Abstract
The invasion-inducing T-lymphoma invasion and metastasis 1 (Tiam1) protein functions as a guanine nucleotide exchange factor (GEF) for the small GTPase Rac1. Differentiation-dependent expression of Tiam1 in the developing brain suggests a role for this GEF and its effector Rac1 in the control of neuronal morphology. Here we show that overexpression of Tiam1 induces cell spreading and affects neurite outgrowth in N1E-115 neuroblastoma cells. These effects are Rac-dependent and strongly promoted by laminin. Overexpression of Tiam1 recruits the α6β1 integrin, a laminin receptor, to specific adhesive contacts at the cell periphery, which are different from focal contacts. Cells overexpressing Tiam1 no longer respond to lysophosphatidic acid– induced neurite retraction and cell rounding, processes mediated by Rho, suggesting that Tiam1-induced activation of Rac antagonizes Rho signaling. This inhibition can be overcome by coexpression of constitutively active RhoA, which may indicate that regulation occurs at the level of Rho or upstream. Conversely, neurite formation induced by Tiam1 or Rac1 is further promoted by inactivating Rho. These results demonstrate that Rac- and Rho-mediated pathways oppose each other during neurite formation and that a balance between these pathways determines neuronal morphology. Furthermore, our data underscore the potential role of Tiam1 as a specific regulator of Rac during neurite formation and illustrate the importance of reciprocal interactions between the cytoskeleton and the extracellular matrix during this process.
Members of the Ras superfamily of small GTPases control a wide variety of cellular responses, which include proliferation, cell cycle regulation, and intracellular transport (Bogusky and McCormick, 1993). A subgroup of this family, the Rho-like GTPases, affect the organization of the actin cytoskeleton. In fibroblasts, Cdc42 is involved in the induction of filopodia, Rac induces the formation of lamellipodia, and activation of Rho leads to the formation of actin stress fibers (Ridley and Hall, 1992; Ridley et al., 1992; Kozma et al., 1995; Nobes and Hall, 1995). In addition, these GTPases promote the assembly of adhesive complexes connecting the actin cytoskeleton with the extracellular matrix (Ridley et al., 1992; Nobes and Hall, 1995). Integrins, cytoskeletal proteins, and signaling molecules converge at these sites, which are known as focal contacts when present at the end of actin stress fibers (Jockusch et al., 1995). Studies using microinjection of activated Rho-like proteins in Swiss 3T3 cells suggest that there is a hierarchy in which these GTPases regulate each others activities. Expression of activated Cdc42 leads to activation of Rac, which subsequently can induce Rho activity (Nobes and Hall, 1995). There are also links between signaling of Ras and Rho family members. In fibroblasts, Ras-induced membrane ruffling is dependent on Rac activity (Ridley et al., 1992) and focus formation induced by Ras involves both Rac and Rho mediated signals (Prendergast et al., 1995; Qiu et al., 1995a ,b). Little is known however, about the mechanisms responsible for this regulation.
Activation of both Ras and Rho proteins in response to growth factors involves the recruitment of guanine nucleotide exchange factors (GEFs),1 which activate these GTPases by catalyzing the exchange of GDP for GTP. A hallmark of GEFs specific for Rho family members is the presence of a Dbl homology domain, flanked by a pleckstrin homology domain. More than 20 proteins with a Dbl homology/ pleckstrin homology domain combination have been identified, and they are considered to be the primary targets of signaling pathways that control the activity of Rho-like GTPases (Cerione and Zheng, 1996; Collard, 1996). Inactivation of Rho proteins is regulated by GTPase activating proteins, (Rho-GAPs), which elevate the intrinsic GTPase activity of Rho proteins, returning them to an inactive state. Several Rho GEFs and Rho GAPs show restricted expression patterns and specificity towards particular Rho family members (Collard, 1996; Lamarche and Hall, 1994), and it remains a challenge to understand how the activity of such proteins directs complex cellular behaviors such as cell migration or differentiation during development and in the adult.
Rho-like GTPases have recently been put forward as potential regulators of neuronal outgrowth (Mackay et al., 1995; Tanaka and Sabry, 1995). Thrombin and the lysophospholipid LPA induce cell rounding and neurite retraction of differentiated mouse neuroblastoma cells (Jalink and Moolenaar, 1992; Jalink et al., 1993). LPA-induced cell rounding can be inhibited by the C3 endotoxin (Jalink et al., 1994) or dominant-negative N19RhoA (Gebbink et al., 1997), establishing a key role for Rho in this process. In addition, ectopic expression of constitutively active or dominant-negative variants of Rac1 and Cdc42 affect neurite formation during Drosophila and mouse development (Luo et al., 1994, 1996).
In earlier work, we have identified the invasion-inducing T-lymphoma invasion and metastasis 1 (Tiam1) gene (Habets et al., 1994). More recently, we showed that the Tiam1 protein functions as GEF for Rac both in vitro and in vivo (Michiels et al., 1995). Tiam1 is expressed at low levels in most tissues but at markedly higher levels in brain and testis (Habets et al., 1995). Expression in the brain is restricted to a subset of neurons, and the onset of expression during development correlates well with neuronal differentiation and/or migration, suggesting a role for Tiam1 in these processes (Ehler et al., 1997). Therefore, we have investigated the effects of Tiam1 on neuronal morphology in mouse N1E-115 neuroblastoma cells. We show that overexpression of Tiam1, by activating Rac1, affects lamellar spreading and neurite formation, and that these effects are strongly promoted by laminin. Overexpression of Tiam1 increases adhesion to laminin and directs the integrin α6β1, a laminin receptor, to specific contacts at the cell periphery. We also find that Tiam1-promoted neurite outgrowth is determined by a balance between Rac- and Rho-mediated signals and that activation of Rac antagonizes the Rho pathway. We propose that interactions between Rac- and Rho-mediated signaling pathways coordinate neurite formation, and that GEFs and GAPs are key regulators in this process.
Materials and Methods
Expression Constructs
The C1199 Tiam1 cDNA was cloned as a BssHII\\ScaI fragment into pRc/ CMV (Invitrogen Corp., Carlsbad, CA) as described earlier (Habets et al., 1994). cDNA's for Rac, Rho, and Cdc42 contained an NH2-terminal myc tag and were cloned into the eukaryotic expression vector pCDNA3 (Invitrogen Corp.). Rac and Rho cDNAs were originally made available by Dr. A. Hall (MRC, London, UK) and cloned as EcoRI fragments into pCDNA3 (Invitrogen Corp.). The pCDNA3 construct containing N19RhoA was obtained from M. Gebbink and W.H. Moolenaar (The Netherlands Cancer Institute, Amsterdam, The Netherlands). V12Cdc42 and N17Cdc42 were derived by PCR from a wild-type Cdc42 cDNA, which was made available by Dr. R. Cerione (Cornell University, Ithaca, NY). A p190 cDNA was obtained from Dr. M. Symons (Onyx Pharmaceuticals, Richmond, CA) and cloned into pcDNA3. Construction, packaging, and infection of recombinant retroviruses containing the C1199 Tiam1 cDNA fused to a IRES-neo sequence will be described in detail elsewhere.
Transient Transfection Assays
N1E-115 cells were seeded in 6-cm dishes at a density of 5 × 105 cells in DME, supplemented with 10% FCS and antibiotics. The next day, plasmids containing the various cDNAs were mixed in a 5:1 ratio with pCMVLacz (Stratagene, La Jolla, CA), and transfections were carried out using the calcium phosphate precipitation method as described earlier (van Leeuwen et al., 1995). The next day, transfected cells were detached from the dish and replated into six-well dishes or onto glass coverslips that were coated overnight with 20 μg/ml laminin-1. Cells were fixed in PBS containing 2% formaldehyde/0.2% glutaraldehyde and assayed for β-galactosidase activity. The morphology of single lacZ-positive cells was scored as either round, flat, or neurite bearing. Cells with at least one process greater than one cell diameter were considered neurite bearing. An average of 250 cells were counted per well and the values presented are the mean percentages (± SEM) of at least two independent transfections.
Immunoprecipitation and Western Blotting
Transfected cells were lysed in a buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 5 mM EDTA supplemented with 20 μg leupeptin, 100 μg aprotinin, and 180 μg PMSF per milliliter. Extracts were clarified by centrifugation. An equal volume of Laemmli loading buffer containing 10% β-mercaptoethanol was added. For immunoprecipitations, clarified lysates were incubated with anti-Tiam1 antibody C16 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 h. Immunocomplexes were removed using a 10% protein A–sepharose slurry (Pharmacia Biotech, Inc., Piscataway, NJ) and washed three times before adding 25 μl of Laemmli sample buffer. Samples were boiled, separated by SDS-PAGE, and subjected to Western blot analysis as described earlier (van Leeuwen et al., 1995). For detection of Tiam1, an affinity-purified rabbit polyclonal antibody (Habets et al., 1994) was used. Myc-tagged GTPases were detected using monoclonal antibody 9E10.
Attachment Assays
Adhesion of cells to laminin was performed in 24-well dishes that were coated in triplicate with increasing concentrations of laminin-1 (Collaborative Biomedical Products, Bedford, MA). After overnight incubation at 4°C, unbound substrate was washed away with PBS and the wells were blocked with PBS containing 1% BSA for 1 h at 37°C. Cells were harvested, washed in DME containing 1% BSA and seeded at a density of 105 cells per well. For antibody-blocking experiments, cells were pretreated in suspension with DME/1%BSA in the presence of 10 μg/ml of affinity- purified antibody GoH3, a rat monoclonal antibody which specifically recognizes the extracellular domain of the α6 integrin (Sonnenberg et al., 1988). Cells were allowed to adhere for 15 min, after which unattached cells were removed by washing with PBS. After fixation in PBS/3% formaldehyde (10 min), cells were stained for 30 min with a 2% crystal violet solution in PBS. Excess stain was washed away with PBS and cells were lysed in PBS containing 1% NP-40. Attachment was quantitated by measuring the absorbance of these lysates at 570 nm. Maximal adhesion was determined on poly-l-lysine–coated dishes and set at 100%. Values depict the average of two experiments. Each experiment consisted of four independent determinations.
Immunofluorescence
Cells grown on (laminin-coated) coverslips were fixed with 3.7% formaldehyde in PBS for 20 min, washed, and permeabilized with 0.2% Triton X-100 in PBS for 5 min. After a brief washing step, fixed cells were blocked with PBS containing 2% BSA (PBS/BSA) for 1 h. All antibody incubations were done in PBS/BSA and coverslips were washed three times between each incubation step. Tiam1 was detected using a rabbit polyclonal anti-Tiam1 antibody (Habets, 1994). Myc-tagged GTPases were detected using antibody 9E10. FITC-labeled secondary antibodies were from Zymed Labs (San Francisco, CA). In the last incubation step, TRITC-labeled phallodin (Molecular Probes, Eugene, OR) was included to allow detection of F-actin. Antibody NM6, directed against B-50/GAP-43, was obtained from Dr. L Schrama (University Utrecht, The Netherlands). Anti–neurofilament 200 (NE14) was from Sigma Chemical Co. (St. Louis, MO). Phosphotyrosine-containing proteins were detected using antibody PY20 (Transduction Laboratories, Lexington, KY). Antipaxillin antibodies (mAb3060) were obtained from Chemicon Int. Inc. (Temecula, CA). Antivinculin antibody VII F9 was obtained from Dr. Marina Glukhova (CNRS Institut Curie, Paris, France), and the α6 integrin subunit was detected with antibody GoH3 (Sonnenberg et al., 1988), which was also used in the adhesion assays. To facilitate detection of these proteins, the signal was enhanced by including a biotinylated secondary antibody (Amersham Corp., Arlington Heights, IL), followed by a third incubation with streptavidin-FITC (Zymed Labs). Coverslips were mounted in vectamount (Vector Laboratories, Burlingame, CA) and viewed under a confocal microscope (model MRC600; Bio-Rad Labs, Hercules, CA).
Results
Tiam1 Expression in Neural Cell Lines
We analyzed Tiam1 protein levels in a number of adherent cell lines by immunoprecipitation in combination with Western blotting (Fig. 1). Whereas no detectable expression was found in NIH 3T3, Rat-1, or COS-7 cells, relatively high levels of expression were observed in N1E-115 cells and PC12 cells, cell lines commonly used to study aspects of neuronal differentiation in vitro. Another neuroblastoma cell line, SK-N-MC, which cannot be induced to produce neurites in vitro, but rather shows growth factor– dependent scattering (van Puijenbroek et al., 1997), did not express Tiam1. In view of the proposed role of Tiam1 in regulating neuronal differentiation in the brain (Ehler et al., 1997), the prominent expression of Tiam1 in neural cell lines may relate to the ability of these cells to form neurites. Mouse N1E-115 neuroblastoma cells can be transfected with high efficiency (Kranenburg et al., 1995) and are rapidly induced to spread and produce neurites after serum withdrawal. Therefore, we decided to use N1E-115 cells as an in vitro model for Tiam1 function in neuronal cells.
Figure 1.
Tiam1 expression in various cell lines. Detection of the Tiam1 protein by immunoprecipitation and Western blotting. The Tiam1 protein was precipitated using antibody C16. Samples were separated on a 7.5% polyacrylamide gel and analyzed by Western blotting using a rabbit polyclonal antibody raised against a COOH-terminal fragment of the Tiam1 protein (Habets et al., 1994).
Effects of C1199 Tiam1, V12Rac1, or V12Cdc42 on N1E-115 Morphology
We studied the consequences of overexpression of Tiam1, constitutively active V12Rac1, or V12Cdc42 on N1E-115 morphology after transient transfection. Rather than using a full-length Tiam1 cDNA, we used an NH2-terminally truncated variant, known as C1199 Tiam1. This variant, which can efficiently activate Rac1 and induces morphological transformation and membrane ruffling in NIH 3T3 cells, is more stably expressed and appears to be more active than the full-length protein (Michiels et al., 1995; van Leeuwen et al., 1995). Cells expressing recombinant proteins were identified 48 h after transfection using immunofluorescence detection and confocal microscopy as described in Materials and Methods.
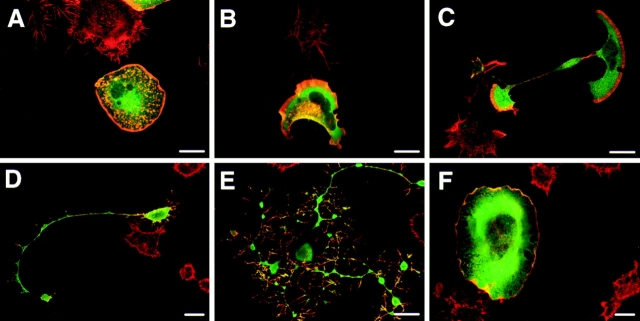
In the presence of serum, cells expressing C1199 Tiam1 showed a characteristic spreading morphology, accompanied by a redistribution of F-actin to the cell periphery (Fig. 2, A and B). Expression of V12Rac1 produced a phenotype very similar to that of Tiam1 (Fig. 2, C and D), which is consistent with earlier observations that Tiam1 activates Rac1. The phenotype induced by Cdc42 resembled that of Rac1 and Tiam1 (Fig. 2, E and F), although most cells expressing Cdc42 were less well spread. Transfection of constitutively active V12Cdc42 in combination with dominant-negative N17Rac1 abolished this phenotype (not shown), indicating that the V12Cdc42-induced phenotype is Rac-dependent. This is in agreement with experiments in Swiss 3T3 cells which show that injection of V12Cdc42 activates Rac (Nobes et al., 1995). Apparently a similar regulation of Rac by Cdc42 occurs in N1E-115 cells.
Figure 2.
Morphological characteristics of N1E-115 cells expressing Tiam1, Rac1, or Cdc42. Transiently transfected cells were grown on glass coverslips for two days, fixed, permeabilized, and stained with anti-Tiam1 antibodies (A) or anti-myc antibody 9E10 to detect V12Rac1 (C) and V12Cdc42 (E). TRITC-labeled phalloidin was used to detect F-actin (B, D, and F). Cells were viewed by confocal microscopy. The untransfected control cells are only visible in the panels stained for F-actin (B, D, and F). Bar, 25 μm.
We next determined the effect of serum withdrawal on these transfected populations. Whereas after 48 h in low serum ∼50% of the non transfected cells had formed neurites, cells expressing C1199 Tiam1, V12Rac1, or V12Cdc42 retained their flat lamellar appearance, similar to what is shown in Fig. 2. We conclude that expression of C1199 Tiam1 or the respective GTPases does not allow neurite formation when grown on plastic.
Tiam1 Promotes the Rapid Formation of Neurite-like Outgrowths and Lamellar Spreading on a Laminin-1 Substrate
Neurite formation in vitro, in response to differentiative signals, is strongly promoted by extracellular matrix components such as laminin, fibronectin, or collagen (Hynes and Lander, 1992). We therefore determined if and how different substrates would affect the phenotype of N1E-115 cells expressing C1199 Tiam1, V12Rac1, or V12Cdc42.
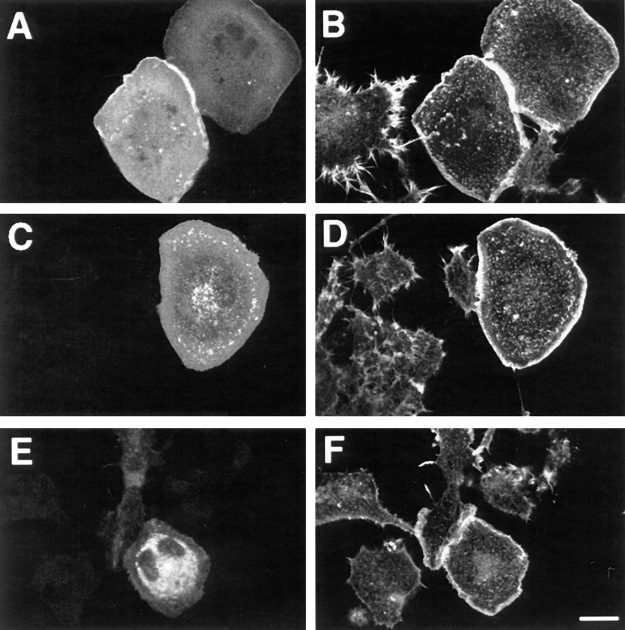
N1E-115 cells grown on fibronectin-coated dishes adhered slowly, and the C1199 Tiam1–expressing cells within this population were mostly flat, a phenotype very similar to that produced on plastic (see Figs. 2 and 5 B). In contrast, on laminin-coated dishes, cells adhered rapidly and the C1199 Tiam1–expressing cells went through a number of dramatic changes in cell morphology and behavior. During the first hours after plating, cells expressing Tiam1 either spread along their entire surface (Fig. 3 A) or became polarized, retracting from the substrate at one end of the cell and carrying a leading lamella at the other end, reminiscent of migrating fibroblasts (Fig. 3 B). After some time, polarized cells began to form neurite-like processes that carried prominent lamellipodia (Fig. 3 C). After overnight growth on laminin, many of these processes had lost their lamellar appearance (Fig. 3 D). The highest expressing cells produced extreme phenotypes overnight, either as a result of uncontrolled branching (Fig. 3 E) or extreme spreading (Fig. 3 F). Surprisingly, these changes took place in the presence of serum, conditions which normally prevent neurite formation.
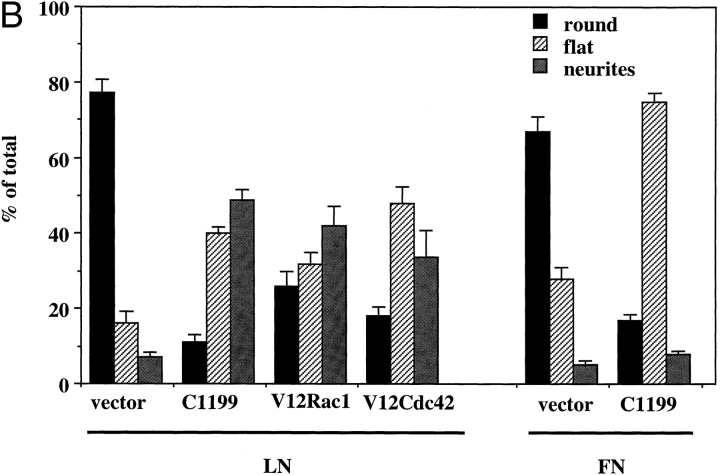
Figure 5.

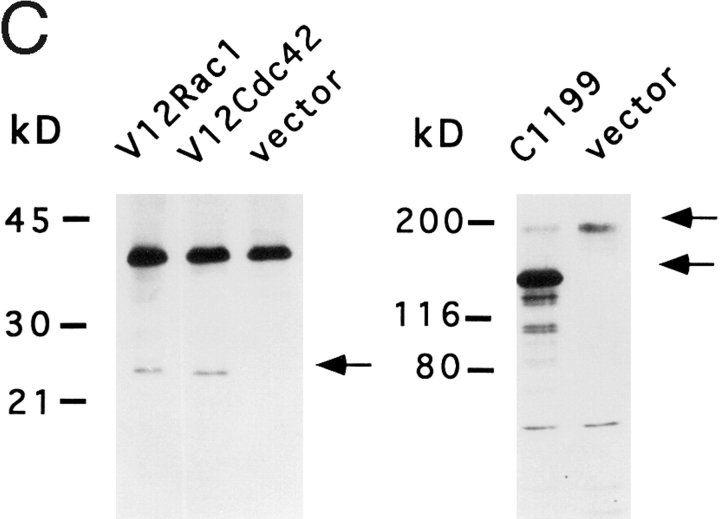
C1199 Tiam1, V12Rac1, and V12Cdc42 promote neurite formation on a laminin-1 substrate. (A) Phase contrast photographs of neurite bearing N1E-115 cells transiently transfected with either C1199 Tiam1, V12Rac1, V12Cdc42, or a control plasmid, and cotransfected with plasmid pCMV-lacZ. 20 h after transfection, cells were seeded onto laminin-coated six-well plates, grown for 4 or 24 h, and subsequently fixed and stained for β-galactosidase activity to identify transfected cells in the population. (B) Quantification of morphological changes. The morphology of transfected (lacZ-positive) cells on either laminin (LN) or fibronectin (FN) was scored as round, flat, or neurite bearing, as described in Materials and Methods. About 250 cells were counted per well. Numbers represent the mean percentage of three independent experiments. Error bars indicate SEM (n = 3). (C) Expression of myc-tagged V12Rac1 or V12Cdc42 (left) or C1199 Tiam1 (right) in transiently transfected N1E-115 cells, as analyzed in B. Total cell lysates were separated on 7.5 or 6% polyacrylamide gels and immunoblotted using anti-Myc or anti-Tiam1 antibodies. In the anti-Tiam1 blot (right), the signal present at 200 kD represents endogenous Tiam1, whereas the smaller 170-kD product is C1199 Tiam1. Bar, 25 μm.
Figure 3.
Morphological transitions observed in Tiam1 cells grown on laminin. C1199 Tiam1–transfected cells were seeded onto laminin-coated coverslips in the presence of serum and allowed to adhere for various lengths of time before fixation and staining. Representative examples of each timepoint are given. (A) Spreading cell, 2 h after seeding. (B) Polarized cell 2 h after seeding, carrying a large lamellipodium at the leading edge. (C) Cell developing neurite-like outgrowths, 4 h after seeding. Note the presence of lamellipodia on both ends of the cell, moving in opposite directions. (D) Neurite-bearing cell after overnight growth on laminin. (E) Extreme branching induced by high expression of C1199 Tiam1 after overnight growth on laminin. (F) Some cells show extreme spreading and an apparent loss of contractility induced by high expression of C1199 Tiam1 after overnight growth on laminin. Tiam1 is shown in green, F-actin in red. Note that D and F represent a 400× magnification, whereas the other images were magnified 600×. The red cells represent untransfected controls. Bars, 25 μm.
To establish the nature of these neurite-like extensions, two different neuronal markers were used. Neurofilament, a marker for mature neurites, could not be detected in these overnight cultures (results not shown). However, the “growth-associated” protein B-50/GAP-43, an early marker for developing neurites and growth cones (Skene et al., 1986), was highly enriched in these processes (Fig. 4). We therefore conclude that these processes have at least neurite-like properties. The expressed C1199 Tiam1, identified by immunofluoresence detection, appeared to be mainly cytosolic and was not particularly enriched at the plasma membrane, neither in spreading cells nor in developing neuronal processes (Fig. 3). This contrasts with our earlier studies in NIH 3T3 cells, where overexpressed Tiam1 colocalized with cortical F-actin (Michiels et al., 1997). In spite of the seemingly cytosolic distribution of C1199 Tiam1 in these cells, biochemical fractionation experiments in N1E-115 cells have demonstrated that part of the endogenously expressed Tiam1 protein is present in a Triton X-100 insoluble fraction, suggesting association with the actin cytoskeleton (Stam et al., 1997).
Figure 4.
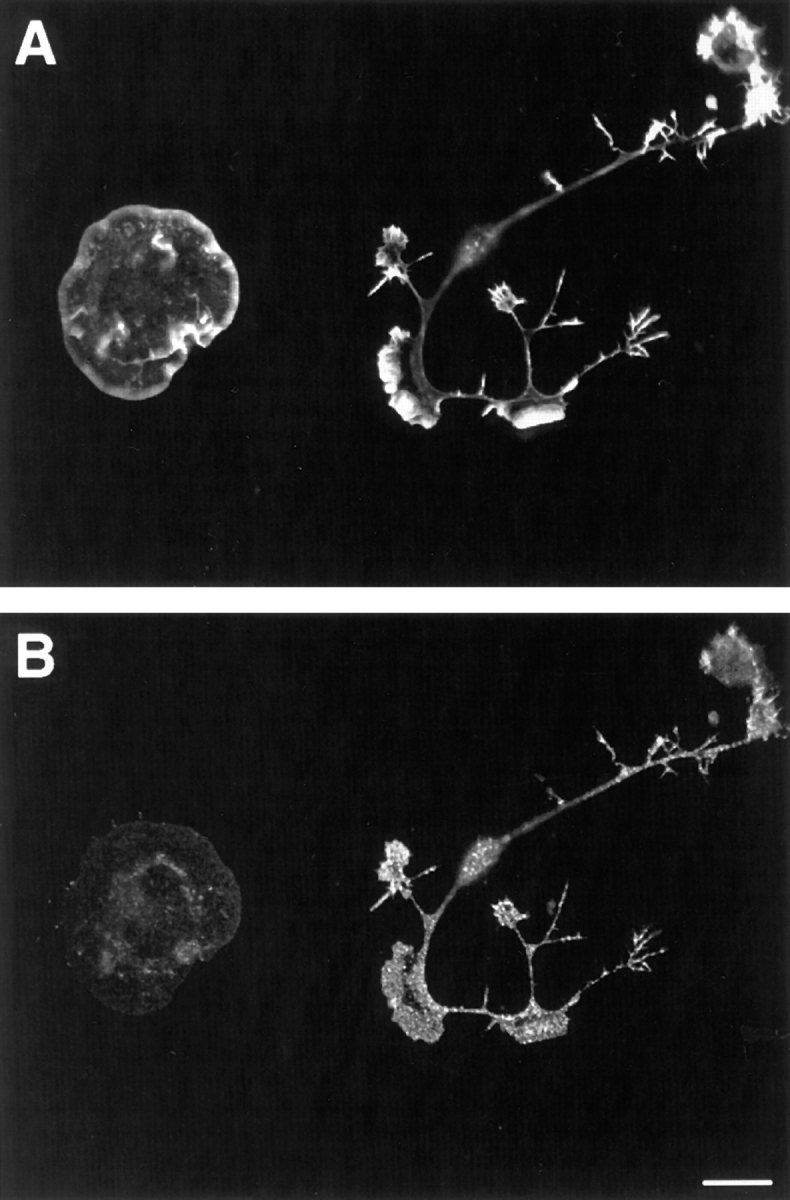
The neurite-specific marker, B-50/GAP-43, is present in neurite-like extensions of C1199 Tiam1–expressing N1E-115 cells. (A) F-actin; (B) B-50/GAP-43. Note how expression of B-50/ GAP-43 is enriched in neuronal processes in comparison to the cell showing a spreading morphology. Bar, 25 μm.
We found that expression C1199 Tiam1, V12Rac1, or V12Cdc42 produced very similar effects, although in cells expressing V12Cdc42, the morphological changes occurred more slowly and fewer cells developed neuronal processes. Since these experiments were performed in the presence of serum, no neuronal processes were induced in the vector control cells. Fig. 5 A shows representative phase-contrast images of cells carrying neurite-like processes within each transfected population, 4 h after plating and after overnight growth on laminin.
To obtain a more quantitative analysis of the effects of C1199 Tiam1, Rac1, or Cdc42 on morphology, phenotypes of individual lacZ-positive cells were scored as either round, flat, or neurite-bearing after overnight growth on laminin (Fig. 5 B). For comparison, C1199 Tiam1–transfected cells and vector-transfected control cells were grown overnight on fibronectin. Whereas on laminin ∼50% of the Tiam1-transfected cells developed neurite-like extensions, on fibronectin most of these cells showed a spreading morphology, and the number of cells that carried neurite-like outgrowths was not increased relative to the control cells (Fig. 5 B). Correct expression of each construct was confirmed by Western blotting (Fig. 5 C).
In summary, expression of C1199 Tiam1 prevents neurite formation on fibronection or plastic, even under conditions that normally favor neuritogenesis, i.e., serum withdrawal. In contrast, when grown on a laminin substrate, neurite-like processes are induced even in the presence of serum. We conclude that, rather than inducing neurite formation per se, overexpression of Tiam1 and consequently activation of Rac1 modulate the ability of these cells to respond to differentiative signals, which is to a large extent determined by specific cell–substrate interactions.
Tiam1 Promotes Adhesion and Spreading on Laminin-1 via the Integrin a6β1
To further explore the role of laminin in Tiam1-induced spreading and the formation of neurite-like outgrowths, we compared adhesion of N1E-115 cells stably expressing C1199 Tiam1 after retroviral transduction and G418 selection with empty vector control cells. The C1199 Tiam1– expressing cells showed a significant increase in adhesion relative to the control cells (Fig. 6). Adhesion was inhibited ∼75% by preincubating cells with a rat monoclonal antibody (GoH3), directed against the α6 integrin subunit (Fig. 6), which is part of the laminin receptor α6β1 (Sonnenberg et al., 1988). Although we cannot exclude the possibility that other laminin-binding integrins may be present on N1E-115 cells, these results implicate α6β1 in adhesion and spreading of N1E-115 cells to laminin.
Figure 6.
Tiam1 overexpression results in increased adhesion to laminin. N1E-115 cells stably expressing C1199 Tiam1 or a control cell line were plated on 24-well plates coated with increasing concentrations of laminin. A small portion of the cells was pretreated in suspension with DME/1% BSA in the presence of 10 μg/ml of affinity-purified anti-α6 antibody (GoH3), or mock treated, and plated in wells coated with 20 μg/ml laminin. Cells were allowed to attach for 15 min, washed, and adhesion was quantitated by crystal violet staining and measuring absorbance at 570 nm (for details see Materials and Methods). Maximal adhesion was determined on poly-l-lysine–coated dishes and set at 100%. Values represent the average of quadruplicate samples from two representative experiments. Error bars indicate SEM (n = 2). The observed differences at laminin concentrations of 20 and 10 μg/ml were found statistically significant by students t test (P < 0.01).
Integrins are known to be involved in regulating neurite formation (for review see Hynes and Lander, 1992). Therefore, the effects of Tiam1 on cell adhesion, spreading, and neurite formation might be explained by changes in expression, activation, or distribution of integrins. Since it was shown in PC12 cells that expression of the integrin α1β1, a laminin/collagen receptor, is upregulated in response to differentiative signals, (Zhang et al., 1993), we determined if expression of α6β1 was altered in the Tiam1-expressing cells. Flow cytometry performed on control cells and C1199 Tiam1–expressing cells, however, did not reveal any differences in cell surface expression of either the α6 or the β1 subunit (not shown). We conclude that the effects of Tiam1 on adhesion and spreading on laminin are not due to increased expression, but may reflect activation and/or redistribution of the laminin receptor α6β1.
Tiam1/Rac1 Overexpression Leads to a Distribution of α6β1 Integrin to Adhesive Contacts at the Leading Edge
Since we did not find any changes in the expression levels of α6β1, we examined the distribution of this integrin in cells expressing Tiam1. For these experiments, we used a population of virally transduced N1E-115 cells that stably expressed C1199 Tiam1. Cells were allowed to adhere on laminin overnight, and the localization of the α6β1 integrin was analyzed by immunofluorescence detection using an antibody against the α6 subunit. In the control cells, we did not see any specific distribution of the α6β1 integrin (Fig. 7 A). In the C1199 Tiam1–expressing cells, however, α6β1 was found in small adhesive complexes along the distal end of advancing lamellae. These complexes were most clearly seen in round spreading cells (Fig. 7 B), but also present in lamellar endings of neurite-like outgrowths (Fig. 7 F). A similar distribution was found using an antibody against β1 (not shown). Neuronal processes produced by control cells after serum withdrawal did not carry prominent lamellipodia. As a consequence, a specific distribution of α6β1 was not found in these cells (not shown).
Figure 7.
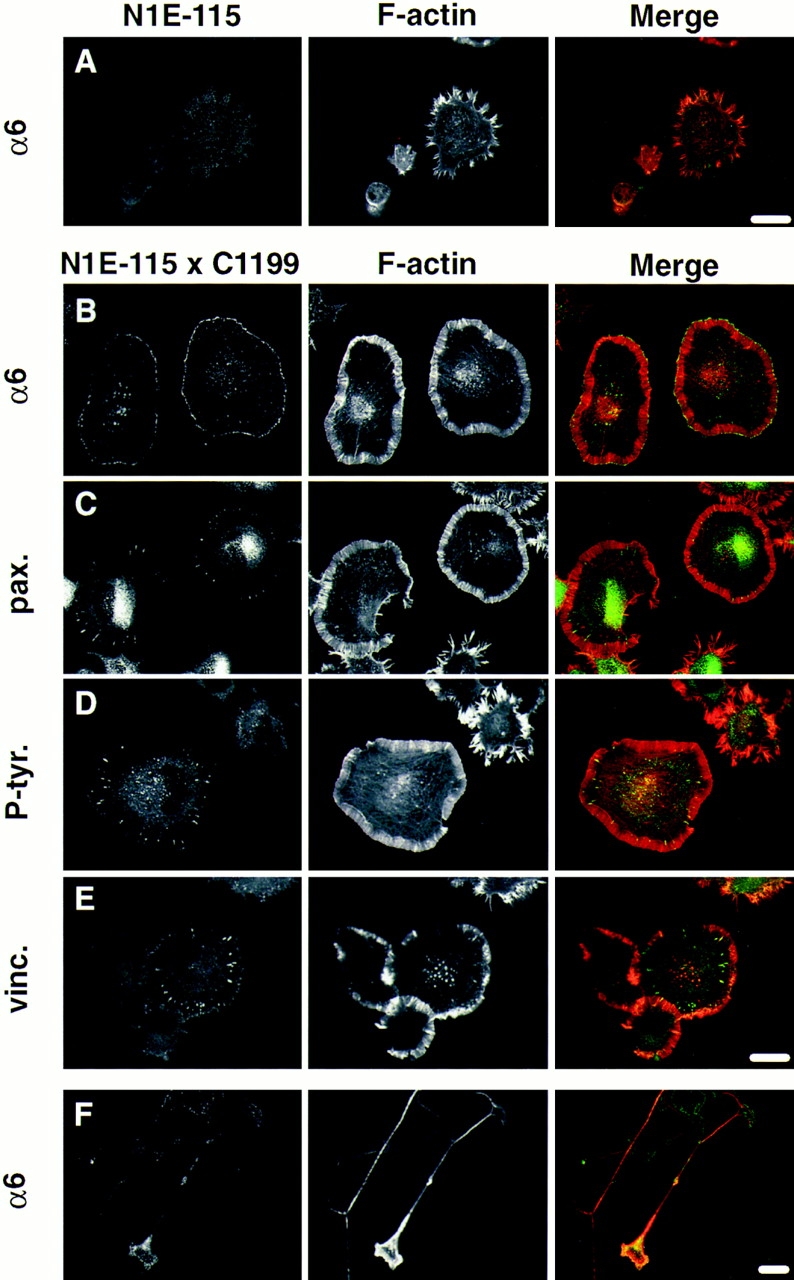
The integrin α6β1 but not focal adhesion components are enriched in adhesive complexes at the cell periphery of cells expressing C1199 Tiam1. Confocal images of C1199 Tiam1– expressing cells grown for 24 h on laminin-coated coverslips before fixation. F-actin was detected using TRITC-labeled phalloidin. (A) Distribution of the α6 integrin subunit in control N1E-115 cells. B–F represent examples of N1E-115 cells expressing C1199 Tiam1. (B) Presence of the α6 integrin subunit in adhesive complexes at the cell border of spreading cells overexpressing C1199 Tiam1. (C–E) Paxillin, phosphotyrosine, and vinculin are only detected in structures that resemble focal contacts. (F) Distribution of the α6 integrin subunit in neurite-bearing cells. For antibodies used, see Materials and Methods. Merge: F-actin in red; α6, paxillin, phosphotyrosine, and vinculin in green. Bars, 25 μm.
In Swiss 3T3 fibroblasts, activation of Rac leads to the formation of similar integrin-containing complexes at the leading edge of cells (Nobes and Hall, 1995). These structures contain a number of cytoskeletal proteins like vinculin and paxillin, components of focal contacts that form at the end of stress fibers. Therefore, we looked at the distribution of paxillin, vinculin, and tyrosine-phosphorylated proteins. Although these proteins were clearly present in focal contacts (Fig. 7, C, E, and D) we did not find paxillin, vinculin or phosphotyrosine-containing proteins in α6β1-containing complexes at the cell periphery. Conversely, we did not find α6β1 in focal contacts (Fig. 7 B). Since the antibody that was used to detect α6 was raised against the ligand binding domain of this integrin, it could be argued that α6 integrins present in these focal contacts may have escaped detection because of antigenic site blocking. However, in other cell types this antibody readily detects focal adhesions (Hogervorst et al., 1993). Therefore, our results suggest that other integrins are present in these structures. The fact that focal contacts were only seen after overnight growth on laminin and could also be detected on fibronectin (not shown) suggests that the formation of these structures is promoted by extracellular matrix components other than laminin. We propose that the Tiam1/Rac1- induced adhesive complexes, as they occur at the cell periphery, are involved in lamellipodia formation and/or may act to stabilize advancing lamellae in developing growth cones. We further conclude that in N1E-115 cells, these α6β1-containing complexes are different from focal contacts.
Relative Levels of Rac and Rho Activity Determine Neurite Formation in N1E-115 Cells
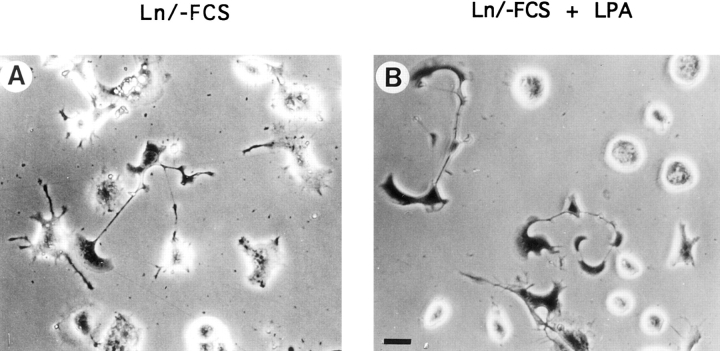
Readdition of serum to serum-starved N1E-115 cells leads to rapid neurite retraction and (transient) cell rounding (Jalink and Moolenaar, 1992; Jalink et al., 1993). Responsible for this phenomenon is the platelet-derived phospholipid LPA (Jalink et al., 1993), which is present in an albumin-bound form in serum (Tigyi and Miledi, 1992). LPA signals through a G protein–coupled receptor to induce contraction of the cortical actin cytoskeleton in a Rho- dependent manner (Jalink et al., 1994). The fact that C1199 Tiam1– or V12Rac1-expressing cells rapidly produced neurite-like outgrowths, even in the presence of serum, suggested that these cells were no longer responsive to LPA.
To substantiate this observation, cells transiently expressing the C1199 Tiam1 protein were plated on laminin-1 and allowed to adhere in the absence of serum for 6 h. Under these conditions, not only did the Tiam1-expressing cells produce neuronal processes, but also the nonexpressing cells in the population began to spread and to produce short extensions (Fig. 8 A). However, 5 min after adding 1 μM LPA to these cultures, ∼80% of the cells that did not express C1199 Tiam1 showed complete cell rounding (Fig. 8 B). In contrast, cells expressing C1199 Tiam1, identified by LacZ staining, did not show any cell rounding or neurite retraction (Fig. 8 B). These results demonstrate that Tiam1-induced activation of Rac prevents Rho-dependent neurite retraction and cell rounding. Also, in view of the apparent loss of contractility accompanied by extreme spreading that occurs in cells highly expressing C1199 Tiam1 (Fig. 3 F), we suspect that activation of Rac1 leads to inhibition of the Rho pathway. Neurite retraction and cell rounding was restored by coexpression of constitutively active V14RhoA (see below), suggesting that regulation of the Rho pathway by Rac occurs at the level of Rho or upstream, rather than downstream.
Figure 8.
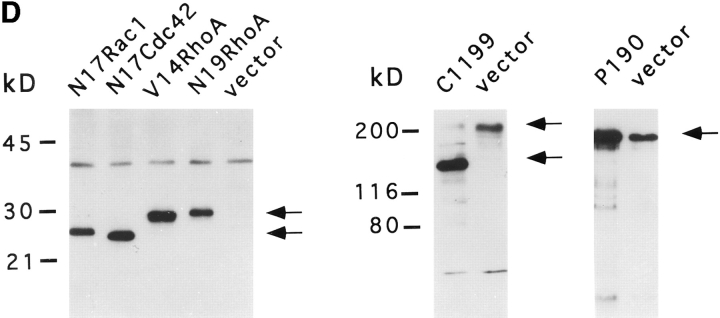
Effects of Rac and Rho activity on Tiam1-induced morphology. (A and B) N1E-115 cells expressing C1199 Tiam1 no longer respond to LPA. Phase contrast micrographs of serum-deprived N1E-115 cells on a laminin substrate. Transfected cells were identified after fixation by LacZ staining. (A) Untreated serum-deprived cells. (B) Serum-deprived cells exposed to 1 μM LPA for 5 min before fixation and lacZ staining. Note that C1199 Tiam1–expressing cells do not respond to LPA in contrast to control cells. (C) Quantification of morphological changes induced by Cdc42, Rac and Rho activity. NIE-115 cells stably expressing C1199 Tiam1 were transiently transfected with myc-tagged constitutively active V14RhoA, dominant-negative N17Rac1, N17Cdc42, and N19RhoA as well as untagged p190. All constructs were cotransfected with plasmid pCMV-lacz. Transfected cells were grown overnight in laminin-coated six-well dishes, fixed, and stained for β-galactosidase activity. Morphology of transfected cells was scored as described in Materials and Methods. About 250 cells were scored per well. Numbers represent the mean percentages of two independent experiments. Error bars indicate S.E.M (n = 2). (D) Expression levels of the transfected constructs in the cell populations as investigated in C. Myc-tagged GTPases (left), Tiam1 (middle), and P190 (right) in N1E-115 cells stably expressing C1199 Tiam1. Total cell lysates were separated on 7.5 or 6% polyacrylamide gels, transferred to nitrocellulose, and immunoblotted. In the anti-myc blot (left), arrows indicate the various transiently expressed GTPases. In the Tiam1 blot (middle), arrows indicate endogenous Tiam1 and C1199 Tiam1. In the p190 blot (right), the signal present in the control lane represents endogenous p190. Bar, 25 μm.
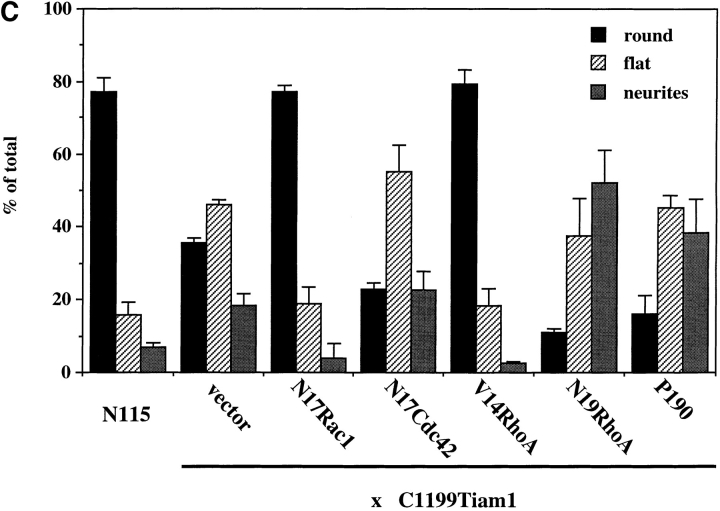
To gain more insight into the contributions of Rac and Rho activity on Tiam1-induced neurite formation, we examined the effects of constitutively active V14RhoA and dominant-negative variants of Rac1, RhoA, and Cdc42 on cells stably expressing Tiam1 after retroviral transduction. These cells expressed moderate levels of the C1199 Tiam1 protein (Fig. 8 D) and showed a less extreme phenotype than cells transiently expressing Tiam1 after transfection. When plated on a laminin substrate and in the presence of serum, ∼50% of these cells showed spreading, whereas another 20% produced neuronal processes. The empty-vector control cells were mostly round and barely produced processes.
Transient transfection of a construct containing either dominant-negative N17 Rac1 or constitutively active V14 RhoA reduced the amount of flat and neurite-bearing cells to levels comparable with the control cells (Fig. 8 C). Conversely, expression of dominant-negative N19RhoA or p190, which has been shown to inactivate RhoA in fibroblasts (Ridley et al., 1993), markedly increased the number of flat or neurite-bearing cells. Transfection of dominant-negative N17 Cdc42 did not affect the Tiam1 phenotype (Fig. 8 C), consistent with Tiam1 activating Rac rather than Cdc42. Expression of C1199 Tiam1 and each of these interfering constructs was confirmed by Western blot analysis (Fig. 8 D). Together, these results lead us to conclude that the induction of these processes by Tiam1 overexpression, is dependent on Rac1- but not Cdc42 activity, and that the relative levels of Rac and Rho activity determine neuronal morphology of N1E-115 cells. Furthermore, our results suggest the existence of a signaling pathway in neuronal cells by which activation of Rac antagonizes Rho activity.
Discussion
Tiam1 Determines N1E-115 Morphology by Activating Rac
Modifications of the actin cytoskeleton and cell–matrix interactions are essential determinants of neurite formation. Rho family members are likely to play an important role in these events (Mackay et al., 1995; Tanaka and Sabry, 1995). Activation of these small GTPases in response to extracellular cues is regulated by GEFs. In this paper, we describe how Tiam1 overexpression affects the morphology of N1E-115 neuroblastoma cells. Transient transfection of a Tiam1 cDNA leads to a sharp change in morphology, characterized by extensive cell flattening and accompanied by a reorganization of F-actin at the cell periphery. Expression of constitutively active V12Rac1, but also V12Cdc42, produces a similar phenotype. The same hierarchical relation between Cdc42 and Rac was shown to exist in Swiss 3T3 cells (Kozma et al., 1995; Nobes et al., 1995). Although we find that activation of Cdc42 can produce a Rac phenotype in N1E-115 cells, the effects of Tiam1 can be reversed by coexpressing dominant-negative N17Rac1, but not N17Cdc42. These results confirm that Tiam1 is involved in Rac signaling, which does not involve Cdc42.
The Effects of Tiam1 on Neurite Formation Are Substrate Dependent
On a laminin substrate, N1E-115 cells expressing C1199 Tiam1, V12Rac1, or V12Cdc42 are rapidly induced to produce neurite-like processes, which at an early stage carry prominent lamellipodia. In contrast, on fibronectin or plastic, expression of C1199 Tiam1 prevents rather than promotes neurite formation, even under conditions that normally induce neurite formation. These differences illustrate the importance of reciprocal interactions between the extracellular matrix and the cytoskeleton during neurite formation. Adhesive interactions between developing neurites and matrix components such as laminin or collagen not only provide the traction that is required to allow directional movement but also induce elaborate changes in the organization of the cytoskeleton (Hynes and Lander, 1992). Moreover, signals downstream of growth factor receptors and integrins converge at several levels to activate the same downstream effectors (Sastry and Horwitz, 1996). For instance, the adaptor protein Grb-2 not only associates with activated receptor tyrosine kinases but also with focal adhesion kinase FAK (Schlaepfer et al., 1994), a component of integrin-containing focal adhesion complexes. Similarly, phosphoinositide-3-kinase is recruited to and activated by growth factor receptors (Cantley et al., 1991), but also binds FAK (Chen and Guan, 1994). Furthermore, lipid second-messengers induced by phosphoinositide-3-kinase have been implicated in the activation of Rac by PDGF and insulin (Wennstrom et al., 1994; Hawkins et al., 1995), which may involve the specific activation and/or localization of guanine nucleotide exchange factors such as Tiam1 (Michiels et al., 1997).
Although the signals responsible for activating Tiam1 and consequently Rac1 in neuronal cells are not known, they may also involve receptor tyrosine kinases. Soluble trophic factors such as NGF and FGF stimulate neurite formation in rat PC12 cells and primary neurons (Chao, 1992). The phenotypic changes that occur in C1199 Tiam1–expressing N1E-115 cells are remarkably similar to the early effects of NGF on the morphology of PC12 cells (Altun-Gultekin and Wagner, 1996). Since Rac was shown to be involved in these responses and PC12 cells express high levels of Tiam1, we are currently using these cells to study the regulation of endogenous Tiam1 in response to tyrosine kinase signaling.
Involvement of the Integrin α6β1 in Tiam1/Rac1-induced Spreading
C1199 Tiam1 expression in N1E-115 neuroblastoma cells promotes adhesion and spreading on laminin, and this adhesion is mediated by the integrin α6β1. Studies in PC12 cells have demonstrated the importance of the integrin α1β1, a laminin/collagen receptor, in mediating cell adhesion and neurite formation (Tawil et al., 1990; Tomaselli et al., 1990; Zhang et al., 1993). Rather than affecting integrin expression, C1199 Tiam1 induces the formation of specific α6β1-containing adhesive complexes at the cell periphery. These complexes are clearly different from focal contacts, which are also detected in Tiam1-overexpressing cells. In fibroblasts, the presence of stress fibers and focal contacts is associated with increased Rho activity and increased contractility (Ridley et al., 1992; Burridge and Chrzanowska-Wodnicka, 1996). In view of our finding that activation of Tiam1 and consequently Rac1 appears to induce loss of contractility and inactivation of the Rho pathway, it may seem contradictory to see prominent focal contacts in the C1199 Tiam1–expressing cells. However, a direct correlation between Rho activity and the formation of focal contacts, as it occurs in fibroblasts, does not appear to exist in N1E-115 cells. In these cells, increased contractility, by activation of Rho, results in cell rounding without the appearance of stress fibers or focal contacts (Jalink et al., 1994).
Opposing Roles for Rac and Rho Regulating Neuronal Morphology
Activation of Rho, induced by the lipid LPA, triggers a rapid contraction of cortical actin cytoskeleton to induce cell rounding and neurite retraction in differentiated N1E-115 cells (Jalink et al., 1994; Kozma et al., 1997; Gebbink et al., 1997). Surprisingly, Tiam1-induced activation of Rac produces the opposite phenotype: cell spreading and the formation of neurites. The fact that cells expressing C1199 Tiam1 no longer respond to LPA suggests that activation of Rac may oppose Rho signaling. A similar conclusion was reached by Kozma et al. (1997), who demonstrated that activation of a muscarinic acetylcholine receptor– induced pathway in N1E-115 cells is dependent on both Cdc42 and Rac and prevents LPA-induced neurite retraction. Since we found that this apparent inhibition of the Rho pathway by Rac is overcome by expression of constitutively active V14RhoA, regulation may occur at the level of Rho or upstream, rather than downstream. The effector molecules responsible for Rac-induced silencing of the Rho pathway are not known. Potential candidates include GAPs, which reduce the activity of Rho-like proteins. At least six RhoGAP proteins have been identified, which show different specificities towards particular Rho family members (Lamarche and Hall, 1994; Reinhard et al., 1995).
We find that one of these proteins, P190, promotes the Tiam1-induced phenotype, presumably by inactivating Rho. In Swiss 3T3 cells, this protein was shown to preferentially inactivate Rho, affecting both cell adhesion and stress fiber formation (McGlade et al., 1993; Ridley et al., 1993). By associating with p120RasGAP, p190 may serve to link the Ras and Rho signaling pathways (McGlade et al., 1993; Settleman et al., 1992). We are currently testing the importance of p190 and other potential GAPs for Rho in the signaling between Rac and Rho.
Role of Tiam1 in Neuronal Development
Expression of Tiam1 in neurons in the brain is developmentally regulated and increases during neuronal migration and differentiation. In the adult, expression remains high in regions of the brain undergoing synaptic remodeling (Ehler et al., 1997). Recently, involvement of Tiam1 in neuronal development was confirmed by Drosophila genetics. Mutations in the Drosophila gene Still-life (sif), which appears to be both structurally and functionally homologous to Tiam1, appear to affect synapse formation during fly development. In addition, transgenic flies which express truncated SIF proteins show specific defects in axonal extension (Sone et al., 1997). The results of our study illustrate how Tiam1, as a specific activator of Rac1, may determine cytoskeletal architecture and adhesion of neuronal cells. Complex interactions with the extracellular matrix involving integrins, and probably other cell adhesion molecules, play an important role in this regulation. Furthermore, a regulatory loop between Rac- and Rho-mediated signaling pathways is revealed. The challenge will be to unravel the molecular mechanisms behind these phenomena.
Acknowledgments
We thank A. Hall, M. Gebbink, R. Cerione, and M. Symons for providing cDNAs or expression constructs; A. Sonnenberg for providing antibody GoH3; Dr. L. Schrama for B-50/GAP-43 antibodies; Dr. M. Glukhova for antivinculin antibodies; L. Oomen for assistance with the confocal microscope and processing of the images; N. Ong for preparing several of the figures; and E. Sander, P. Hordijk, J. Stam, and A. Sonnenberg for critically reviewing the manuscript. We thank W. Moolenaar for suggesting the use of and providing N1E-115 cells.
This work was supported by grants from the Dutch Cancer Society (NKB 94-878) and The Netherlands Organization for Scientific Research (NWO) to J.G. Collard.
Abbreviations used in this paper
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- LPA
lysophosphatidic acid
- NGF
nerve growth factor
- Tiam1
T-lymphoma invasion and metastasis 1
Footnotes
Address all correspondence to Dr. J.G. Collard, The Netherlands Cancer Institute, Division of Cell Biology (H1), Plesmanlaan 121, 1066 XC Amsterdam, The Netherlands. Fax: (+31) 20 512 1944. E-mail: JCOLL@ NKI.NL
References
- Altun-Gultekin ZF, Wagner JA. Src, Ras, and Rac mediate the migratory response elicited by NGF and PMA in PC12 cells. J Neurosci Res. 1996;44:308–327. doi: 10.1002/(SICI)1097-4547(19960515)44:4<308::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bogusky MS, McCormick F. Proteins regulating Ras and its relatives. Nature (Lond) 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Guan JL. Stimulation of phosphatidylinositol 3′ kinase- association with focal adhesion kinase by platelet-derived growth factor. J Biol Chem. 1994;269:31229–31233. [PubMed] [Google Scholar]
- Collard JG. Signaling pathways regulated by Rho-like proteins: a possible role in tumor formation and metastasis. Int J Oncol. 1996;8:131–138. [PubMed] [Google Scholar]
- Ehler E, van Leeuwen F, Collard JG, Salinas PC. Expression of Tiam1 in the developing brain suggests a role for the Tiam1-Rac signaling pathway in cell migration and neurite outgrowth. Mol Cell Neurosci. 1997;9:1–12. doi: 10.1006/mcne.1997.0602. [DOI] [PubMed] [Google Scholar]
- Gebbink MFBG, Kranenburg O, Poland M, van Horck FPG, Houssa B, Moolenaar WH. Identification of a novel putative Rho specific GDP–GTP exchange factor and a Rho binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets GGM, Scholtes EHM, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Habets GGM, van der Kammen RA, Stam JC, Michiels F, Collard JG. Sequence of the human invasion-inducing Tiam1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene. 1995;10:1371–1376. [PubMed] [Google Scholar]
- Hawkins PT, Eguinoa A, Qui RG, Stokoe D, Cooke FT, Walters R, Wenstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide-3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Hogervorst F, Admiraal LG, Niessen C, Kuikman I, Janssen H, Daams H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Lander AD. Contact adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neuronal cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Diff. 1993;4:247–255. [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rüdiger M, Schlüter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42HS and Bradykinin promote the formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1 and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Scharnhorst V, Van der Eb AJ, Zantema A. Inhibition of cyclin-dependent kinase activity triggers neuronal differentiation of mouse neuroblastoma cells. J Cell Biol. 1995;131:227–234. doi: 10.1083/jcb.131.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;10:436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Luo L, Hensh TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature (Lond) 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Nobes CD, Hall A. The Rho's progress: a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. [DOI] [PubMed] [Google Scholar]
- McGlade J, Brunkhorst DA, Anderson D, Mbamalu G, Settleman J, Dedhar S, Rozakis-Adckock M, Chen LB, Pawson T. The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO (Eur Mol Biol Organ) J. 1993;12:3073–3081. doi: 10.1002/j.1460-2075.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GGM, Stam JC, van der Kammen RA, Collard JG. A role for Rac-1 in Tiam1 induced membrane ruffling and T-lymphoma invasion. Nature (Lond) 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Michiels F, Stam JC, Hordijk PL, van der Kammen RA, Ruuls van Stalle L, Feltkamp CA, Collard JG. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-jun NH2-terminal kinase activation. J Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTP-ases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Prendergast GC, Khosravi FR, Solski PA, Kurzawa H, Lebowitz PF, Der CJ. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature (Lond) 1995a;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995b;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Scheel AA, Diekmann D, Hall A, Ruppert C, Bahler M. A novel type of myosin implicated in signalling by rho family GTPases. EMBO (Eur Mol Biol Organ) J. 1995;14:697–704. doi: 10.1002/j.1460-2075.1995.tb07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Self AJ, Kasmi F, Paterson H, Hall A, Marshall CJ, Ellis C. Rho family GTPase activating proteins p190, bcr, and rhoGAP show distinct specificities in vitro and in vivo. . EMBO (Eur Mol Biol Organ) J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Adhesion-growth factor interactions during differentiation: and integrated response. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature (Lond) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature (Lond) 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- Skene JHP, Jacobson RD, Snipes GJ, MacGuire CB, Norden J, Freeman JA. A protein induced during nerve growth, GAP-43, is a major component of growth cone membranes. Science (Wash DC) 1986;233:783–785. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Sone M, Hoshiro M, Suzuki E, Shinya K, Kaibuchi K, Nakagoshi H, Saigo K, Nabeshima Y, Hama C. Still life, a protein in synaptic terminals of Drosophilahomologous to GDP-GTP exchangers. Science (Wash DC) 1997;275:543–547. doi: 10.1126/science.275.5299.543. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature (Lond) 1988;336:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stam, J.C., E.E. Sander, F. Michiels, F.N. van Leeuwen, H.E.T. Kain, R.A. van der Kammen, and J.G. Collard. 1997. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. In press. [DOI] [PubMed]
- Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Tawil NJ, Houde M, Blacher R, Esch F, Reichardt LF, Turner DC, Carbonetto S. α1β1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry. 1990;29:6540–6544. doi: 10.1021/bi00479a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF. A neuronal cell line (PC12) expresses two β1 class integrins -α1β1 and α3β1 that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FN, van der Kammen RA, Habets GGM, Collard JG. Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene. 1995;11:2215–2221. [PubMed] [Google Scholar]
- Van Puijenbroek, A.A.F.L., D.H.J. van Weering, C.E. van den Brink, J.L. Bos, P.T. van der Saag, S.W. de Laat, and J. Hertog. Cell scattering of SK-N-MC neuroepithelioma cells in response to Ret and FGF receptor tyrosine kinase activation is correlated with sustained ERK2 activation. Oncogene. 14:1147– 1157. [DOI] [PubMed]
- Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tarone G, Turner DC. Expression of integrin α1β1 is regulated by nerve growth factor and dexamethasone in PC12 cells. J Biol Chem. 1993;268:5557–5565. [PubMed] [Google Scholar]