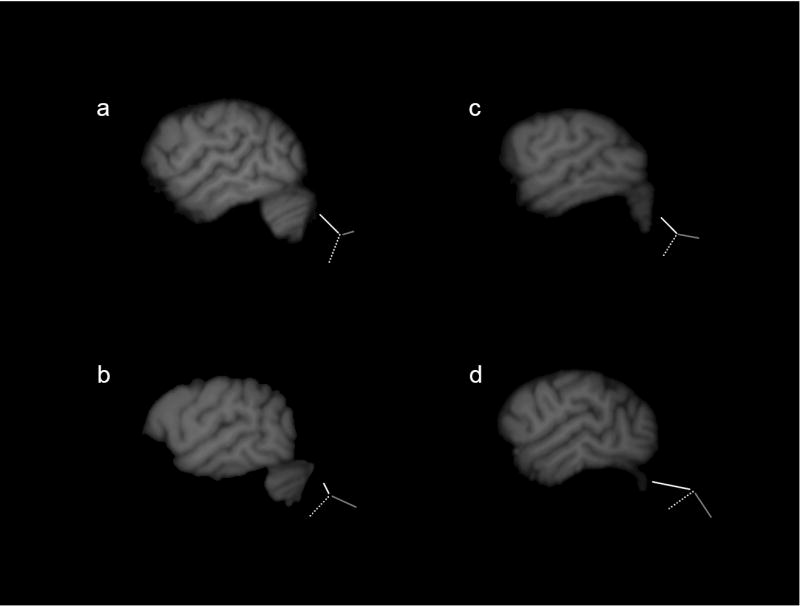
Bifurcation patterns of the sylvian fissure. Single sagittal slices of magnetic resonance images depicting the four sylvian fissure bifurcation patterns, a) superior, b) inferior, c) symmetric, and d) reversed. Traces of the bifurcations appear alongside each image. Dashed line depicts the sylvian fissure, grey solid line is the PDR and the white solid line is the PAR. Images a and b are left hemispheres, whereas c and d are right hemispheres.
Data Analysis
For both the PAR and PDR, asymmetry quotients (AQ) were derived following the formula [AQ=(R-L)/(R + L) * .5)] where R and L refer to the summed values from all slices in which the rami were traced for the right and left hemispheres, respectively. Positive values indicated a rightward bias and negative values indicated a leftward bias. Prior to statistical analysis, SPSS 15.0 (SPSS, Inc., Chicago, Illinois, USA) was used to construct box plots to identify extreme AQ values for the PAR. Box plots include the interquartile range and the 5% and 95% confidence intervals as indicated by error bars located outside of the box. Two subjects (both females) with PAR AQ values outside the confidence intervals were identified as outliers and were removed from further analysis.
Behavioral Measure of Hand Use
A composite measure of handedness was derived from four measures of hand use previously described in these subjects including manual gestures [18], simple reaching [16], bimanual feeding [11], and a task measuring coordinated bimanual actions, referred to as the TUBE task [12]. These four measures were selected on which to derive a single measure of handedness because a) they are uncorrelated with each other [see 19], b) they each elicit consistent hand preferences in the chimpanzees and c) these measures were available in the largest cohort of subjects. A brief description of each measure is provided below.
Bimanual Feeding (FEED)
Each afternoon, the primates housed at the YNPRC receive fruits and vegetables as part of their daily diet. Each subject usually receives 2 oranges, 1 banana, some celery stalks, and/or carrots. Upon retrieving the food, the subjects typically move to a seating place and consume the food. The chimpanzees generally hold the extra pieces of food with one hand and feed with the opposite hand. Hand use was recorded when the subjects were feeding with one hand for a minimum duration of 3 seconds and the non-feeding hand was holding the remaining portions of food. The dominant hand was recorded as the one feeding. A minimum of 50 responses were obtained from each subject.
Coordinated Bimanual Actions (TUBE)
The second handedness measure was a task requiring bimanual coordinated actions, referred to as the TUBE task (Hopkins, 1995). For the TUBE task, peanut butter is smeared on the inside edges of poly-vinyl-chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use one hand to hold the tube and the other hand to remove the substrate. The PVC tubes were handed to the subjects in their home cages and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter and brought it to their mouth, the hand used was recorded as left or right. As with the feeding measure, a minimum of 50 responses were obtained from each subject.
Manual Gestures
At the onset of each trial, an experimenter would approach the chimpanzee’s home cage and center themselves in front of the chimpanzee at a distance of approximately 1.0–1.5 m. If the chimpanzee was not already positioned in front of the experimenter at the onset of the trial, the chimpanzee would immediately move towards the front of the cage when the experimenter arrived with the food. The experimenter then called the chimpanzee’s name and offered a piece of food until the chimpanzee produced a manual gesture. Only responses in which the chimpanzee’s unimanually extended the digit(s) through the cage mesh to request the food were considered a response. Other possible manual responses such as cage banging or clapping were not counted as a gesture. Two-handed gestures, although rare, were not scored as were gestures that were produced by the chimpanzee prior to the experimenter arriving in front of the chimpanzee’s home cage.
Simple Reaching
On each trial, a raisin was thrown into the subject’s home cage. The raisin was thrown by the experimenter to a location at least three meters from the focal subject so that the chimpanzees had to locomote to position to the raisin, pick up the raisin and bring it to their mouth for consumption. When the chimpanzee acquired the raisin, the experimenter recorded the hand used as left or right. One, and only one, reaching response was recorded each trial to assure independence of data points [see 14 for contrasting views, 27]. Thus, raisins were not randomly scattered in home cages but rather an individual raisin was thrown into cages and subjects retrieved the raisin before another was thrown into the cage. Subjects were required to locomote at least three strides between reaching responses to maintain postural readjustment between trials.
All the chimpanzees were tested in the outdoor portion of their home cages. The number of responses obtained from each subject differed within and between tasks. Notwithstanding, a minimum of 30 responses were obtained for each individual for each task. Individuals recording the hand use data were blind to the anatomical asymmetries of the subjects as well as to the hypotheses of this study.
For each measure, a handedness index (HI) was determined following the formula HI = (R − L)/R + L), where R and L reflect the frequency of left and right hand use. HI values varied from −1.0 to 1.0 with negative values reflecting left hand biases and positive values reflecting right hand biases. Rather than consider each handedness measure separately in the analyses, we derived a single composite handedness value by averaging the HI values for the 4 tasks. In addition, we classified subjects as right and left handed based on the sign of the HI. Subjects with positive composite handedness values were classified as right-handed, whereas those with negative values were classified as left-handed.
Results
Descriptive Statistics
Of the 76 individuals, bilateral bifurcation of the sylvian fissure into ascending and descending rami was present in 58 individual apes (76 %). For the remaining 18 individuals (24 %), only unilateral or no bifurcation occurred at the terminal point of the SF. A comparison of bifurcated vs. non-bifurcated SF as a function of sex using a Fisher’s exact probability test failed to reveal a significant association. However, Fisher’s exact test did indicate a significant difference between right and left handed subjects. These data are presented in Table 1.
Table 1.
Bifurcation of the posterior sylvian fissure by sex and handedness categories. Fisher’s exact tests were conducted on total values (indicated in bold). Hemisphere information is included in regular type (RH = right hemisphere, LH = left hemisphere).
| Bilateral | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bifurcation
|
No Bilateral Bifurcation
|
|||||||
| Sex | RH only | LH only | Neither | |||||
|
|
|
|||||||
| Male | 26 | 5 | = | 1 | 3 | 1 | ||
| Female | 32 | 13 | = | 4 | 5 | 4 | ||
| Handedness | ||||||||
|
|
||||||||
| * | 28 | 11 | = | 5 | 5 | 1 | ||
| 19 | 1 | = | 0 | 0 | 1 | |||
p < .05; Fisher’s exact test, two-tailed.
Table 2 describes the distribution of the different bifurcation patterns by hemisphere (Table 2a) and sex (Table 2b). McNemar-Bowker tests indicated a significant difference in bifurcation patterns between the left and right hemispheres (Table 2a). In the left hemisphere, subjects most frequently have the Inferior bifurcation pattern whereas the Symmetric pattern is most common in the right hemisphere. Fisher’s exact tests failed to reveal significant differences between males and females with regard to posterior bifurcation pattern in the right or the left hemispheres.
Table 2.
(a) Distribution of the four bifurcation types in each hemisphere. Significant differences were found between the left and right hemispheres (McNemar-Bowker Test X2(6) = 13.74, p < .05). (b) Distribution of the four bifurcation types in each hemisphere for females (F) and males (M). No significant sex differences were found for either the left or right hemispheres.
| Superior | Inferior | Symmetric | Inverted | |
|---|---|---|---|---|
| a | ||||
| Left Hemisphere | 7 | 27 | 17 | 7 |
| Right Hemisphere | 12 | 11 | 23 | 12 |
|
| ||||
| b | ||||
| Left Hemisphere | 4 F
3 M |
18 F
9 M |
5 F
12 M |
5 F
2 M |
| Right Hemisphere | 7 F
5 M |
6 F
5 M |
16 F
7 M |
3 F
9 M |
The Posterior Ascending Ramus, the Posterior Descending Ramus, and Handedness
The presence of a PAR and PDR were observed in both hemispheres in 58 of the subjects (26 males, 32 females). In the initial analysis, we compared the AQ values for the PAR as a function of the sex and the handedness of the subjects using a univariate analysis of variance. The asymmetry quotient for the PAR was the dependent variable whereas sex (male or female) and handedness (left or right) served as the between-subject factor. A significant main effect for sex was found F(1, 46) = 4.37, p = .04, however no main effect for handedness F(1, 46) = 0.98, p = .33, or interaction F(1,46) = 1.79, p = .19, was observed. The mean PAR AQ’s for males and females are shown in Figure 3.
Figure 3.
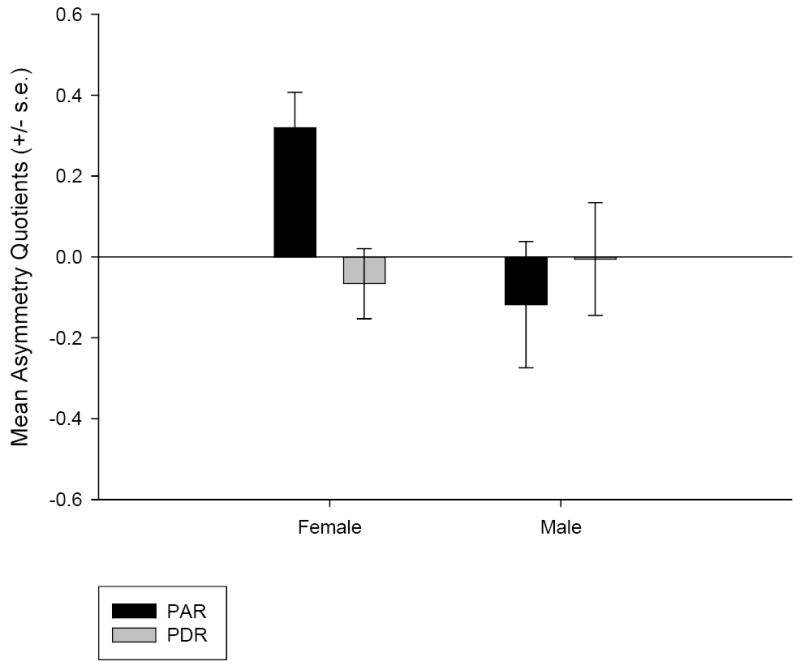
Mean asymmetry quotients (AQ) and standard errors of the mean for the planum parietale (PP) and posterior descending ramus (PDR) in male and female subjects.
Because there was a main effect for sex on the PAR asymmetry, separate one-sample t-tests were performed on male and female PAR AQ scores to assess whether or not either sex showed a population-level asymmetry. Females were significantly rightward for the PAR t(31) = 3.63, p = .001, whereas males were not t(25) = -0.76, p = .46.
Similarly, we compared the AQ values for the PDR as a function of the sex of the subjects using a univariate analysis of variance. The AQ values for the PDR was the dependent variable whereas sex and handedness served as the between-subject factors. No main effects or interactions were observed. No population-level asymmetry for the PDR was evident t(57) = -0.50, p = .62.
Scan Type and the PAR and PDR
Given that our sample was made up of both cadaver specimens as well as MR scans acquired in vivo and the fact that two different magnet strengths were used to acquire images, we compared the AQ values for the PAR as a function of the scan type (1.5 tesla cadaver, 1.5 tesla in vivo, 3.0 tesla in vivo) using a univariate analysis of variance. The AQ values for the PAR was the dependent variable whereas scan type served as the between subject factor. No main effect for scan type was found F(2, 57) = 0.95, p = .39. In addition, Table 3 lists the means and standard deviations for the PAR and PDR in the left and right hemispheres by scan type.
Table 3.
Means and standard deviations of posterior ascending rami and posterior descending rami of the sylvian fissure
| Posterior Ascending Ramus (mm) | Posterior Descending Ramus (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LEFT | RIGHT | L > R | R > L | LEFT | RIGHT | L > R | R > L | ||
|
| |||||||||
| Mean | 7.14 | 7.19 | 7.32 | 7.64 | |||||
| Cadaver specimens | SD | 3.81 | 3.66 | 2.44 | 2.53 | ||||
| N | 15 | 7 | 8 | 15 | 7 | 8 | |||
|
| |||||||||
| Mean | 7.23 | 7.41 | 9.32 | 8.32 | |||||
| 1.5 Tesla in vivo | SD | 2.42 | 2.27 | 3.88 | 2.86 | ||||
| N | 32 | 15 | 17 | 32 | 19 | 13 | |||
|
| |||||||||
| Mean | 6.26 | 7.71 | 10.31 | 7.86 | |||||
| 3 Tesla in vivo | SD | 3.30 | 3.03 | 4.26 | 2.26 | ||||
| N | 11 | 3 | 8 | 11 | 8 | 3 | |||
|
| |||||||||
| Mean | 7.02 | 7.41 | 8.99 | 8.06 | |||||
| Entire Sample | SD | 2.97 | 2.78 | 3.74 | 2.65 | ||||
| N | 58 | 25 | 33 | 58 | 34 | 24 | |||
|
| |||||||||
| Mean | 5.40 | 7.27 | 9.02 | 9.44 | |||||
| Gannon et al. (2005) | SD | 2.87 | 2.70 | 4.24 | 2.94 | ||||
| N | 23 | 9 | 14 | 23 | 11 | 11 | |||
Discussion
The data provided in this report indicate that a majority of chimpanzees possess a posterior bifurcation of the SF. In addition, the results suggest that the PAR is larger in the right hemisphere as compared to the left, revealing an asymmetric PP. This asymmetry is influenced by the sex of the subjects with females showing a rightward asymmetry for the PP compared to males. These results are consistent with earlier reports identifying the presence of population-level asymmetries in perisylvian regions in great apes [3, 7, 8, 13, 15].
With regard to the pattern of posterior bifurcation of the sylvian fissure, chimpanzees did not show significant sex differences. Notwithstanding, the majority of female subjects have a shorter PAR compared to PDR in the left hemisphere, and tend to have relatively symmetric posterior rami in the right hemisphere (Table 2b). In contrast, males tend to have posterior rami of similar lengths in the left hemisphere, and no one type as the majority in the right hemisphere. Ide et al., [21] report that the Superior bifurcation pattern is the predominant one in both males and females. In contrast, relatively few of the subjects in this study were found to have a larger PAR compared to PDR irrespective of hemisphere or sex. Gannon et al. [8], reported that the most common bifurcation pattern observed in their sample was the Inverted type described by Ide et al. [21]. However, as indicated in Table 2, this pattern was observed in only a very small percentage of our subjects. It is possible, that this qualitative difference was the result of the different sample types (i.e. MRI vs. cadaver specimens).
One of the goals of this study was to determine if similar patterns of asymmetry in the PP would be evident from MR images in vivo as they were from surface sulci in cadaver specimens [8]. To this end, we averaged the lengths of the PAR and PDR from every slice in both hemispheres in order to compare our results directly with those reported by Gannon et al. [8]. As indicated in Table 3, the data are similar between the two studies. These results suggest that quantification of MR images provide an alternative assessment technique of PAR asymmetry in a larger homogenous sample of chimpanzees. However, our data do differ from those reported by Gannon et al. [8] with respect to the pattern of posterior bifurcation of the sylvian fissure. This difference may in fact be the result of the different sampling methods (i.e. surface sulci in cadaver specimens vs. MR imaging). In order to evaluate this hypothesis, we constructed whole brain 3-dimensional (3D) renderings from the MR images of our subjects. Although classification of the posterior bifurcation of the sylvian fissure was possible from these 3D renderings, these results did not correlate strongly with the patterns observed from MR images of the most lateral slice in the sagittal plane. Therefore, it is possible that the bifurcation patterns visible on the surface of a cadaver specimen differ from those visible in MR images just within the brain tissue itself. Notwithstanding, as depicted in Table 3, our results are quite similar to those reported by Gannon et al. [8].
In humans, handedness and sex influence PP asymmetry [23]. Although no main effect for handedness was observed in our sample, whether or not the sylvian fissure bifurcated bilaterally at its posterior end was influenced by the sex of the subjects. Specifically, a greater proportion of chimpanzees with negative composite HI scores (i.e. those that predominantly use their left hand) have a bilateral bifurcation of the sylvian fissure as compared to those subjects that most frequently employ their right hand. To further explore the relation between subject handedness and planum parietale asymmetry, we examined the association between AQ values for the PAR and composite HI scores. Pearson correlations did not reveal significant correlations for females (r = .32, p = .10) or for males (r = .11, p = .63). Recently, it has been reported that chimpanzees that reliably employ their right hand for manual gestures have larger inferior frontal gyri in the left hemisphere than those apes that do not show consistent hand use for gestures [33]. In addition, handedness has been shown to modulate the association between corpus callosum size and brain asymmetries in the frontal orbital sulcus but not the planum temporale in chimpanzees [20]. Collectively, these data support the view that the neurobiological foundations of language in humans may have evolved from substrates that originally supported a multimodal communication system.
It should be noted that, despite these findings, relatively little is known about the functional role of the PP, especially with regards to the relevance of this differential anatomical asymmetry. However, recent data have implicated the PP in the processing of voice spectral information in humans bilaterally [24]. Furthermore, Foundas et al., [5] reported that the size of the right PAR predicted writing handedness in human subjects. These are intriguing findings, especially given the reported sex and handedness differences in humans [23] as well as those differences identified in this report. Other studies have also examined the relation between the PP and language disorders and dyslexia. Whereas normal PP asymmetry has been reported in children with developmental language disorder [30], significantly fewer dyslexics show a rightward PP asymmetry when compared to individuals without dyslexia [9, but see 31]. Still, the PP is regarded as a region involved in the processing of linguistic auditory information. The data presented in this report provide evidence that the study of the PP may provide further insight into the evolution of lateralized language regions in the human brain.
It has been proposed that human spoken language evolved from a multimodal form of communication that included the concomitant use of gestural and auditory signals [22, 28]. Although a great deal about the role of the PP in human language evolution has yet to be learned, extant data indicate that the PP plays a role in linguistic and paralinguistic communication in humans [24, 29]. The PP asymmetry described in this report suggests that this region may have its evolutionary roots as a neurobiological substrate underlying multimodal communication in a common ape and human ancestor.
Acknowledgments
This work was supported in part by NIH grants F32DC007823 to JPT, NS-36605 and NS-42867 to WDH and RR-00165. The authors would like to thank the YNPRC veterinary staff for their assistance. Please send correspondences and requests for reprints to William D. Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road, Atlanta, Georgia, 30329, whopkin@emory.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- 2.Broca P. Remarques sur le siege de la faculte du langage articule; suivies d’une observation d’aphemie. Bull Soc Anat Paris. 1861;6:330–357. [Google Scholar]
- 3.Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- 4.Falk D, Hildebolt C, Cheverud J, Vannier M, Helmkamp RC, Konigsberg L. Cortical asymmetries in the frontal lobe of rhesus monkeys (Macaca mulatta) Brain Research. 1990;512:40–45. doi: 10.1016/0006-8993(90)91167-f. [DOI] [PubMed] [Google Scholar]
- 5.Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: Do right- and left handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 6.Galaburda AM. Anatomic basis of cerebral dominance. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- 7.Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee Planum Temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- 8.Gannon PJ, Kheck NM, Braun AR, Holloway RL. Planum parietale of chimpanzees and orangutans: A comparative resonance of human-like planum temporale asymmetry. The Anatomical Record. 2005;287:1128–1141. doi: 10.1002/ar.a.20256. [DOI] [PubMed] [Google Scholar]
- 9.Heiervang E, Hugdahl K, Steinmetz H, Smievoll AI, Stevenson J, Lund A, Ersland L, Lundervold A. Planum temporale, planum parietale and dichotic listening in dyslexia. Neuropsychologia. 2000;38:1704–1713. doi: 10.1016/s0028-3932(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 10.Heilbronner PL, Holloway RL. Anatomical brain asymmetries in New World and Old World monkeys. Stages of temporal lobe development in primate evolution. American Journal of Physical Anthropology. 1988;76:39–48. doi: 10.1002/ajpa.1330760105. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes) : Rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in non-human primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- 15.Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure length asymmetries in primates revisited: A comparative MRI study. Brain, Behavior and Evolution. 2000;56:293–299. doi: 10.1159/000047213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere specialization in motor skill. Journal of Experimental Psychology: General. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins WD, Russell JL, Freeman H, Buehler N, Reynolds E, Schapiro SJ. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins WD. Hemispheric specialization in chimpanzees: Evolution of hand and brain. In: Shackelford T, Keenan JP, Platek SM, editors. Evolutionary Cognitive Neuroscience. MIT Press; Boston: 2007. pp. 99–120. [Google Scholar]
- 20.Hopkins WD, Dunham L, Cantalupo C, Taglialatela JP. The association between handedness, brain asymmetries, and corpus callosum size in chimpanzees (Pan troglodytes) Cerebral Cortex. doi: 10.1093/cercor/bhl086. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ide A, Rodriguez E, Zaidel E, Aboitiz F. Bifurcation patterns in the human sylvian fissure: hemispheric and sex differences. Cerebral Cortex. 1996;6:717–725. doi: 10.1093/cercor/6.5.717. [DOI] [PubMed] [Google Scholar]
- 22.Iverson JM, Thelen E. Hand, mouth and brain. Journal of Consciousness Studies. 1999;6:19–40. [Google Scholar]
- 23.Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. NeuroReport. 1994;5:1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- 24.Lattner S, Meyer ME, Friederici AD. Voice perception: Sex, pitch, and the right hemisphere. Human Brain Mapping. 2005;24:11–20. doi: 10.1002/hbm.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeMay M, Geschwind N. Hemispheric differences in the brains of great apes. Brain, Behavior, and Evolution. 1975;11:48–52. doi: 10.1159/000123623. [DOI] [PubMed] [Google Scholar]
- 26.LeMay M. Asymmetries of the brains and skulls of nonhuman primates. In: Glick SD, editor. Cerebral lateralization in nonhuman species. Academic Press; New York: 1985. pp. 223–245. [Google Scholar]
- 27.McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- 28.McNeil D. Hand and mind: What gestures reveal about thought. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 29.Meyer M, Zaehle T, Gountouna V-E, Barron A, Jancke L, Turk A. Spectro-temporal processing during speech perception involves left posterior auditory cortex. NeuroReport. 2005;16 doi: 10.1097/00001756-200512190-00003. [DOI] [PubMed] [Google Scholar]
- 30.Preis S, Jancke L, Schittler P, Huang Y, Steinmetz H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia. 1998;36:849–855. doi: 10.1016/s0028-3932(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 31.Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Archives of Neurology. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz H, Rademacher J, Jancke L, Huang Y, Thron A, Zilles K. Total surface of temporoparietal intrasylvian cortex: Diverging left-right asymmetries. Brain and Language. 1990;39:357–372. doi: 10.1016/0093-934x(90)90145-7. [DOI] [PubMed] [Google Scholar]
- 33.Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan U, Caliskan S. Asymmetries in the cerebral dimensions and fissures of the dog. International Journal of Neuroscience. 1987;32:943–952. doi: 10.3109/00207458709043351. [DOI] [PubMed] [Google Scholar]
- 35.Tan U. Similarities between sylvian fissure asymmetries in cat brain and planum temporale asymmetries in human brain. International Journal of Neuroscience. 1992;66:163–175. doi: 10.3109/00207459209003303. [DOI] [PubMed] [Google Scholar]
- 36.Tan U, Kutlu N. The endopoint of the sylvian fissure is higher on the right than the left in cat brain as in human brain. International Journal of Neuroscience. 1993;68:11–17. doi: 10.3109/00207459308994255. [DOI] [PubMed] [Google Scholar]
- 37.Wernicke C. Der aphasische Symptomenkomplex. Breslau: Cohn, Weigert; 1874. [Google Scholar]
- 38.Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. Journal of Comparative Neurology. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- 39.Yeni-Komshian G, Benson D. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 1976;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]


