Abstract
Background
This study describes cigarette smoking’s effect on development of physical disability following initial musculoskeletal-related hospitalization.
Methods
We followed 15,140 US Army personnel hospitalized for common musculoskeletal disorders between 1989–1996 for up to 8 years (1997) to assess risk for long-term physical disability.
Results
Trends between increased smoking level and long-term disability were identified for persons with knee injuries, rotator cuff injuries, and intervertebral disc displacement. In proportional hazards models, disability was significantly associated with heavy smoking among all subjects (relative hazard (RH) = 1.21). Both heavy smokers (RH = 1.49) and light to moderate smokers (RH = 1.44) were at greater risk for disability following meniscal injuries. Excess fraction due to smoking among subjects with meniscal injuries who currently smoke was 38%.
Conclusions
Findings suggest an association between smoking and development of disability following meniscal injury. Given the high excess fraction of disability associated with smoking, other studies are needed to confirm this association.
Keywords: disability, musculoskeletal disorders, smoking, cohort, survival analysis, army
INTRODUCTION
Since the initial surgeon general’s report in 1964 [USDHHS, 1964], smoking has been recognized as one of the most important public health issues of our time and the most important preventable cause of death and disease in the United States [USDHHS, 1989; McGinnis and Foege, 1993]. While smoking is well recognized as a contributor to a wide variety of illnesses, its role in the incidence of injuries is rarely considered [Amoroso et al., 1998]. A recent meta-analysis suggests that smoking is related to an increased incidence of fatal injuries [Leistikow et al., 1998], thereby corroborating the evidence linking cigarette smoking with both acute injuries [Sacks and Nelson, 1994; Kwiatkowski et al., 1996] and musculoskeletal disorders [Reynolds et al., 1994, 1999; Dettori et al., 1996]. However, the role of smoking in the development of physical disability following a musculoskeletal injury or disorder has been virtually unrecognized despite longstanding laboratory studies [Mosely et al., 1978; Siana et al., 1989] and recent clinical reviews [Towler, 2000; Krueger and Rohrich, 2001] demonstrating mechanisms and clinical evidence that smoking impairs wound healing.
Musculoskeletal disorders represent a tremendous burden to society, comprising the largest proportion of reported work-related injuries and illnesses [BLS, 1997], resulting in an estimated 315 million physician visits annually, and costing approximately $150 billion in direct and indirect costs in 1992 [Yelin and Callahan, 1995]. Musculoskeletal disorders also result in various levels of disability, ranging from limitations in activities of daily living [Deyo and Diehl, 1988; Williams et al., 1998] to an inability to return to work [MacKenzie et al., 1987; Volinn et al., 1991; Lancourt and Kettelhut, 1992; Hazard et al., 1996; MacKenzie et al., 1998], with significant ramifications for family members and coworkers alike. Several studies have examined the relationship of smoking and the development of work-related disability for a variety of conditions [Leigh, 1985; Mäkelä et al., 1993; Hubert and Fries, 1994; Eriksen et al., 1998; Rothenbacher et al., 1998; Feuerstein et al., 1999], but little is known regarding its impact following the occurrence of a musculoskeletal condition. Recognizing work-related disability to be a multifactoral development [Cats-Baril and Frymoyer, 1991; Feuerstein and Thebarge, 1991; Bongers et al., 1993; Katz et al., 1997], it is neither appropriate nor possible to identify a single factor as the source of such a complex process as the development of disability. The detailed investigation of all possible risk factors that contribute to the development of disability, particularly those that are amenable to intervention, is essential. Unfortunately, most studies on disability have not examined smoking, in part because of the difficulty and expense in following individuals over time.
One population where it is possible to examine both smoking practices and development of disability is the US Army. Army personnel engage in a variety of physically demanding tasks, many with the potential for injury. As a result, musculoskeletal disorders are common, associated with the majority (51%) of diagnoses resulting in disability discharge from service [Feuerstein et al., 1997], and are a critical concern in terms of military readiness and cost. However, despite the burden of disabilities, few studies have examined the health outcome associated with discharge from the service due to disability [Feuerstein et al., 1997, 1999]. Recently, the role of smoking has received greater attention in relation to the incidence of injuries among Army personnel [Jones et al., 1993; Reynolds et al., 1994, 1999; Amoroso et al., 1996a,b,c; Dettori et al., 1996], though none of these articles has addressed smoking in relation to developing disability following injury. This study evaluates the role of smoking as an independent risk factor in the development of disability. Our objectives were to determine whether cigarette smoking is associated with the development of long-term physical disability among persons hospitalized with a musculoskeletal disorder; to determine which diagnoses are most strongly associated with smoking; and to suggest mechanisms of smoking’s effects based on these findings.
METHODS
Study Design and Cohort Definition
A retrospective cohort design was used to follow US Army personnel from their initial musculoskeletal-related hospitalization, which occurred between the years 1989 and 1996, through the development of long-term physical disability, up through 1997. To be included in the study, cohort subjects must have met several criteria: (1) been on active duty at the time of hospitalization; (2) been hospitalized for a specified musculoskeletal disorder or severe sprain/strain during the period 1989–1996; and (3) completed an Army Health Risk Appraisal (HRA) survey at some point during the same time period. The HRA is a self-administered questionnaire that offers data on health practices, behaviors, and stress levels. There were 16,348 persons who met those initial criteria. Because the goal of the study was to capture subjects at their first hospital admission for one of the diagnoses of interest, those hospitalized for the same condition prior to 1989 (N = 1,053) or having a disability rating preceding the initial musculoskeletal hospitalization (N = 27) were disqualified and excluded from the cohort. An additional 148 subjects lacked information on cigarette smoking practice from the HRA and were excluded, leaving 15,120 eligible subjects.
Eligibility required a hospitalization record with one of 40 possible principal diagnoses selected a priori representing either “acute” injuries within International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [USDHHS, 1988] diagnostic codes 836, 840, or 844, or “chronic” musculoskeletal conditions (710–739, 354) that represent similar clinical presentations. These 40 diagnoses were classified into 13 functional groupings for analysis based on similar mechanisms of injury (Table I). We included only musculoskeletal-related diagnoses that were commonly admitted and likely to be coded accurately rather than the entire widely discrepant group of musculoskeletal conditions. Some of the diagnoses may not typically require hospitalization, but may have been included as a day surgery case (considered as a hospital admission) or an in-patient admission during the earlier part of the study period when the threshold for hospitalization of soldiers was lower.
TABLE I.
Diagnostic Categories of Musculoskeletal Disorders and Sprain/Strains, US Army, 1989–1997
| Diagnostic category | ICD-9-CMa code |
|---|---|
| Synovitis and tenosynovitis | 727.0 Synovitis and tenosynovitis (includes .0, .00, .01, .02, .03, .04, .05, .06, .09) |
| Carpal and cubital tunnel syndromes | 354.0 Carpal tunnel syndrome |
| 354.2 Lesion of ulnar nerve (Cubital tunnel syndrome) | |
| Rotator cuff injury | 726.1 Rotator cuff syndrome of shoulder and allied disorders (includes .1, .10, .11, .12, .19) |
| 840.3 Infraspinatus (muscle) (tendon) | |
| 840.4 Rotator cuff (capsule) | |
| 840.5 Subscapularis (muscle) | |
| 840.6 Supraspinatus (muscle) (tendon) | |
| Ganglion and cyst of synovium, tendon, and bursa | 727.4 Ganglion and cyst of synovium, tendon, and bursa (includes .4, .40, .41, .42, .43, .49) |
| Bunion and deformities of toe | 727.1 Bunion |
| 735.0 Hallux valgus (acquired) | |
| Malunion and nonunion of fracture | 733.8 Malunion and nonunion of fracture (includes .8, .81, .82) |
| Meniscal injury | 717.0 Old bucket handle tear of medial meniscus |
| 717.1 Derangement of anterior horn of medial meniscus | |
| 717.2 Derangement of posterior horn of medial meniscus | |
| 717.3 Other and unspecified derangement of medial meniscus | |
| 717.4 Derangement of lateral meniscus (includes .4, .40, .41, .42, .43, .49) | |
| 717.5 Derangement of meniscus, not elsewhere classified | |
| 836.0 Tear of medial cartilage or meniscus of knee, current | |
| 836.1 Tear of lateral cartilage or meniscus of knee, current | |
| 836.2 Other tear of cartilage or meniscus of knee, current | |
| Cruciate ligament injury | 717.83 Old disruption of anterior cruciate ligament |
| 717.84 Old disruption of posterior cruciate ligament | |
| 844.2 Sprain/strain of cruciate ligament of knee | |
| Collateral ligament injury | 717.81 Old disruption of lateral collateral ligament |
| 717.82 Old disruption of medial collateral ligament | |
| 844.0 Sprain/strain of lateral collateral ligament | |
| 844.1 Sprain/strain of medial collateral ligament | |
| Chondromalacia | 717.7 Chondromalacia of patella |
| Non-specific back pain | 724.2 Lumbago |
| 724.5 Backache, unspecified | |
| Displacement of intervertebral disc | 722.0 Displacement of cervical intervertebral disc without myelopathy |
| 722.1 Displacement of thoracic or lumbar intervertebral disc without myelopathy (includes .1, .10, .11) | |
| 722.2 Displacement of intervertebral disc, site unspecified, without myelopathy | |
| Degeneration and other disc disorders | 722.4 Degeneration of cervical intervertebral disc |
| 722.5 Degeneration of thoracic or lumbar intervertebral disc (includes .51, .52) | |
| 722.6 Degeneration of intervertebral disc, site unspecified | |
| 722.7 Intervertebral disc disorder with myelopathy (includes .70, .71, .72, .73) | |
| 722.8 Postlaminectomy syndrome (includes .80, .81, .83) | |
| 722.9 Other and unspecified disc disorder (includes .90, .91, .92, .93) |
International Classification of Diseases, Ninth Revision, Clinical Modification.
Data Sources
Data were obtained from the Total Army Injury and Health Outcomes Database (TAIHOD), a collection of available databases in the Army that was recently compiled for injury prevention and health research [Amoroso et al., 1999]. This study involved linking five of the databases: personnel, hospitalization, disability, loss from service, and HRA at the level of the individual soldier. These databases include information on health practices (HRA), demographics (personnel), clinical parameters (hospitalization), outcomes (disability), and follow-up time (hospitalization, disability, and loss from service). Physical demands were obtained from Army Regulation 611-201, which provides a summary of all enlisted jobs and a rating of physical demand based on upper body strength requirements [Department of the Army, 1994]. Unique identifiers (encrypted social security numbers) enabled us to link information across databases, in effect permitting us to track the natural history of a subject’s condition from initial hospitalization to long-term disability. The completeness of the Army’s administrative databases (less than 3% missing data for all covariates) provided excellent follow-up with minimal loss of cohort subjects. The study procedures were approved by institutional review boards at Johns Hopkins University and the US Army Research Institute for Environmental Medicine.
Health Behavior Data
Health behavior data was obtained from elements of the 75 item HRA, a derivative of the Rhode Island Wellness Survey and the Carter Center Health Risk Appraisal and similar to the widely used CDC Behavioral Risk Factor Surveys [Bell et al., 2000]. There are several occasions when the HRA is administered, including when a soldier joins the military or reports to a new post, during semi-annual physical exams, and during routine physical health exams such as an outpatient or occupational health visit. Given this non-random administration of the HRA, a recent assessment of the external validity of the HRA was performed by Bell et al. [2000] to determine whether those at greater risk for adverse health problems are oversampled. Results revealed differences of no more than 1% among demographic characteristics of those who took the HRA and those who did not.
Based on self-reported daily cigarette consumption from the HRA, smoking status was defined as nonsmoker (i.e., one who never smoked), former smoker, and current smoker. Current smokers were further classified as either a light to moderate smoker (<1 pack/day) or a heavy smoker (1 + pack/day) in a manner similar to that used in the worldwide survey of smoking in the US military [Kroutil et al., 1994]. The status reported at the first survey was used to represent smoking as a fixed, rather than time-dependent, variable because few subjects (<10%) had taken the HRA on more than one occasion. “How many cigarettes a day do you smoke?” was the only question asked of current smokers. Because there was no measure of years smoked available in the HRA, pack-years could not be used as an exposure variable.
Alcohol use was assessed in terms of typical weekly quantities consumed. Given the sensitive nature of the Army asking its soldiers about their alcohol use, there was a concern that respondents may underreport their alcohol consumption habits, particularly if they were under the legal drinking age of 21 years. However, other studies have found self-reported drinking behavior to be largely valid and reliable [O’Hare et al., 1991; Embree and Whitehead, 1993; Thompson et al., 1993; Smith et al., 1995]. Furthermore, Bell et al. [2000] found that risky behaviors are in fact reported, as evidenced by the large number of subjects (including minors) who reported drinking heavily (15 drinks or more per week) and the consistency of reported drinking levels with other studies of similar demographic groups.
Outcome
The outcome considered in this analysis was follow-up time (number of months) to disability evaluation following initial musculoskeletal-related hospitalization. Long-term disability was based upon determinations made by a Medical Evaluation Board that decided whether the individual met medical retention standards, and a Physical Evaluation Board that assessed whether the individual was fit or unfit for duty [Peck, 1999]. The following ratings were selected to represent long-term disability status: (1) permanent disability/retirement; (2) severance with a lump sum payment but no benefits; or (3) temporary disability. Persons can remain on temporary disability for a maximum of five years and must be re-evaluated within 18 months, although most ultimately receive permanent disability retirements [Amoroso and Canham, 1999].
Follow-up time was calculated from the date of the initial musculoskeletal hospitalization until the subject experienced one of the following events: disability discharge; discharge from service (censored) for another reason (e.g., honorable discharge); or remain on active duty through the entire follow-up period (censored). The standardized disability evaluation process provided a consistent outcome measure regardless of subjects’ geographic location.
Excess fraction, an alternative term to attributable risk (AR) used when causality has not been firmly established [Greenland and Robins, 1988], was calculated to assess the association of smoking with disability discharge specifically in smokers (the exposed group) and in the total population. Calculations were based on the assumption of a binary exposure (current smokers vs. nonsmokers) and their respective overall rates of disability discharge. The excess fraction within the group of smokers was based on the equation
where Ir is the rate of disability discharge among current smokers and I0 is the rate for nonsmokers [Kahn and Sempos, 1989]. The excess fraction in the total population due to smoking was
where It is the rate of disability discharge among the total study population.
Time-to-Event Analysis
Kaplan-Meier estimates of survival time for each diagnostic category were used to assess the five year cumulative risk of disability discharge among smoking levels. A log-rank test for trend was performed to evaluate the association between disability discharge and the four levels of smoking exposure.
Cox proportional hazards models provided estimates of the combined effect of multiple risk factors and the contribution of each covariate independently [Collett, 1994]. Covariates other than smoking included factors known to be associated with disability, such as age, education, and gender, as well as race/ethnicity, pay grade/rank, job class, frequency of reported work stress, length of service, and type of injury or condition. Many other predictors, including alcohol use, were initially examined but excluded from the final models based on negative preliminary findings [Lincoln et al., 2002]. Nonsmokers were selected as the reference group, such that a relative hazard that exceeded one indicated an increased risk of disability relative to nonsmokers while controlling for other potential confounders. Separate models were generated for each of the 13 diagnostic categories using a forced entry approach to determine the effect of smoking and the variation in smoking’s influence across diagnoses. The proportionality assumption was verified by examining log minus log plots of survival functions [Collett, 1994]. All covariates were analyzed in categorical form using SPSS for Windows, Release 7.5.2 (Chicago, IL).
RESULTS
This cohort was generally young (mean age = 31 years), male (85%), white (63%), and educated (>99% graduated high school). Of the 15,120 study subjects, 7,799 (52%) were nonsmokers; 2,610 (17%) were former smokers; 2,766 (18%) were light to moderate smokers; and 1,945 (13%) were heavy smokers.
The mean follow-up time was 38.4 months (SD = 26.9 months), although light to moderate smokers (35.9 months) and heavy smokers (36.6 months) had shorter mean follow-up times than nonsmokers (39.3 months) and former smokers (39.9 months) (mean difference = 3.2 months, P <0.001). Heavy smokers smoked an average of 24.9 cigarettes/day (SD = 7.7) and light to moderate smokers smoked an average of 9.7 cigarettes/day (SD = 4.4).
Distribution of Covariates Among Smoking Levels
The distribution of demographic and occupational characteristics among smoking levels is presented in Table II. Pearson chi-square tests identified significant differences (P <0.001) in the distribution of smoking levels across each covariate.
TABLE II.
Demographic and Occupational Characteristics of the Study Population, US Army, 1989–1997*
| Characteristic and Strata | Nonsmoker (N = 7,799) | Former smoker (N = 2,610) | Light to moderate smoker (N = 2,766) | Heavy smoker (N =1,945) | Total (N =15,120) |
|---|---|---|---|---|---|
| Mean follow-up time (months) | 39.3 (SD = 27.1) | 39.9 (SD = 27.1) | 35.9 (SD = 26.1) | 36.6 (SD = 26.4) | 38.4 (SD = 26.9) |
| Gender (P < 0.001) | |||||
| Male | 83.8% | 86.3% | 83.5% | 91.8% | 85.2% |
| Female | 16.2% | 13.7% | 16.5% | 8.2% | 14.8% |
| Age (mean years) (P <0.001) | 30.1 (SD = 7.3) | 33.5 (SD = 7.9) | 30.0 (SD = 6.9) | 32.0 (SD = 7.0) | 30.9 (SD = 7.4) |
| <21 | 7.6% | 4.3% | 9.0% | 4.6% | 6.9% |
| 21–25 | 26.5% | 16.1% | 25.5% | 19.0% | 23.6% |
| 26–34 | 38.0% | 32.1% | 37.4% | 35.0% | 36.5% |
| 35 + | 27.8% | 47.5% | 28.1% | 41.4% | 33.0% |
| Race/ethnicity (P <0.001) | |||||
| White | 57.5% | 67.1% | 58.0% | 86.3% | 62.9% |
| Black | 33.6% | 22.9% | 33.6% | 9.5% | 28.6% |
| Hispanic | 4.2% | 5.2% | 3.5% | 1.7% | 3.9% |
| American Indian/Alaskan | 0.6% | 0.6% | 0.5% | 0.5% | 0.6% |
| Asian/Pacific Islander | 1.7% | 1.1% | 1.6% | 0.7% | 1.4% |
| Other | 2.5% | 3.0% | 2.6% | 1.3% | 2.4% |
| Education level (P <0.001) | |||||
| No H.S. diploma | 0.2% | 0.2% | 0.5% | 0.4% | 0.3% |
| H.S. grad/GED | 69.2% | 69.1% | 87.5% | 87.4% | 74.9% |
| Some college | 6.3% | 9.6% | 5.9% | 6.3% | 6.8% |
| College degree | 24.3% | 21.2% | 6.0% | 5.9% | 8.0% |
| Pay gradea (P <0.001) | |||||
| E1–E3 | 11.4% | 7.5% | 15.2% | 10.2% | 11.3% |
| E4–E6 | 54.8% | 50.3% | 67.9% | 62.7% | 57.4% |
| E7–E9 | 10.4% | 20.2% | 11.7% | 20.8% | 13.7% |
| W1–W5 | 2.7% | 5.0% | 1.8% | 2.6% | 2.9% |
| 01–03 | 12.9% | 6.2% | 2.4% | 1.7% | 8.4% |
| 04–010 | 7.7% | 10.8% | 1.0% | 2.0% | 6.3% |
| Physical demand (of enlisted jobs only, P <0.001) | |||||
| Light | 2.6% | 2.8% | 2.4% | 2.5% | 2.6% |
| Medium | 10.0% | 8.6% | 8.6% | 5.4% | 8.8% |
| Moderately heavy | 19.5% | 21.3% | 18.0% | 17.5% | 19.2% |
| Heavy | 1.6% | 1.7% | 1.1% | 1.4% | 1.5% |
| Very heavy | 66.2% | 65.6% | 70.0% | 73.2% | 68.0% |
| Work stress (P <0.001) | |||||
| Often | 7.9% | 7.2% | 8.9% | 12.3% | 8.5% |
| Sometimes | 23.9% | 25.3% | 23.9% | 27.1% | 24.6% |
| Seldom | 39.4% | 41.5% | 38.0% | 37.7% | 39.3% |
| Never | 28.8% | 26.0% | 29.1% | 22.9% | 27.6% |
| Job satisfaction (P < 0.001) | |||||
| Not satisfied | 12.9% | 13.2% | 16.3% | 19.0% | 14.4% |
| Somewhat | 24.7% | 22.8% | 24.6% | 25.9% | 24.5% |
| Mostly | 38.3% | 39.9% | 37.8% | 36.7% | 38.3% |
| Totally | 24.1% | 24.0% | 21.3% | 18.4% | 22.8% |
Totals may not equal 100% because of missing data. Percentages represent column percentages. Weekly alcohol consumption not significant in univariate analyses. (Data not shown).
Pay grade is listed in increasing order.
Unadjusted associations indicated that heavy smokers were over represented among males, whites, persons older than 34 years of age, those with more than 10 years of service, those with lower levels of education (no college), and middle (pay grade E4–E6) and higher (pay grade E7–E9) ranking enlisted persons. Nonsmokers were over represented among females, persons younger than 35 years of age, persons of black, Hispanic, and Asian/Pacific Islander racial/ethnic backgrounds, officers, those with 10 or fewer years of service, and those with college degrees. In contrast to heavy smokers’ perceptions of work stress and job satisfaction, greater proportions of nonsmokers reportedly “never” experienced work stress (29% vs. 23%) and were “totally satisfied” with their job (24% vs. 18%). Recurrent hospitalizations occurred in 19% of all subjects, but did not vary by smoking status.
Distribution of Disability Across Smoking Levels
Disability was assigned to 9.5% of the subjects, with 1.1% receiving permanent disability (disability rating of at least 30% or having at least 20 years of service), 7.4% receiving lump sum severance payment (disability rating of less than 30% and having less than 20 years of services), and 1.0% receiving temporary disability (same requirements as for permanent disability but used for a long-term condition that has not yet stabilized). Among those with a disability discharge, in 87% the primary cause of disability was the same as that of the initial hospitalization. A comparison of the five-year cumulative risk for disability across smoking levels and the number of subjects for each diagnostic category is presented in Table III. For all musculoskeletal diagnoses combined, heavy smokers had the highest cumulative risk of disability (17.6%), followed by light to moderate smokers (15.8%), nonsmokers (12.2%), and then former smokers (10.3%), suggesting a dose-response relationship (P <0.001).
TABLE III.
Comparison of FiveYear Cumulative Risk of Disability Among Different Levels of Smoking by Diagnostic Category, US Army, 1989–1997
| Diagnostic category | Nonsmoker (%) | Former smoker (%) | Light to moderate smoker (%) | Heavy smoker (%) | Log-rank test for trend |
|---|---|---|---|---|---|
| Synovitis and tenosynovitis (n = 810) | 11.7 | 6.9 | 14.6 | 13.8 | 1.48 (P = 0.224) |
| Carpal and cubital tunnel syndromes (n = 542) | 13.8 | 9.5 | 15.2 | 17.7 | 1.09 (P = 0.297) |
| Rotator cuff injury (n = 325) | 6.9 | 5.9 | 13.9 | 21.8 | 6.55 (P = 0.011) |
| Ganglion and cyst of synovium, tendon, and bursa(n =1,344) | 4.8 | 5.6 | 4.6 | 13.1 | 2.35 (P = 0.125) |
| Bunion and deformities of toe (n =1,517) | 8.6 | 3.1 | 11.9 | 6.3 | 0.00 (P = 0.978) |
| Malunion and nonunion of fracture (n = 807) | 17.1 | 16.1 | 9.1 | 17.4 | 0.92 (P = 0.338) |
| Meniscal injury (n = 3,653) | 10.0 | 7.5 | 17.2 | 16.4 | 18.81 (P < 0.001) |
| Cruciate ligament injury (n = 2,247) | 14.2 | 13.0 | 18.9 | 17.2 | 3.07 (P = 0.080) |
| Collateral ligament injury (n = 560) | 9.1 | 7.3 | 19.3 | 27.6 | 8.71 (P = 0.003) |
| Chondromalacia (n = 915) | 14.0 | 13.0 | 17.3 | 24.1 | 4.67 (P = 0.031) |
| Non-specific back pain (n = 683) | 17.3 | 18.4 | 16.4 | 14.0 | 1.08 (P = 0.300 |
| Displacement of intervertebral disc (n =1,588) | 19.4 | 16.8 | 24.3 | 26.6 | 3.84 (P = 0.050) |
| Degeneration and other disc disorders (n =129) | 19.1 | 16.2 | 31.9 | 8.9 | 0.94 (P = 0.333) |
| All diagnostic categories (n =15,120) | 12.2 | 10.3 | 15.8 | 17.6 | 31.75 (P <0.001) |
The influence of smoking exposure for all conditions combined was further demonstrated by significant differences in their survival functions (P <0.001) (Fig. 1). The diagnostic categories most prominently demonstrating the trend of increasing risk of disability with increasing level of smoking were meniscal injury (P <0.001), collateral ligament injury (P = 0.003), rotator cuff injury (P = 0.011), chondromalacia (P = 0.031), intervertebral disc displacement (P = 0.050), and cruciate ligament injury (P = 0.080) (Table III). Meniscal injuries in particular demonstrated a dramatic difference in risk between current smokers (including both light to moderate and heavy) and nonsmokers (Fig. 2). Former smokers demonstrated a lower risk of disability than did nonsmokers in all but two categories (ganglion/cyst and nonspecific back pain).
FIGURE 1.
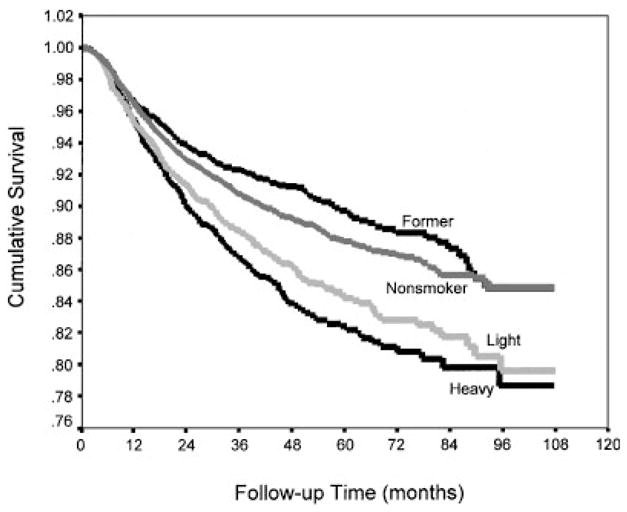
Time to disability following hospitalization for all musculoskeletal diagnostic categories by cigarette smoking level, US Army,1989–1997.
FIGURE 2.
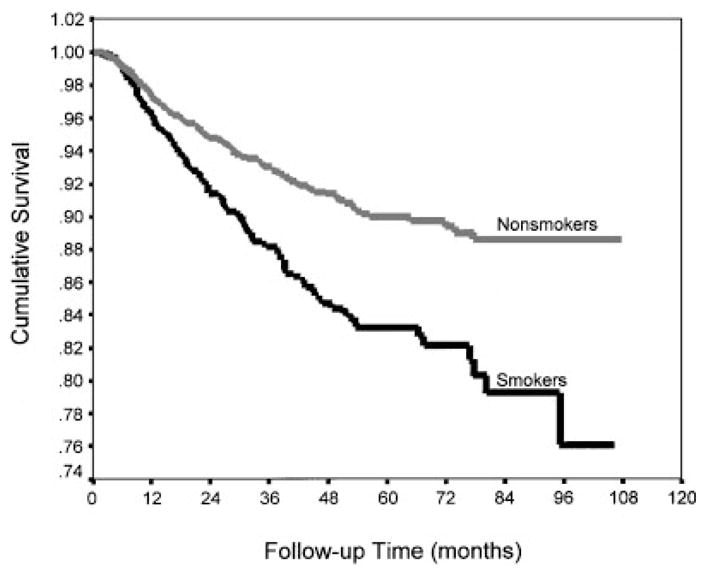
Time to disability following hospitalization for meniscal injury by cigarette smoking level, US Army,1989–1997.
Role of Smoking in Disability Discharge
The relative hazards (RH) and 95% confidence intervals (95% CI) for developing disability across smoking levels for each diagnostic group are presented in Table IV. Multivariate Cox models were adjusted for age group, gender, race/ethnicity, education, pay grade/rank, length of service, and work stress. Occupational specialty was excluded from the models because of collinearity with pay grade/rank and gender. Interaction terms involving age, length of service, physical demands, alcohol use, and work stress by smoking were examined but not included in the final models as they were not statistically significant.
TABLE IV.
Cox Proportional Hazards Models for Disability by Diagnostic Group, US Army, 1989–1997*
| Nonsmoker
|
Former smoker
|
Light to moderate smoker
|
Heavy smoker
|
||||
|---|---|---|---|---|---|---|---|
| Diagnostic category | Relative hazard | Relative hazard | 95% CI | Relative hazard | 95% CI | Relative hazard | 95% CI |
| Synovitis and tenosynovitis | 1.00 | 0.68 | 0.30,1.53 | 1.00 | 0.54,1.86 | 1.10 | 0.48, 2.48 |
| Carpal and cubital tunnel syndromes | 1.00 | 0.97 | 0.36, 2.61 | 1.65 | 0.73, 3.72 | 1.74 | 0.67, 4.55 |
| Rotator cuff injury | 1.00 | 0.84 | 0.20, 3.55 | 1.46 | 0.44, 4.88 | 2.99 | 0.95, 9.38 |
| Ganglion and cyst of synovium, tendon, and bursa | 1.00 | 1.11 | 0.51, 2.42 | 0.69 | 0.29,1.61 | 1.90 | 0.90, 4.01 |
| Bunion and deformities of toe | 1.00 | 0.39 | 0.14,1.11 | 1.46 | 0.87, 2.44 | 0.54 | 0.16,1.82 |
| Malunion and nonunion of fracture | 1.00 | 1.12 | 0.59, 2.11 | 0.71 | 0.38,1.31 | 0.97 | 0.52,1.81 |
| Meniscal injury | 1.00 | 1.06 | 0.72,1.54 | 1.44a | 1.07,1.94 | 1.49a | 1.06, 2.11 |
| Cruciate ligament injury | 1.00 | 0.96 | 0.65,1.44 | 1.10 | 0.79,1.54 | 0.96 | 0.62,1.49 |
| Collateral ligament injury | 1.00 | 0.78 | 0.29, 2.13 | 1.63 | 0.79, 3.34 | 1.80 | 0.73, 4.43 |
| Chondromalacia | 1.00 | 1.42 | 0.79, 2.56 | 1.04 | 0.60,1.79 | 1.47 | 0.87, 2.50 |
| Nonspecific back pain | 1.00 | 1.14 | 0.65,1.97 | 0.92 | 0.50,1.68 | 0.85 | 0.42,1.73 |
| Displacement of intervertebral disc | 1.00 | 0.75 | 0.51,1.10 | 0.90 | 0.64,1.27 | 1.12a | 0.79,1.60 |
| Degeneration and other disc disorders | 1.00 | 0.82 | 0.16, 4.38 | 1.19 | 0.19, 7.33 | 0.09 | 0.01,1.37 |
| All diagnostic categories | 1.00 | 0.94 | 0.80,1.11 | 1.11 | 0.97,1.27 | 1.21a | 1.04,1.42 |
| All diagnostic categories except meniscal injuries | 1.00 | 0.92 | 0.77,1.10 | 1.04 | 0.89,1.22 | 1.16 | 0.97,1.38 |
The risk estimates have been adjusted for age group, gender, race/ethnicity, education, pay grade/rank, length of service, and work stress.
P <0.05.
Disability following a meniscal injury was significantly associated with heavy smoking (RH = 1.49, 95% CI 1.06–2.11) and light to moderate smoking (RH = 1.44, 95% CI 1.07–1.94). When all diagnostic groups were considered together, heavy smokers had a significantly higher risk for disability (RH = 1.21, 95% CI 1.04–1.42). The light to moderate smokers were also at elevated risk, though risk differences did not reach statistical significance (RH = 1.11, 95% CI 0.97–1.27). When cases with meniscal injuries were removed from the group of all diagnostic categories, the trend of heavy smokers having the greatest risk persisted, but was no longer statistically significant (RH = 1.16, 95% CI 0.97–1.38). Elevated RH indicating an association between smoking and long-term disability were also evident for the diagnoses of carpal tunnel syndrome, rotator cuff injury, collateral ligament injury, and chondromalacia, though the confidence intervals did not exclude one.
The excess fraction of disability discharge associated with smoking was calculated for cases with a meniscal injury. Among all subjects with meniscal injuries (excluding former smokers), the excess fraction of disability discharge due to smoking was 18% (95% CI 9–27%). Specifically among subjects with meniscal injuries who were also current smokers, the excess fraction was 38%.
DISCUSSION
Results of this study indicate an association between smoking and the likelihood of developing a long-term disability subsequent to musculoskeletal hospitalization in a young, active, and healthy cohort of employed persons. Kaplan-Meier estimates illustrate statistically distinct survival functions among different smoking levels and log-rank tests for trend demonstrate linear associations between smoking level and cumulative risk for disability discharge for six different diagnostic categories. Multivariate Cox proportional hazards models identified smoking to be an independent predictor of disability among heavy smokers hospitalized for a musculoskeletal diagnosis (RH = 1.21). In particular, soldiers hospitalized with a meniscal injury who smoked were at increased risk for subsequent disability where heavy smokers experienced nearly a 50% increased risk (RH = 1.49) and light to moderate smokers were only slightly lower (RH = 1.44). Results from Cox models for several other diagnostic categories suggest that smoking may affect disability following these diagnoses as well, though the effect may be overshadowed by stronger predictors of disability such as age, education, and pay grade.
To our knowledge, this represents the first identified association between smoking and development of long-term disability among persons with meniscal injuries. Since this finding persists after adjusting for various psychosocial and occupational factors, it suggests the presence of an underlying physiological mechanism. Studies suggest the menisci may be especially vulnerable to the effect of tobacco because of a vascular structure that only penetrates the peripheral 10–25% of the meniscus and that “… isolated lesions in this (avascular) area would lack the blood supply necessary for an inflammatory and reparative response” [Arnoczky and Warren, 1982]. Given that the menisci are provided with a limited blood supply in even the healthiest person, the biological effects of smoking (e.g., arteriolar vasoconstriction, cellular hypoxia, demineralization of bone, delayed revascularization, and immune suppression) [Amoroso et al., 1996b; Hoogendoorn et al., 2002] may further decrease the supply of nutrients to the damaged tissue and result in the interruption of the healing process and increase the risk of long-term dysfunction. Similar arguments have been espoused to explain the associations between smoking and poor wound healing, bone metabolism, low back pain (particularly related to a herniated disc), postoperative infection, and, in general, decreased healing of any injured tissue with limited vascularization [Kwiatkowski et al., 1996; Kinsella et al., 1999; Towler, 2000; Adams et al., 2001; Hoogendoorn et al., 2002].
Meniscal injuries represent perhaps the most properly coded diagnostic group of injuries in this cohort and are more likely to undergo a standardized treatment regimen (including surgery) than other groups, such as back conditions. The relative uniformity associated with this condition and the fact that it was the largest single category (N = 3,653) may have contributed to the significant association between smoking and subsequent disability, whereas associations for other musculoskeletal diagnoses did not reach statistical significance. For many of the other injury categories, the sample sizes may have been too small to detect a meaningful difference, thereby resulting in elevated risks that were not statistically significant. This is quite possibly the case for rotator cuff injury (n = 325), which exhibited a statistically significant test for trend (P = 0.011) and an elevated RH among heavy smokers (RH = 2.99), although the 95% CI included unity (0.95–9.38).
It is surprising that the multivariate analyses did not identify a significant association between smoking and disability following any of the back disorders or carpal tunnel syndrome, given the substantial literature associating smoking with the incidence (though not necessarily the development of disability) of these conditions [Frymoyer et al., 1980; Svensson et al., 1983; Kelsey et al., 1984; Owen and Damron, 1984; Biering-Sørensen and Thomsen, 1986; Saraste and Hultman, 1987; Battié et al., 1989; Deyo and Bass, 1989; Vessey et al., 1990; Heliövarra et al., 1991; Tsai et al., 1992; Boshuizen et al., 1993; O’Connor and Marlowe, 1993; Reynolds et al., 1994; Finkelstein, 1995; Nathan et al., 1996; Tanaka et al., 1997]. Perhaps there are many more causal pathways and mediating factors for back disorders that dilute the effect of smoking. One possible explanation for the muted effect of smoking among persons with back disorders may be that physical activity compensates for some of the harmful effects of smoking, as suggested by Eriksen et al. [1998]. Especially in a young and fit population such as the active duty Army, smoking’s effect may be reduced by the Army’s high level of physical activity. The possibility that smoking contributes to the development of back disorders but not to full-blown disability must also be considered. However, additional research is warranted before the divergence of these results from what is suggested in previous literature can be adequately interpreted.
Because of the substantial excess fraction of disability associated with smoking (38% among current smokers with meniscal injuries), the introduction of smoking cessation efforts at the time of diagnosis has great potential to prevent the development of physical disability and to reduce the large associated direct and indirect costs of musculoskeletal-related disability payments to veterans (lifetime costs of $485 million to newly disabled Army personnel in 1993 [AFEB, 1996] and average costs of $274,000 per permanent disability case [Amoroso and Canham, 1999]). Secondary prevention initiatives that alleviate the tissue deoxygenation induced by smoking, such as post-operative hyperbaric and ultrasound treatments [Upson, 1986; Cook et al., 1997], may also be helpful.
The strong association between smoking and development of disability is alarming in such a young and active population. Aggressive campaigns that focus on smoking’s associations with acute health outcomes, such as poor wound healing or impotence [Shabsigh et al., 1991; Mannino et al., 1994], are likely to be better received in smoking cessation campaigns focused on young adults and adolescents than the long-term risks of cancer and heart disease. This is also consistent with the recent studies of antismoking campaigns that found aggressive and hard line campaigns to be the most effective [Siegel, 1998; Farrelly et al., 2002].
It must also be remembered that we were limited in looking at only those cases severe enough to require hospitalization. In the case of back disorders, the degree that a hospitalized patient population represents the total population of individuals with back disorders may be quite biased. Also, fewer subjects with back disorders received a surgical procedure (58%) than subjects with meniscal injuries (93%). Perhaps the difference in disability risk reflects a greater susceptibility to the effects of tobacco on wound healing following surgery and the interaction of smoking on the physiological repair mechanisms may be responsible for the greater risk among meniscal injury subjects.
Our results suggest that former smokers are the least likely to incur a disability discharge. Although they were never determined to be at significantly lower risk, former smokers may be protected because they quit smoking as part of a series of behavioral changes that contribute to improved health and decreased likelihood of developing a disability. The difference in risk between heavy smokers and former smokers does imply that the effects of smoking prior to injury are not permanent and may, in fact, be reversible. Though they require verification, these findings have considerable importance in highlighting the benefits of smoking cessation.
Among studies that investigate the effect of smoking in association with physical disability [Leigh, 1985; Pinsky et al., 1985, 1987; Guralnik and Kaplan, 1989; Hubert and Fries, 1994; Rothenbacher et al., 1998] smoking’s relatively small risks and modest dose-response relationship are largely consistent. Ours, however, is the only one to address disability subsequent to a musculoskeletal condition, per se, while the others typically do not stratify by underlying condition.
In addition to a physiological mechanism, a psychosocial mechanism may contribute to smokers’ greater risk of injury as well as disability stemming from greater risk-taking behavior or exposure to environments less conducive to healing [Amoroso et al., 1996b]. Psychosocial factors such as being unmarried or divorced [Volinn et al., 1991; Lehmann et al., 1993; Badley and Ibanez, 1994; Cheadle et al., 1994], having less education [Deyo and Tsui-Wu, 1987; Deyo and Diehl, 1988; Mäkelä et al., 1993; Badley and Ibanez, 1994; Hubert and Fries, 1994; MacKenzie et al., 1998; Dionne et al., 2001], having a negative perception of the workplace [Bigos et al., 1992; Lancourt and Kettelhut, 1992; Williams et al., 1998; Feuerstein et al., 1999], and having fewer coping mechanisms [Habeck et al., 1991; Lancourt and Kettelhut, 1992; Feuerstein et al., 2001; Shaw et al., 2002] may result in a person having less motivation or fewer personal resources that enable him or her to experience a successful recovery. In our study, we found heavy smoking to be associated with lower levels of socioeconomic status, more physically demanding jobs, and higher levels of job stress. Perhaps a combination of such psychosocial factors and the direct physiological effects of tobacco contribute to the heightened risk of disability demonstrated by heavy smokers in this study.
There are several limitations of this study, some of which involve the HRA data. As with any source of self-reported health behaviors, reporting biases stemming from under-reporting or recall deficiency are possible. Not having available a measure of years smoked among current smokers from the HRA prohibited the calculation of pack-years, although Kinsella et al. [1999] findings suggest that the acute (rather than chronic) effects of smoking have a greater impact on healing mechanisms. Nonetheless, future studies that address the association between smoking and disability should be based on this preferred measure of cumulative cigarette smoking burden.
Despite the many covariates available for analysis as a result of database linkages, no measures of rehabilitation services (e.g., physical therapy) were available in a centralized administrative record. Another concern relates to the stability and accuracy of behavioral practices in Army personnel over time and whether the measures are likely to be comparable at the times of taking the HRA and initial musculoskeletal hospitalization. To address this issue, the stability of smoking practices before and after hospitalization was assessed in a subset of 1,482 subjects who completed more than one HRA. Excellent agreement between self-reported smoking status at time 1 and 2 was found (kappa = 0.74, 95% CI 0.71–0.77). This provides confidence in the reliability of the smoking status measure and supports the concept that smoking and hospitalization are independent, thereby minimizing the risk of simultaneous equation bias [Leigh, 1985]. However, there is some potential for misclassification of smoking exposure due to differences in smoking practice between the time of the HRA survey and the time of the injury, though it is largely expected to be nondifferential. The prevalence of smoking reported in this cohort is similar to that of a worldwide survey of US military personnel [Kroutil et al., 1994] in terms of age, gender, race/ethnicity, and pay grade. Although we attempted to exclude subjects with a previous musculoskeletal hospitalization (which may have served to mute the effect of smoking), there is the small possibility that some may have experienced such an event prior to Army entry beyond our data collection, although rigorous physical standards of recruitment reduce this likelihood. Lastly, our definition of disability is based on the initial, rather than final assessment by the Army medical board. Therefore, a very small proportion (1%) of “temporary disability” cases have yet to be assigned to return to duty or granted a permanent disability, though most eventually will receive permanent disability status.
The primary strengths of this study are the use of a wide array of pre-injury exposure data and the ability to follow a large population-based cohort over many years. The creative linkage of relevant data systems on exposures and outcomes are increasingly being realized as effective and efficient tools for etiologic research [Mittleman et al., 1997; NORA Traumatic Injury Team, 1998]. This cohort study provided insight into one of the potential risk factors for development of disability while adjusting for many potential confounders. The determination of disability was not affected by the antagonistic employee–employer relationship that is often evident with civilian workers’ compensation cases. In addition, we used a standardized service-wide protocol for determination of disability, unlike private sector disability policies.
The association between smoking and musculoskeletal-related disability raises important clinical and public health questions. Should meniscal injuries in smokers be given the same treatment as nonsmokers? How much disability, lost quality of life, and expense could be avoided if smoking cessation were introduced following injury? Despite these unanswered questions, clinicians should be aware of the potential detriment smoking may have on efforts to rehabilitate young and active patients, a population previously thought to be largely unaffected by smoking. Given the high excess fraction among subjects with meniscal injuries who were also current smokers, other studies are needed to confirm this association.
Acknowledgments
The authors thank Michelle Yore for her programming assistance and construction of the datasets, Dr. Richard Hinton and Dr. Tamara Lauder for their clinical guidance, and Dr. Susan Baker, Dr. Jacqueline Agnew, Dr. Mei-Cheng Wang, Dr. Ellen MacKenzie, and Dr. Clifford Mitchell for their editorial suggestions.
Contract grant sponsor: Defense Women’s Health Research Program (Army Medical Research and Materiel Command); Contract grant numbers: DA MD17-95-1-5066, W4168044; Contract grant sponsor: National Institute for Occupational Safety and Health; Contract grant number: 5 R01 OH03703-02; Contract grant sponsor: Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health; Training (to Dr. Lincoln); Contract grant number: T42/CCT310419; Contract grant sponsor: William Haddon, Jr. Fellowship in Injury Prevention (to Dr. Lincoln); Contract grant sponsor: National Institute on Alcohol Abuse and Alcoholism (to Dr. Bell); Contract grant number: 1 R29 AA11407; Contract grant sponsor: National Institute on Alcohol Abuse and Alcoholism (to Dr. Smith); Contract grant number: 1 R29 AA0770; Contract grant sponsor: University of Auckland Injury Prevention Research Centre (to Dr. Smith).
Footnotes
The use of Army medical records in the preparation of this material is acknowledged, but it is not to be construed as implying official Department of the Army approval of the conclusions presented. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, Department of Veterans Affairs, National Institute on Alcohol Abuse and Alcoholism, or the US Government.
Presented at the American Public Health Association 127th Annual Meeting and Exposition, Chicago, Illinois, November1999.
This article is a US Government work and, as such, is in the public domain in the United States of America.
References
- Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32:61–65. doi: 10.1016/s0020-1383(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Amoroso PJ, Canham ML. Disabilities related to the musculoskeletal system: Physical evaluation board data. Mil Med. 1999;164(8 Suppl):4.1–4.73. [PubMed] [Google Scholar]
- Amoroso PJ, Dettori JR, Reynolds KL, Schneider GA, Lavin P, Ryan JB, Jones BH. Tobacco use and injury risk among military parachutists. Presented at the Third International Conference for Injury Prevention and Control; Melbourne, Australia. 1996a. [Google Scholar]
- Amoroso PJ, Reynolds KL, Barnes JA, White DJ. Tobacco and Injury: An Annotated Bibliography. Natick, MA: US Army Research Institute of Environmental Medicine; 1996b. Technical note 96-1. [Google Scholar]
- Amoroso PJ, Reynolds KL, Dettori JR. A review of the relationship between tobacco use and musculoskeletal injury. Presented at the Third International Conference for Injury Prevention and Control; Melbourne, Australia. 1996c. [Google Scholar]
- Amoroso PJ, Reynolds KL, Bell NS. Benzodiazepine use and crash risk in older patients (letter) JAMA. 1998;279:113. [PubMed] [Google Scholar]
- Amoroso PJ, Yore MM, Weyandt B, Jones BH. A model comprehensive research database: Total army injury and health outcomes database. Milit Med. 1999;164(8 Suppl):8.1–8.36. [PubMed] [Google Scholar]
- AFEB (Armed Forces Epidemiological Board) Injuries in the Military. In: Jones BH, Hansen BC, editors. A Hidden Epidemic. Aberdeen Proving Ground, MD: US Army Center for Health Promotion and Preventive Medicine; 1996. Technical Report 29 HA 4844 97. [Google Scholar]
- Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- Badley EM, Ibanez D. Socioeconomic risk factors and musculoskeletal disability. J Rheumatol. 1994;10:90–95. [PubMed] [Google Scholar]
- Battié MC, Bigos SJ, Fisher LD, Hansson TH, Nachemson AL, Spengler DM, Wortley MD, Zeh J. A prospective study of the role of cardiovascular risk factors and fitness in industrial back pain complaints. Spine. 1989;14:141–147. doi: 10.1097/00007632-198902000-00001. [DOI] [PubMed] [Google Scholar]
- Bell NS, Amoroso PJ, Yore MM, Smith GS, Jones BH. Self-reported risk-taking behaviors and hospitalization for motor vehicle injury among active duty army personnel. Am J Prev Med. 2000;18(3S):85–95. doi: 10.1016/s0749-3797(99)00168-3. [DOI] [PubMed] [Google Scholar]
- Biering-Sørensen F, Thomsen C. Medical, social, and occupational history as risk indicators for low-back trouble in a general population. Spine. 1986;11:720–725. doi: 10.1097/00007632-198609000-00011. [DOI] [PubMed] [Google Scholar]
- Bigos SJ, Battie MC, Spengler DM, Fisher LD, Fordyce WE, Hansson T, Nachemson AL, Zeh J. A longitudinal, prospective study of industrial back injury reporting. Clin Orthop. 1992;279:21–34. [PubMed] [Google Scholar]
- Bongers PM, de Winter CR, Konpier MAJ, Hildebrandt VH. Psychosocial factors at work and musculoskeletal disease. Scand J Work Environ Health. 1993;19:297–312. doi: 10.5271/sjweh.1470. [DOI] [PubMed] [Google Scholar]
- Boshuizen HC, Verbeek JHAM, Broersen JPJ, Weel ANH. Do smokers get more back pain? Spine. 1993;18:35–40. doi: 10.1097/00007632-199301000-00007. [DOI] [PubMed] [Google Scholar]
- BLS (Bureau of Labor Statistics) Lost-worktime injuries: Characteristics and resulting time away from work, 1995. Washington DC: US Department of Labor; 1997. Publication no. 97-188. [Google Scholar]
- Cats-Baril WL, Frymoyer JW. Identifying patients at risk of becoming disabled because of low back pain. Spine. 1991;16:605–607. doi: 10.1097/00007632-199106000-00001. [DOI] [PubMed] [Google Scholar]
- Cheadle A, Franklin G, Wolfhagen C, Savarino J, Liu PY, Salley C, Weaver M. Factors influencing the duration of work-related disability: A population-based study of Washington State workers’ compensation. Am J Public Health. 1994;84:190–196. doi: 10.2105/ajph.84.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D. Modeling Survival Data in Medical Research. New York, NY: Chapman and Hall; 1994. [Google Scholar]
- Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orthop. 1997;337:198–207. doi: 10.1097/00003086-199704000-00022. [DOI] [PubMed] [Google Scholar]
- Department of the Army (DAPE-MB) Enlisted Career Management Fields and Military Occupational Specialty, US Army Regulation 611-201. Washington DC: 1994. [Google Scholar]
- Dettori JR, Reynolds KL, Amoroso PJ, Barnes JA, Westphal KA, Lavin PT. Smoking and injury risk among female US Army basic combat trainees. Presented at the Third International Conference for Injury Prevention and Control; Melbourne, Australia. 1996. [Google Scholar]
- Deyo RA, Bass JE. Lifestyle and low-back pain; the influence of smoking and obesity. Spine. 1989;14:501–506. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Diehl AK. Psychosocial predictors of disability in patients with low back pain. J Rheumatol. 1988;15:1557–1564. [PubMed] [Google Scholar]
- Deyo RA, Tsui-Wu Y-J. Functional disability due to back pain. A population-based study indicating the importance of socioeconomic factors. Arthritis Rheum. 1987;30:1247–1253. doi: 10.1002/art.1780301107. [DOI] [PubMed] [Google Scholar]
- Dionne C, Koepsell TD, Von Korff M, Deyo RA, Barlow WE, Checkoway H. Formal education and back pain: A review. J Epidemiol Comm Health. 2001;55:455–468. doi: 10.1136/jech.55.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embree BG, Whitehead PC. Validity and reliability of self-reported drinking behavior: Dealing with the problem of response bias. J Stud Alcohol. 1993;54:334–344. doi: 10.15288/jsa.1993.54.334. [DOI] [PubMed] [Google Scholar]
- Eriksen W, Natvig B, Rutle O, Bruusgaard D. Smoking as a predictor of long-term work disability in physically active and inactive people. Occup Med. 1998;48:315–320. doi: 10.1093/occmed/48.5.315. [DOI] [PubMed] [Google Scholar]
- Farrelly MC, Healton CG, Davis KC, Messeri P, Hersey JC, Haviland ML. Getting to the truth: Evaluating national tobacco counter marketing campaigns. Am J Public Health. 2002;92:901–907. doi: 10.2105/ajph.92.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein M, Thebarge RW. Perceptions of disability and occupational stress as discriminators of work disability in patients with chronic pain. J Occup Rehabil. 1991;3:185–195. doi: 10.1007/BF01073455. [DOI] [PubMed] [Google Scholar]
- Feuerstein M, Berkowitz SM, Peck CA. Musculoskeletal-related disability in US Army personnel: Prevalence, gender, and military occupational specialties. J Occup Environ Med. 1997;39:68–78. doi: 10.1097/00043764-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Feuerstein M, Berkowitz SM, Huang GD. Predictors of occupational low back disability: Implications for secondary prevention. J Occup Environ Med. 1999;41:1024–1031. doi: 10.1097/00043764-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Feuerstein M, Berkowitz SM, Haufler AJ, Lopez MS, Huang GD. Working with low back pain: Workplace and individual psychosocial determinants of limited duty and lost time. Am J Ind Med. 2001;40:627–638. doi: 10.1002/ajim.10000. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM. Back pain and parenthood. Occup Environ Med. 1995;52:51–53. doi: 10.1136/oem.52.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frymoyer JW, Pope MH, Costanza MC, Rosen JC, Goggin JE, Wilder DG. Epidemiological studies of low-back pain. Spine. 1980;5:419–423. doi: 10.1097/00007632-198009000-00005. [DOI] [PubMed] [Google Scholar]
- Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128:1185–1197. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Kaplan GA. Predictors of healthy aging: Prospective evidence from the Alameda County study. Am J Public Health. 1989;79:703–708. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck RV, Leahy MJ, Hunt HA, Chan F, Welch EM. Employer factors related to workers’ compensation claims and disability management. Rehab Counsel Bull. 1991;34:210–226. [Google Scholar]
- Hazard RG, Haugh LD, Reid S, Preble JB, MacDonald L. Early prediction of chronic disability after occupational low back injury. Spine. 1996;21:945–951. doi: 10.1097/00007632-199604150-00008. [DOI] [PubMed] [Google Scholar]
- Heliövarra M, Makela M, Knekt P, Impivaara O, Aromaa A. Determinants of sciatica and low-back pain. Spine. 1991;16:608–614. doi: 10.1097/00007632-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Hoogendoorn JM, Simmermacher RK, Schellekens PP, van der Werken C. Adverse effects of smoking on healing of bones and soft tissues. Unfallchirurg. 2002;105:76–81. doi: 10.1007/s113-002-8170-8. [DOI] [PubMed] [Google Scholar]
- Hubert HB, Fries JF. Predictors of physical disability after age 50. Six-year longitudinal study in a runners club and a university population. Ann Epidemiol. 1994;4:285–294. doi: 10.1016/1047-2797(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Jones BH, Cowan DN, Tomlinson JP, Robinson JR, Polly DW, Frykman PN. Epidemiology of injuries associated with physical training among young men in the army. Med Sci Sports Exerc. 1993;25:197–203. [PubMed] [Google Scholar]
- Kahn HA, Sempos CT. Statistical methods in epidemiology. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Katz JN, Keller RB, Fossel AH, Katz JN, Keller RB, Fossel AH. Predictors of return to work following carpal tunnel release. Am J Ind Med. 1997;31:85–91. doi: 10.1002/(sici)1097-0274(199701)31:1<85::aid-ajim13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Githens PB, O’Conner T, Weil U, Calogero JA, Holford TR, White AA, 3rd, Walter SD, Ostfeld AM, Southwick WO. Acute prolapsed lumbar intervertebral disc: An epidemiologic study with special reference to driving automobiles and cigarette smoking. Spine. 1984;9:608–613. doi: 10.1097/00007632-198409000-00012. [DOI] [PubMed] [Google Scholar]
- Kinsella JB, Rassekh CH, Wassmuth ZD, Hokanson JA, Calhoun KH. Smoking increases facial skin flap complications. Ann Otol Rhinol Laryngol. 1999;108:139–142. doi: 10.1177/000348949910800206. [DOI] [PubMed] [Google Scholar]
- Kroutil LA, Bray RM, Marsden ME. Cigarette smoking in the US military: Findings from the 1992 worldwide survey. Prev Med. 1994;23:521–528. doi: 10.1006/pmed.1994.1071. [DOI] [PubMed] [Google Scholar]
- Krueger JK, Rohrich RJ. Clearing the smoke: The scientific rationale for tobacco abstention with plastic surgery. Plast Reconstr Surg. 2001;108:1063–1073. doi: 10.1097/00006534-200109150-00042. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TC, Hanley EN, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25:590–597. [PubMed] [Google Scholar]
- Lancourt J, Kettelhut M. Predicting return to work for lower back pain patients receiving worker’s compensation. Spine. 1992;17:629–640. doi: 10.1097/00007632-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Lehmann TR, Spratt KF, Lehmann KK. Predicting long-term disability in low back injured workers presenting to a spine consultant. Spine. 1993;18:1103–1112. doi: 10.1097/00007632-199306150-00023. [DOI] [PubMed] [Google Scholar]
- Leigh JP. An empirical analysis of self-reported, work-limiting disability. Med Care. 1985;23:310–319. doi: 10.1097/00005650-198504000-00003. [DOI] [PubMed] [Google Scholar]
- Leistikow BN, Martin DC, Jacobs J, Rocke DM. Smoking as a risk factor for injury death: A meta-analysis of cohort studies. Prev Med. 1998;27:871–878. doi: 10.1006/pmed.1998.0374. [DOI] [PubMed] [Google Scholar]
- Lincoln AE, Smith GS, Amoroso PJ, Bell NS. The natural history and risk factors of musculoskeletal conditions resulting in disability among US Army personnel. Work. 2002;18:99–113. [PMC free article] [PubMed] [Google Scholar]
- MacKenzie EJ, Shapiro S, Smith RT, Siegel JH, Moody M, Pitt A. Factors influencing return to work following hospitalization for traumatic injury. Am J Public Health. 1987;77:329–334. doi: 10.2105/ajph.77.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie EJ, Morris JA, Jurkovich GJ, Yasui Y, Cushing BM, Burgess AR, DeLateur BJ, McAndrew MP, Swiontkowski MF. Return to work following injury: Factors influencing outcome. Am J Public Health. 1998;88:1630–1637. doi: 10.2105/ajph.88.11.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Kievens RM, Flanders WD. Cigarette smoking: An independent risk factor for impotence? Am J Epidemiol. 1994;140:1003–1008. doi: 10.1093/oxfordjournals.aje.a117189. [DOI] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- Mittleman MA, Maldonado G, Gerberich SG, Smith GS, Sorock GS. Alternative approaches to analytical designs in occupational injury epidemiology. Am J Ind Med. 1997;32:129–141. doi: 10.1002/(sici)1097-0274(199708)32:2<129::aid-ajim4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mosely LH, Finseth F, Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg. 1978;61:570–575. doi: 10.1097/00006534-197804000-00013. [DOI] [PubMed] [Google Scholar]
- Mäkelä M, Heliövaara M, Sievers K, Knekt P, Maatela J, Aromaa A. Musculoskeletal disorders as determinants of disability in Finns aged 30 years or more. J Clin Epidemiol. 1993;46:549–559. doi: 10.1016/0895-4356(93)90128-n. [DOI] [PubMed] [Google Scholar]
- Nathan PA, Keniston RC, Lockwood RS, Meadows KD. Tobacco, caffeine, alcohol, and carpal tunnel syndrome in American industry. J Occup Environ Med. 1996;38:290–298. doi: 10.1097/00043764-199603000-00015. [DOI] [PubMed] [Google Scholar]
- NORA Traumatic Injury Team. Traumatic Occupational Injury Research Needs and Priorities. US Department of Health and Human Services. DHHS (NIOSH); 1998. Publication 98-134. [Google Scholar]
- O’Connor FG, Marlowe SS. Low back pain in military basic trainees: A pilot study. Spine. 1993;18:1351–1354. doi: 10.1097/00007632-199308000-00015. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Bennett P, Leduc D. Reliability of self-reports of alcohol use by community clients. Hosp Comm Psychiatry. 1991;42:406–408. doi: 10.1176/ps.42.4.406. [DOI] [PubMed] [Google Scholar]
- Owen BD, Damron CF. Personal characteristics and back injury among hospital nursing personnel. Res Nurs Health. 1984;7:305–313. doi: 10.1002/nur.4770070409. [DOI] [PubMed] [Google Scholar]
- Peck CA. The US Army Physical Disability System. Rehabilitation of the Injured Combatant. In: Belandres PV, Dillingham TR, editors. Textbook of Military Medicine. II. Washington DC: Office of the Surgeon General, US Department of the Army, and Borden Institute; 1999. [Google Scholar]
- Pinsky JL, Branch LG, Jette AM, Haynes SG, Feinleib M, Cornoni-Huntley JC, Bailey KR. Framingham disability study: Relationship of disability to cardiovascular risk factors among persons free of diagnosed cardiovascular disease. Am J Epidemiol. 1985;122:644–656. doi: 10.1093/oxfordjournals.aje.a114144. [DOI] [PubMed] [Google Scholar]
- Pinsky JL, Leaverton PE, Stokes J., III Predictors of good function: The Framingham Study. J Chronic Dis. 1987;40(Suppl 1):159S–167S. doi: 10.1016/s0021-9681(87)80045-0. [DOI] [PubMed] [Google Scholar]
- Reynolds KL, Heckel HA, Witt CE, Martin JW, Pollard JA, Knapik JJ, Jones BH. Cigarette smoking, physical fitness, and injuries in infantry soldiers. Am J Prev Med. 1994;10:145–150. [PubMed] [Google Scholar]
- Reynolds KL, White JS, Knapik JJ, Witt CE, Amoroso PJ. Injuries and risk factors in a 100-mile (161 km) infantry road march. Prev Med. 1999;28:167–173. doi: 10.1006/pmed.1998.0396. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Arndt V, Fraisse E, Zschenderlein B, Fliedner TM, Brenner H. Early retirement due to permanent disability in relation to smoking in workers of the construction industry. J Occup Environ Med. 1998;40:63–68. doi: 10.1097/00043764-199801000-00012. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Nelson DE. Smoking and injuries: An overview. Prev Med. 1994;23:515–520. doi: 10.1006/pmed.1994.1070. [DOI] [PubMed] [Google Scholar]
- Saraste H, Hultman G. Life conditions of persons with and without low-back pain. Scand J Rehabil Med. 1987;19:109–113. [PubMed] [Google Scholar]
- Shabsigh R, Fishman IJ, Schum C, Dunn JK. Cigarette smoking and other vascular risk factors in vasculogenic impotence. Urology. 1991;38:227–231. doi: 10.1016/s0090-4295(91)80350-g. [DOI] [PubMed] [Google Scholar]
- Shaw WS, Feuerstein M, Lincoln AE, Miller VI, Wood PM. Ergonomic and psychosocial factors affect daily function in workers’ compensation claimants with persistent upper extremity disorders. J Occup Environ Med. 2002;44:606–615. doi: 10.1097/00043764-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Siana JE, Rex S, Gottrup F. The effect of cigarette smoking on wound healing. Scand J Plast Reconstr Surg Hand Surg. 1989;23:207–209. doi: 10.3109/02844318909075119. [DOI] [PubMed] [Google Scholar]
- Siegel M. Mass media antismoking campaigns: A powerful tool for health promotion. Ann Intern Med. 1998;129:128–132. doi: 10.7326/0003-4819-129-2-199807150-00013. [DOI] [PubMed] [Google Scholar]
- Smith GT, McCarthy DM, Goldman MS. Self-reported drinking and alcohol-related problems among early adolescents: Dimensionality and validity over 24 months. J Stud Alcohol. 1995;56:383–394. doi: 10.15288/jsa.1995.56.383. [DOI] [PubMed] [Google Scholar]
- Svensson HO, Vedin A, Wilhelmsson C, Andersson GBJ. Low-back pain in relation to other diseases and cardiovascular risk factors. Spine. 1983;8:277–285. doi: 10.1097/00007632-198304000-00008. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Wild DK, Cameron LL, Freund E. Association of occupational and non-occupational risk factors with the prevalence of self-reported carpal tunnel syndrome in a national survey of the working population. Am J Ind Med. 1997;32:550–556. doi: 10.1002/(sici)1097-0274(199711)32:5<550::aid-ajim18>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Thompson DC, Rivara FP, Thompson RS, Salzberg PM, Wolf ME, Pearson DC. Use of behavioral risk factor surveys to predict alcohol-related motor vehicle events. Am J Prev Med. 1993;9:224–230. [PubMed] [Google Scholar]
- Towler J. Cigarette smoking and its effects on wound healing. J Wound Care. 2000;9:100–104. doi: 10.12968/jowc.2000.9.3.25962. [DOI] [PubMed] [Google Scholar]
- Tsai SP, Gilstrap EL, Cowles SR, Waddell LC, Jr, Ross CE. Personal and job characteristics of musculoskeletal injuries in an industrial population. J Occup Med. 1992;34:606–612. [PubMed] [Google Scholar]
- Upson AV. Topical hyperbaric oxygenation in the treatment of recalcitrant open wounds. A clinical report. Phys Ther. 1986;66:1408–1412. doi: 10.1093/ptj/66.9.1408. [DOI] [PubMed] [Google Scholar]
- USDHHS (US Department of Health and Human Services) Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service, 1964. Washington DC: US Department of Health and Human Services; 1964. Public Health Service publication no. 1103. [Google Scholar]
- USDHHS (US Department of Health and Human Services) Clinical Modification. 9. Washington DC: Public Health Service; 1988. International Classification of Diseases. [Google Scholar]
- USDHHS (US Department of Health and Human Services) Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General. Washington DC: US Department of Health and Human Services; 1989. DHHS (CDC) publication no. 89-8411. [Google Scholar]
- Vessey MP, Villard-Mackintosh L, Yeates D. Epidemiology of carpal tunnel syndrome in women of childbearing age. Findings in a large cohort study. Int J Epidemiol. 1990;19:655–659. doi: 10.1093/ije/19.3.655. [DOI] [PubMed] [Google Scholar]
- Volinn E, Van Koevering D, Loeser JD. Back sprain in industry: The role of socioeconomic factors in chronicity. Spine. 1991;16:542–548. doi: 10.1097/00007632-199105000-00010. [DOI] [PubMed] [Google Scholar]
- Williams RA, Pruitt SD, Doctor JN, Epping-Jordan JE, Wahlgren DR, Grant I, Patterson TL, Webster JS, Slater MA, Atkinson JH. The contribution of job satisfaction to the transition from acute to chronic low back pain. Arch Phys Med Rehabil. 1998;79:366–374. doi: 10.1016/s0003-9993(98)90135-6. [DOI] [PubMed] [Google Scholar]
- Yelin E, Callahan LF. The economic cost and social and psychological impact of musculoskeletal conditions. National Arthritis Data Work Groups. Arthritis Rheum. 1995;38:1351–1362. doi: 10.1002/art.1780381002. [DOI] [PubMed] [Google Scholar]


