Abstract
Lysosomes are dynamic structures capable of fusing with endosomes as well as other lysosomes. We examined the biochemical requirements for homotypic lysosome fusion in vitro using lysosomes obtained from rabbit alveolar macrophages or the cultured macrophage-like cell line, J774E. The in vitro assay measures the formation of a biotinylated HRP–avidin conjugate, in which biotinylated HRP and avidin were accumulated in lysosomes by receptor-mediated endocytosis. We determined that lysosome fusion in vitro was time- and temperature-dependent and required ATP and an N-ethylmaleimide (NEM)-sensitive factor from cytosol. The NEM-sensitive factor was NSF as purified recombinant NSF could completely replace cytosol in the fusion assay whereas a dominant-negative mutant NSF inhibited fusion. Fusion in vitro was extensive; up to 30% of purified macrophage lysosomes were capable of self-fusion. Addition of GTPγs to the in vitro assay inhibited fusion in a concentration-dependent manner. Purified GDP-dissociation inhibitor inhibited homotypic lysosome fusion suggesting the involvement of rabs. Fusion was also inhibited by the heterotrimeric G protein activator mastoparan, but not by its inactive analogue Mas-17. Pertussis toxin, a Gαi activator, inhibited in vitro lysosome fusion whereas cholera toxin, a Gαs activator did not inhibit the fusion reaction. Addition of agents that either promoted or disrupted microtubule function had little effect on either the extent or rate of lysosome fusion. The high value of homotypic fusion was supported by in vivo experiments examining lysosome fusion in heterokaryons formed between cells containing fluorescently labeled lysosomes. In both macrophages and J774E cells, almost complete mixing of the lysosome labels was observed within 1–3 h of UV sendai-mediated cell fusion. These studies provide a model system for identifying the components required for lysosome fusion.
Organelles within the endocytic pathway are dynamic structures, resulting from the fusion and fission of newly internalized vesicles with preexisting structures. The early portion of the endocytic pathway exists in a “steady state.” In the absence of continued membrane internalization the early endocytic apparatus virtually disappears and the constituent membrane and contents are then directed to the cell surface and the lysosome (Tsuruhara et al., 1990; Ward et al., 1995). Whereas the lysosome retains its identity in the absence of continued membrane internalization, it is a dynamic organelle capable of undergoing changes both in morphology and size (Kornfeld and Mellman, 1989; Storrie and Desjardins, 1996). Studies have indicated that lysosomes can undergo self, or homotypic fusion. Acidification of macrophage cytosol results in the fragmentation of lysosomes into vesicles that are removed from their normal perinuclear location to a more peripheral location (Heuser, 1989; Perou and Kaplan, 1993a ). Upon normalization of cytosolic pH, these fragmented lysosomes return to their normal perinuclear location and re-fuse to generate a population of similar size to that found in untreated cells. Perhaps the most dramatic example of homotypic lysosome fusion results from studies in which cells are fused to form a heterokaryon. Lysosomes from each partner (which can be identified through either content markers or species-specific lysosomal membrane proteins [e.g., Lamp-1,2]) within the heterokaryons show complete intermixing of contents and membrane (Deng and Storrie, 1988; Deng et al., 1991; Ferris et al., 1987; Perou and Kaplan, 1993b ).
Although there is abundant evidence that lysosomes can fuse, the mechanisms that regulate fusion, however, are unknown. A diverse series of GTP-binding proteins, ADP ribosylation factors (ARFs) (Balch et al., 1992; Lenhard et al., 1992), rabs (Novick and Grennwald, 1993; Zerial and Stenmark, 1993; Feng et al., 1995), and trimeric G proteins (Bomsel and Mostov, 1992; Colombo et al., 1992b ; Leyte et al., 1992; Pimplikar and Simons, 1993; Vitale et al., 1993; Beron et al., 1995; Nurnberg and Ahnert-Hilger, 1996), have been implicated in regulating fusion events in both the secretory and endocytic pathways (for review see Rothman, 1994; Novick and Garret, 1994; Aridor and Balch, 1996; Rothman and Wieland, 1996). No such proteins have been identified to be either associated with lysosomes or involved in defining lysosome size and shape (Ali et al., 1989). The only known protein that affects lysosome size is the Chediak/beige protein (Perou et al., 1996), which when absent or defective results in abnormally large lysosomes. Recently, the Chediak/beige protein has been identified (Barbosa et al., 1996; Nagle et al., 1996; Perou et al., 1996), although the deduced sequence of the protein yields no clues as to its function.
In an effort to discern the factors responsible for lysosomal fusion we have devised an in vitro homotypic lysosome fusion assay. In this communication we report on the biochemical characteristics of the assay system, its specificity and the minimal requirements for lysosome fusion in vitro. We demonstrate that lysosomes derived from macrophages have a high capacity for homotypic fusion. Whereas fusion appears to require a heterotrimeric G protein and rab(s), it is independent of microtubules.
Materials and Methods
Cells
Rabbit alveolar macrophages were obtained by bronchial lavage (Myrvik et al., 1961) and maintained as previously described (Kaplan, 1980). J774E cells were grown in αMEM (GIBCO BRL, Gaithersburg, MD) containing 10 μg/ml 2-amino-6-mercaptopurine (Sigma Chemical Co., St. Louis, MO) supplemented with 10% FBS (HyClone Laboratories Inc., Logan, UT).
Materials
Unless otherwise noted, all reagents were obtained from Sigma Chemical Co. Human holo-transferrin was iodinated as described previously (Ward et al., 1982). αMacroglobulin was isolated and trypsinized as previously described (Kaplan and Nielson, 1979). Biotinylated horseradish peroxidase (b-HRP)1, avidin D, and anti-avidin were obtained from Vector Labs, Inc. (Burlingame, CA). Mastoparan and mastoparan derivatives were obtained from Peninsula Labs (Belmont, CA). Recombinant NSF and dominant-negative NSF (D1E-Q, Glu329 to Gln) were obtained from Dr. S.W. Whiteheart (University of Kentucky, Lexington, KY). Canine guanine nucleotide–dissociation inhibitor (GDI) and bovine GDI were generous gifts from Dr. A. Wandinger-Ness (Northwestern University, Evanston, IL). ELISA plates were obtained from Corning Glass Works (Corning, NY) and prepared with a 1:200 dilution of anti-avidin as described by Braell (1992). Cytosol was obtained from either J774E, alveolar macrophages, or rat liver. Filtered cytosol was prepared using spin columns (Bio-Rad Laboratories, Hercules, CA).
Subcellular Fractionation
Cells were incubated in 1 mg/ml b-HRP in HMEM/BSA at 37°C for 45 min, washed extensively, and then chased for an additional 90–120 min. Cells were then incubated in 10−8 M 125I-transferrin (125I-Tf[Fe]2) in HMEM/BSA for 45 min at 37°C. Cells were washed extensively, and then homogenized in homogenization buffer (HB) (250 mM sucrose, 20 mM Hepes, 0.5 mM EGTA, pH 7.2, KOH) at 35–50 × 106 cells/ml using a ball-bearing homogenizer to ∼50–75% break up. Homogenates were centrifuged at 800 g for 5 min at 4°C to obtain a postnuclear supernatant (PNS). PNSs were centrifuged for 3 min at 16,000 g to yield crude cytosols and vesicle pellets. Vesicle pellets were resuspended in a small volume of HB and fractionated over 23% Percoll (J774 E cells) or 27% Percoll (alveolar macrophages) as described previously (Ward et al., 1990a ,b). Gradients were fractionated and HRP activity was assayed on an ELISA plate using 0.8 mg/ml O-phenylenediamine and 0.02% H2O2 in citrate-phosphate buffer, pH 5.0. The reaction was stopped by addition of 6N HCl and read at 490 nm on a kinetic microplate reader (Molecular Devices, Sunnyvale, CA). β-Galactosidase activity was assayed using methylumbelliferyl-β galactoside (Sigma Chemical Co.) as a substrate. 125I-Tf activity was measured on an auto-gamma 5780 counter (Packard Instrument Co., Meriden, CT).
In Vitro Fusion Reaction
Cells were incubated with either 0.5 mg/ml b-HRP or avidin at 37°C for 45–60 min in Hanks' minimal essential medium (HMEM). Cells were washed extensively and chased for an additional 90–120 min. Cells were washed and homogenized in HB, and then a PNS was obtained as described above. (Typically the vesicle protein concentration was 1–2 mg/ml with a cytosol protein concentration ranging from 2–4 mg/ml). PNSs and/ or lysosomes (isolated as described) were combined at 4°C in the presence of excess (20–100 μg/ml) b-insulin. Fusion reactions were performed in 250 mM sucrose, 2 mM DTT, 1 mM MgCl2, 0.5 mM EGTA, 50 mM KCl, and 20 mM Hepes, pH 7.2 (fusion buffer), plus or minus an ATP regenerating system as described previously (Colombo et al., 1992a ) using O-phenylenediamine. Reactions were stopped by placing samples at 0°C and solubilizing in 0.05% Triton X-100, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1 mg/ml heparin, 20–100 μg/ml biotinylated insulin. Samples were centrifuged at 16,000 g, for 3 min, and the supernatants assayed for avidin– b-HRP complex formation using precoated anti-avidin ELISA plates as described (Braell, 1992). In vitro fusion was expressed as a percentage of the maximum avidin–b-HRP complex formation in samples solubilized in the absence of biotinylated insulin. All experiments were performed a minimum of three times.
Lysosome Isolation
Lysosomes were isolated by fractionating over Percoll gradients (Ward et al., 1990a ), pooling of fractions corresponding to HRP and lysosomal enzyme activity (typically 3–7 of a 20-fraction gradient). The Percoll was then removed by high speed centrifugation (220,000 g for 2 h).
Microscopy Studies
J774E cells or rabbit alveolar macrophages were incubated in HMEM containing either 1 mg/ml fluorescein-dextran (10,000 mol wt) or 0.5 mg/ml Texas red–dextran (10,000 mol wt) (Molecular Probes, Inc., Eugene, OR) at 37°C for 30 min. Cells were washed and incubated in HMEM at 37°C for an additional 90 min. The two cell populations were then washed, mixed, and fused using UV sendai fusion as per Perou and Kaplan (1993b). After specified times cells were visualized using an inverted fluorescent microscope (Nikon Inc., Torrance, CA) with a ×100 oil immersion objective (Nikon Inc.). Images were acquired using Oncor image analysis software as previously described (Ward et al., 1995).
Preparation of Microtubules
Phosphocellulose-purified bovine brain tubulin was a gift from Dr. D. Gard (Department of Biology, University of Utah, Salt Lake City, UT). Microtubules were prepared by serially adjusting 0.66 mg/ml tubulin in 80 mM Pipes, pH 6.8, 1 mM EGTA, 1 mM MgCl2, and 1 mM GTP to 0.2 μM, 2.0 μM, and 10 μM taxol with incubations at 4°C for 5 min at each step. This solution was added to reaction mixtures containing 10 μM taxol to achieve a final concentration of 0.033 mg/ml tubulin. The presence of polymerized microtubules was confirmed by immunofluorescence using a monoclonal anti–β-tubulin (ICN Biomedicals, Inc., Costa Mesa, CA) and a Texas red– conjugated goat anti–mouse secondary (Molecular Probes Inc.). For in vitro studies on microtubules, cytosol was treated with microtubule depolymerizing agents before the in vitro fusion reaction. Protein determinations were performed as described (Lowry et al., 1951).
Results
Demonstration of In Vitro Homotypic Lysosome Fusion
The fusion assay relies on the formation of a b-HRP–avidin complex within lysosomes, with the subsequent capture of the complex by anti-avidin antibodies as originally described by Braell (1992). One population of cells was incubated with b-HRP, whereas the other cells were incubated with avidin. Cells were exposed to the ligands and subsequently chased in ligand-free media such that the ligands were localized in the lysosome. PNSs or purified lysosomes were mixed together in the presence of b-insulin to capture any released avidin, and samples were incubated at different temperatures and conditions. At the end of the incubation samples were treated with detergent, avidin– b-HRP complex captured by immobilized antibody, and the amount of complex was determined by measuring HRP activity. Both b-HRP and avidin are mannose-terminal glycoproteins, and are internalized via the mannose-terminal glycoprotein receptor. Experiments were performed using rabbit alveolar macrophages or a clone of the murine macrophage cell line, J774E, as these cell types express high levels of mannose-terminal glycoprotein receptors.
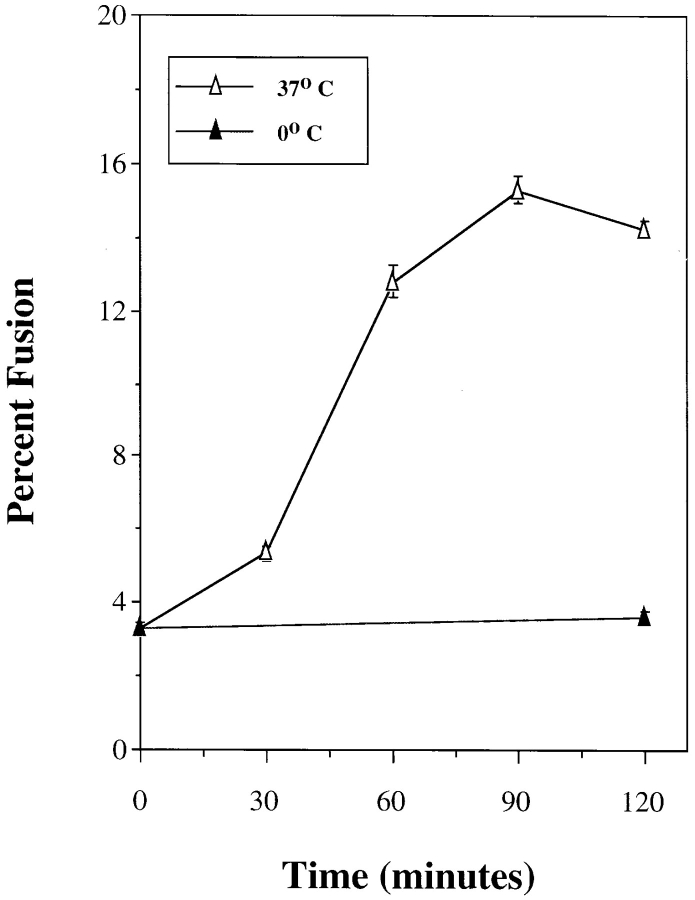
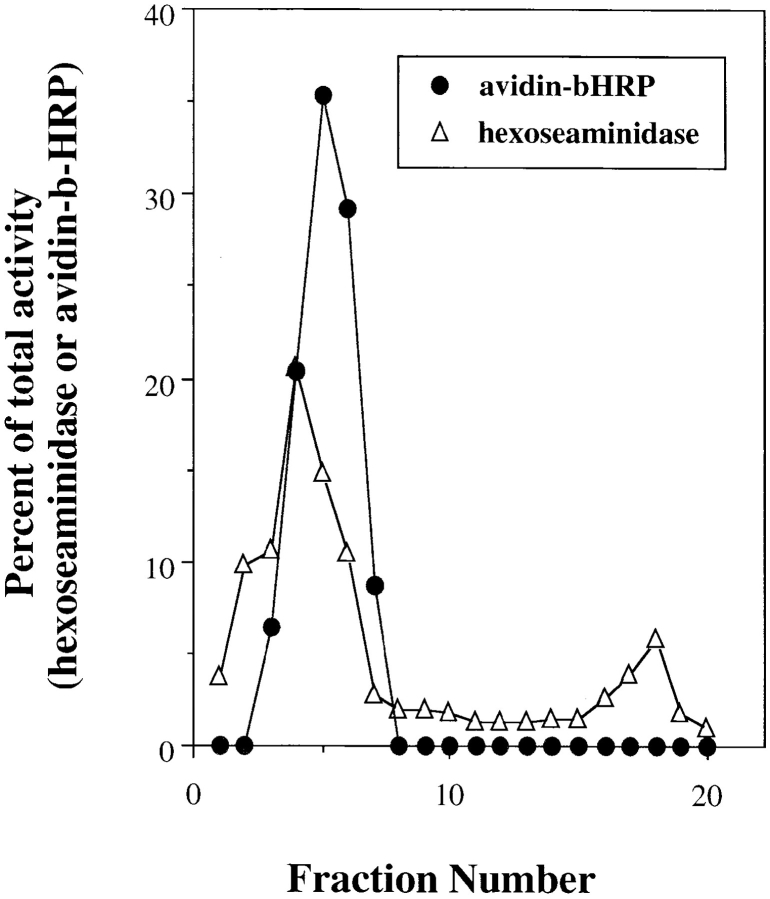
Using the protocol described in Materials and Methods we determined that the pulse–chase procedure resulted in the localization of >95.0% of the cell-associated ligand within lysosomes as assessed by Percoll gradients. When PNSs were mixed and incubated in vitro, there was a time- and temperature-dependent formation of avidin–b-HRP complexes. Approximately 10–20% of the initial b-HRP activity was found associated with avidin in PNSs incubated at 37°C for 60 min; whereas at 0°C, minimal avidin– b-HRP complex formation was observed (Fig. 1). Similar results were obtained using rabbit alveolar macrophages (data not shown). Since the presence of b-insulin blocked the formation of adventitious avidin–b-HRP complexes released as a result of vesicle breakage, formation of avidin–b-HRP complexes could only result from vesicle fusion. The contents of an alveolar macrophage fusion assay were fractionated on Percoll gradients before addition of detergent. Avidin–b-HRP complexes cosedimented with the lysosomal marker hexoseaminidase indicating that the complex was a result of fusion and not binding of free ligands (Fig. 2). The data indicate that the products of the in vitro fusion may result in a slight change in the buoyant density of lysosomes, the avidin–b-HRP peak and hexoseaminidase peaks do not completely coincide. One explanation of this result is that the fusion products may be larger and consequently be less dense.
Figure 1.
Time and temperature dependent in vitro lysosome–lysosome fusion. J774E cells were incubated at 37°C for 45–60 min with either 0.5 mg/ml avidin or 0.5 mg/ml b-HRP. Cells were washed extensively and chased for an additional 90–120 min, and then homogenized. The homogenate was centrifuged at 800 g, for 5 min to obtain a PNS. Typically the vesicle concentration was 1–2 mg/ml with a cytosol protein concentration ranging from 2–4 mg/ml. PNSs from cells labeled with either b-HRP or avidin were combined in the presence of excess b-insulin (20–100 μg/ml), 1 mM MgCl2, 2 mM DTT, 50–75 mM KCl, ± an ATP-regenerating system at 0°C or 37°C for specified times. At the end of the incubation period, vesicles were solubilized in a Triton X-100–containing buffer with excess b-insulin and assayed as described in Materials and Methods. The percent in vitro fusion was calculated using the maximum avidin–b-HRP complex formation in the absence of b-insulin as the denominator.
Figure 2.
Percoll Gradients of in vitro lysosome–lysosome fusion. In vitro fusion was performed as described in Fig. 1 using alveolar macrophages. At the end of the fusion reaction, the PNSs were loaded onto 27% Percoll gradients and subcellular fractionation performed as described in Materials and Methods. Alveolar macrophages yield a single peak of lysosomal activity (Ward et al., 1990). Fractions were assayed for hexoseaminidase activity as well as avidin–b-HRP complex formation in the presence and absence of excess b-insulin. The data are expressed as a percentage of the total activity.
Specificity of Lysosome Fusion
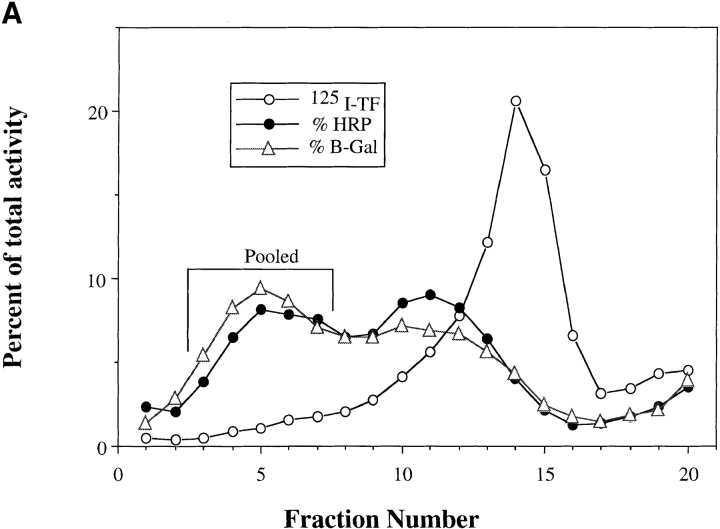
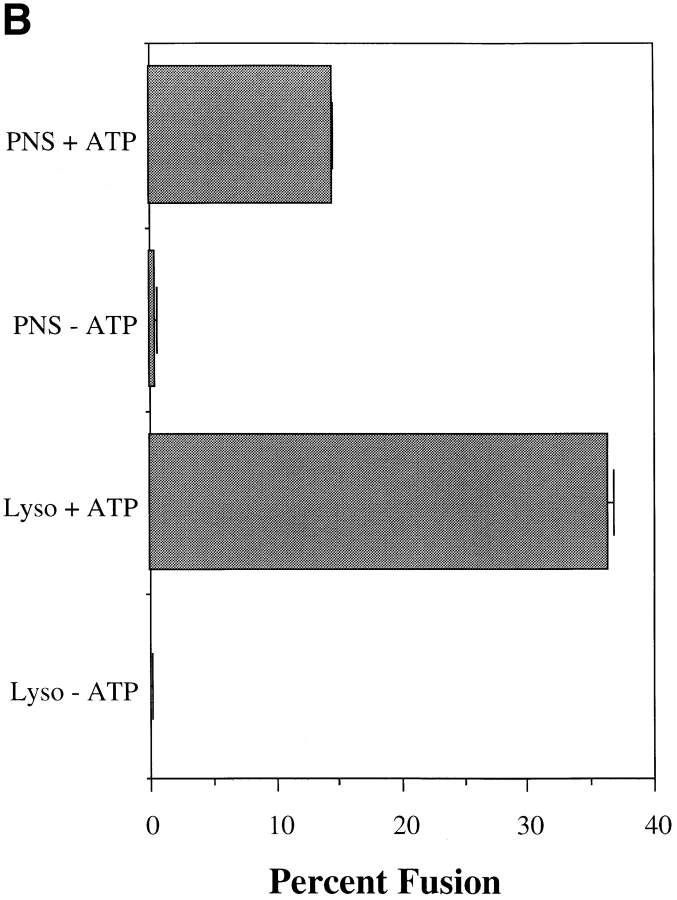
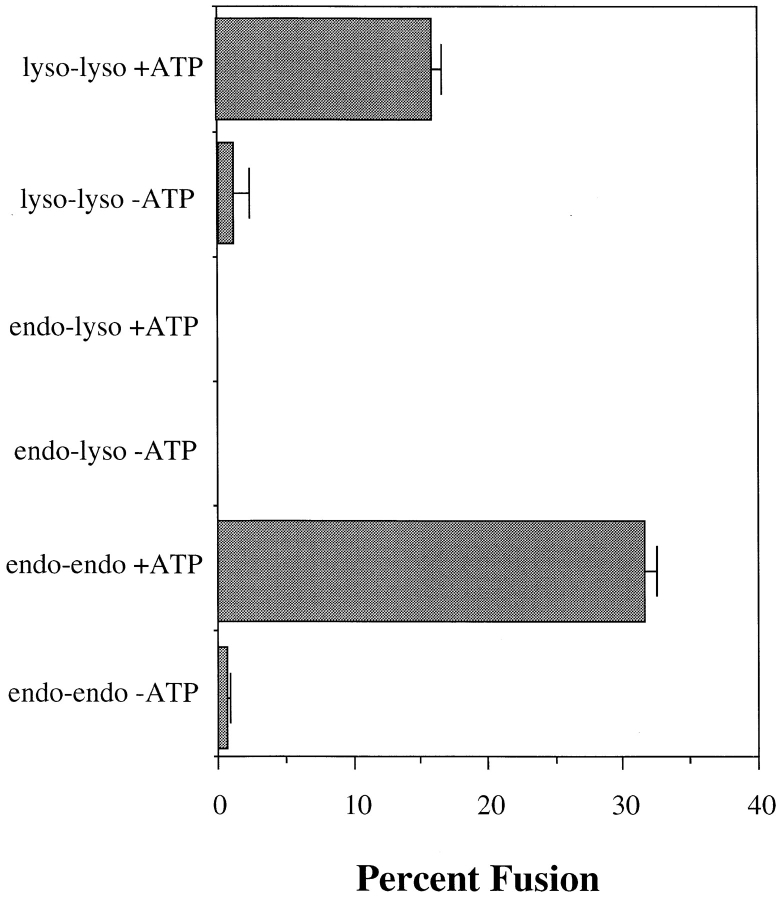
PNSs contain many vesicle populations. It is possible that the ligand may not be solely restricted to lysosomes, and that complex formation results from other vesicles, e.g., endosomes fusing with each other or with lysosomes. To address this point, lysosomes purified from Percoll gradients were used in the fusion assay. PNSs from either avidin- or b-HRP–loaded J774E cells were layered over 23% Percoll and centrifuged to separate lysosomes from endosome/Golgi vesicles. Previously, we demonstrated that Golgi as well as late endosomes are fairly coincident with early endosomes, but are well separated from lysosomes on Percoll gradients (Ajioka and Kaplan, 1987; Ward et al., 1990a ,b). As demonstrated in Fig. 3 A, lysosomes in J774E cells show a biphasic buoyant density in which ∼50% of the lysosomes, as defined by β-galactosidase or hexoseaminidase activity (data not shown), were well separated from 125I-Tf–containing endosomes. Lysosomes in many cell types show heterogeneous buoyant densities (Kjeken et al., 1995; Cuervo et al., 1997). Alternatively, the less dense peak of hexoseaminidase may also represent a late endosomal population. Studies are currently underway to determine the fusogenic nature of different vesicle populations along the endocytic pathway. To avoid the possibility of endosomal contamination, the dense fractions of lysosomal activity (fractions 3–7), were pooled and used for a fusion assay in the presence or absence of an ATP-regenerating system (Fig. 3 B). The degree of fusion seen using this enriched lysosome preparation was equal to or greater than that seen using the corresponding PNS. In many experiments the degree of fusion was as high as 30% of the input b-HRP.
Figure 3.
Lysosome isolation and in vitro fusion. J774E cells were incubated with either b-HRP or avidin as described in Fig. 1. Cells were then incubated with 125I-Tf(Fe)2 at 37°C for 30 min. PNSs were obtained and centrifuged at 10,000 g, for 30 min. Pellets were resuspended in homogenization buffer and layered over 23% Percoll density gradients. Samples were centrifuged at 59,000 g for 27 min and fractionated into 20 samples. A is a representative gradient showing endosomes (125I-TF) and lysosomes (HRP as well as β-Gal activity). Fractions 3–7 were pooled and centrifuged at 220,000 g for 90 min to remove Percoll. The lysosomal pellet was obtained and washed once in HB before resuspension in either fresh or frozen cytosol. In vitro fusion was performed on PNS as well as lysosome pellets and the percent fusion determined (B) as described in Materials and Methods.
The specificity of the fusion reaction was further demonstrated using rabbit alveolar macrophages. Percoll gradient fractionation of cellular homogenates showed a single buoyant density peak of lysosomes that was well separated from both early and late endosomes (Ward et al., 1990a ,b). Previous studies using macrophages determined that brief exposure of cells to endocytic ligands results in the localization of those ligands strictly to the early endocytic apparatus (Diaz et al., 1988; Ward et al., 1990b ). Homotypic in vitro fusion can readily be demonstrated for purified early endosomes as well as purified lysosomes (Fig. 4). However, no evidence of fusion between early endosomes and lysosomes was observed, indicating again the highly selective nature of the fusion process. These results support other studies that have demonstrated the specificity of homotypic vesicle fusion (Diaz et al., 1988; Gruenberg et al., 1989; Colombo et al., 1992).
Figure 4.
In vitro endosome–endosome and lysosome–lysosome in alveolar macrophages. Cells were incubated at 37°C with 0.5 mg/ml b-HRP or avidin for either 5 min (endosome) or 45 min, followed by a 90 minute chase period (lysosome). Cells were homogenized, PNS was obtained and layered over separate 27% Percoll gradients, and then subcellular fractionation was performed as described in Fig. 3. Endosomes (fractions 11–16) and lysosomes (fractions 3–7) were isolated and Percoll removed before in vitro vesicle fusion as described in Materials and Methods.
Homotypic Lysosome Fusion In Vivo
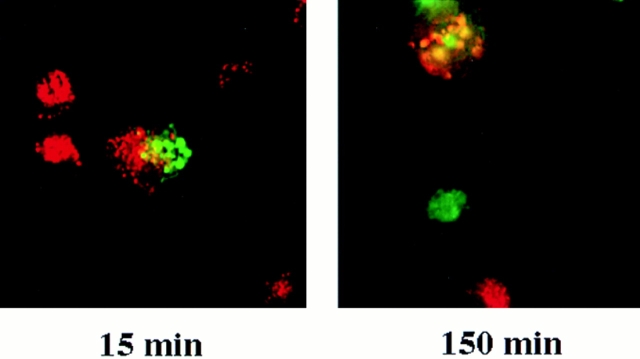
To demonstrate that the high degree of fusion was not due to an in vitro artifact, we assessed the degree and rate of lysosome fusion using an in vivo experiment. Cells, containing different fluorescently labeled lysosomes, were fused together and lysosome fusion assessed by the coincidence of the two fluorescent dyes. Cells, labeled separately with either fluorescein-dextran or Texas red dextran, were fused using UV sendai virus as described (Ferris et al., 1987; Perou and Kaplan, 1993b ). Heterokaryons began showing a small amount of coincidence of fluorescent dextrans as early 15 min with almost complete coincidence evident at 1–3 h (Fig. 5). Nonfused cells on the same cover slip did not contain the second dye, eliminating the possibility that cells released and reaccumulated the fluorescent dye. Multiple fields were scanned and in fused cells the majority (70–90%) of lysosomes contained both dyes. This result indicates that homotypic lysosome fusion is not a rare event but in fact is highly frequent.
Figure 5.
Lysosome–lysosome fusion in vivo. To determine if lysosome–lysosome fusion occurred in vivo, alveolar macrophages were loaded with either 1 mg/ml FITC-dextran or 0.5 mg/ml Texas red–dextran at 37°C for 30 min. Cells were washed and incubated for an additional 90 min in HMEM. Cells were then fused using UV sendai virus protocols as described in Materials and Methods. Cells were washed, plated onto glass coverslips, and then placed back in HMEM at 37°C for various times and examined by fluorescence microscopy.
Biochemical Requirements of Homotypic Fusion
In vitro fusion was dependent upon cytosol, but the origin of the cytosol was irrelevant. Similar results were obtained using cytosol from cultured cells, isolated rabbit macrophages, or mouse liver homogenates (data not shown). Fusion in vitro was ATP dependent, as depletion of endogenous ATP by addition of glucose and hexokinase resulted in the complete inhibition of lysosome fusion (Table I). Treatment of cytosol with NEM resulted in the complete loss of fusion activity, suggesting that homotypic lysosome fusion, like almost all other fusion events, was dependent on an NEM-sensitive factor that may be NSF (Block et al., 1988). Addition of recombinant NSF in the absence of cytosol reconstituted fusion activity. Further, addition of purified NSF completely reconstituted fusion activity in the presence of NEM-treated cytosol, and did not enhance fusion activity when added to untreated cytosol. Under similar conditions a mutant NSF, unable to hydrolyze ATP (D1E-Q, Glu329 to Gln), did not promote fusion. Further, this mutant NSF acted as a dominant negative and inhibited fusion when added back to a complete reaction with cytosol. These results demonstrate that NSF and its ability to hydrolyze ATP are required for homotypic lysosome fusion. The data also suggest that the only soluble protein required is NSF; all other proteins are lysosome bound.
Table I.
Biochemical Requirements for In Vitro Lysosome–Lysosome Fusion
| Experimental treatment | Percent fusion | |
|---|---|---|
| − ATP | 3.6 ± 0.9 | |
| + ATP | 21.4 ± 1.8 | |
| + ATP, wt NSF | 21.0 ± 2.0 | |
| + ATP, + d.m. neg. NSF | 1.5 ± 0.4 | |
| + ATP, − cytosol | 1.8 ± 0.2 | |
| + ATP, + NEM cytosol | 0.8 ± 0.5 | |
| + ATP, − cytosol, + wt. NSF | 21.5 ± 2.0 | |
| + ATP, + NEM cytosol, + wt NSF | 18.0 ± 1.0 | |
| + ATP, + Hexokinase | 1.8 ± 0.0 |
Purified lysosomes from cells incubated with either avidin or b-HRP were isolated as described in Materials and Methods. Lysosomes were pelleted at 16,000 g for 3 min to either remove cytosol or treat cytosol with 1 mM NEM as per Braell (1992). Lysosomes from avidin and b-HRP cells were resuspended in either HB, filtered cytosol, wild-type NSF, dominant-negative NSF (D1E-Q, a Glu329 to Gln), or NEM-treated cytosol in the presence or absence of an ATP-regenerating system and fusion buffer. Hexokinase (25 U/ml) and glucose were added to the fusion reaction as described by Colombo et al. (1992a) and in vitro fusion assessed as described in Materials and Methods.
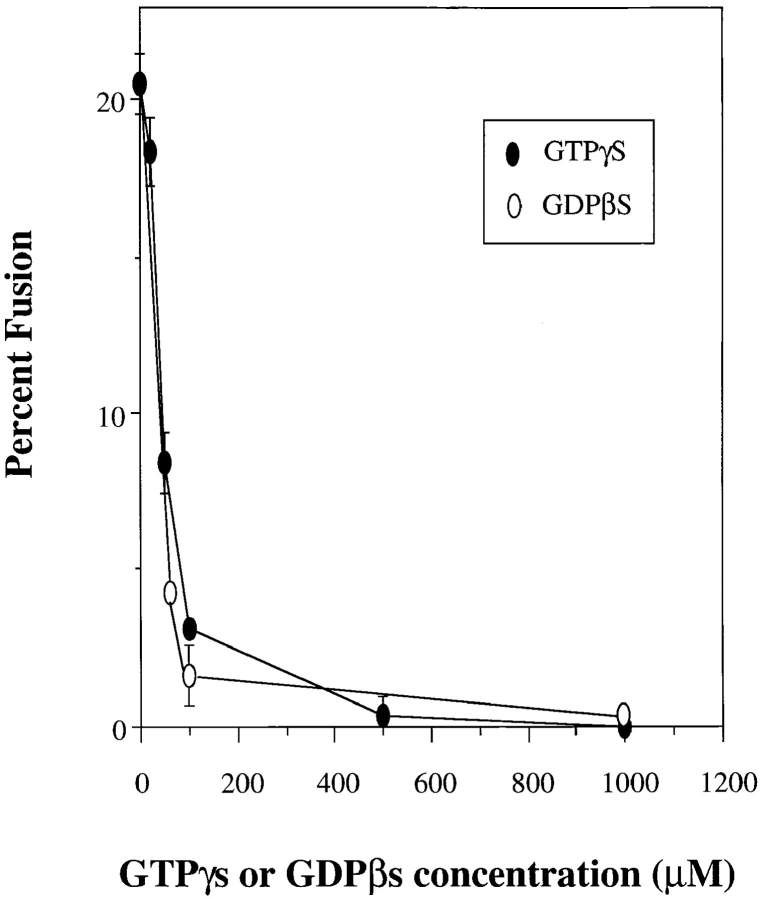
Previous studies on in vivo, as well as in vitro vesicle fusion have demonstrated the role of GTP-binding proteins (for review see Gruenberg and Maxfield, 1995; Robinson et al., 1996). No GTP-binding proteins have been identified that are associated with lysosomes (Ali et al., 1989). Studies in yeast have demonstrated the role of yeast Ypt7 in vacuolar inheritance and vacuole fusion in vitro (Conradt et al., 1992; Haas et al., 1994, 1995). The mammalian analogue of Ypt7 is rab7, which has been shown to be associated with late endosomes (Ali et al., 1989; Aniento et al., 1993; Feng et al., 1995). To determine if a GTP-binding protein may be involved in lysosome–lysosome fusion, we added the nonhydrolyzable analogues GTPγs or GDPβs to the in vitro fusion reactions. Fig. 6 demonstrates that GTPγs and GDPβs inhibited fusion in a concentration- dependent manner.
Figure 6.
Effects of GTPγs and GDPβs on in vitro lysosome–lysosome fusion. Lysosomes from alveolar macrophages loaded with avidin or b-HRP were combined in gel- filtered cytosol and in vitro fusion reactions performed in the presence of an ATP- regenerating system plus or minus various concentrations of GTPγs or GDPβs.
The concentration of GTP analogues that inhibited fusion ranged from 50–200 μM, a range slightly higher than that required for inhibition of other vesicle fusions (Bomsel and Mostov, 1992; Colombo et al., 1992b; Beron et al., 1995). This concentration was similar, however, to that required to inhibit homotypic yeast vacuolar fusion (Conradt et al., 1992; Haas et al., 1994, 1995). The fact that GTPγs inhibits lysosome–lysosome fusion in vitro suggests that a GTP-binding protein(s) is involved. GDI prevents the dissociation of GDP from a variety of rab proteins (Sasaki et al., 1990) and has been shown to inhibit several in vitro vesicle fusion reactions (Dirac-Svejstrup et al., 1994; Elazar et al., 1994; Peter et al., 1994; Acharya et al., 1995; Haas et al., 1995; Ikonen et al., 1995; Turner et al., 1997). Addition of GDI inhibits lysosome–lysosome fusion in vitro, whereas heat-inactivation of GDI before addition to the fusion reaction completely blocked the inhibitory effect (Table II). These data suggest that a rab protein is required for homotypic lysosome fusion.
Table II.
GDI Addition to In Vitro Lysosome–Lysosome Fusion
| Sample | Percent fusion | |
|---|---|---|
| + ATP | 20.9 ± 0.5 | |
| − ATP | 1.0 ± 0.1 | |
| 10 μg/ml bGDI | 0.9 ± 0.6 | |
| 50 μg/ml bGDI | 0.0 ± 0.1 | |
| HI 50 μg/ml bGDI | 15.5 ± 0.7 | |
| 10 μg/ml cGDI | 0.7 ± 0.0 | |
| 50 μg/ml cGDI | 0.0 ± 0.0 | |
| HI 50 μg/ml cGDI | 14.9 ± 0.8 |
Lysosomes were isolated as in Table I. In vitro reactions were performed as described with or without various concentrations of purified canine or bovine GDI. Samples of GDI were also heat inactivated by boiling for 5 min before addition to the fusion reaction. cGDI, is GDI purified from canine; bGDI, is purified from bovine; and HI, heat inactivation.
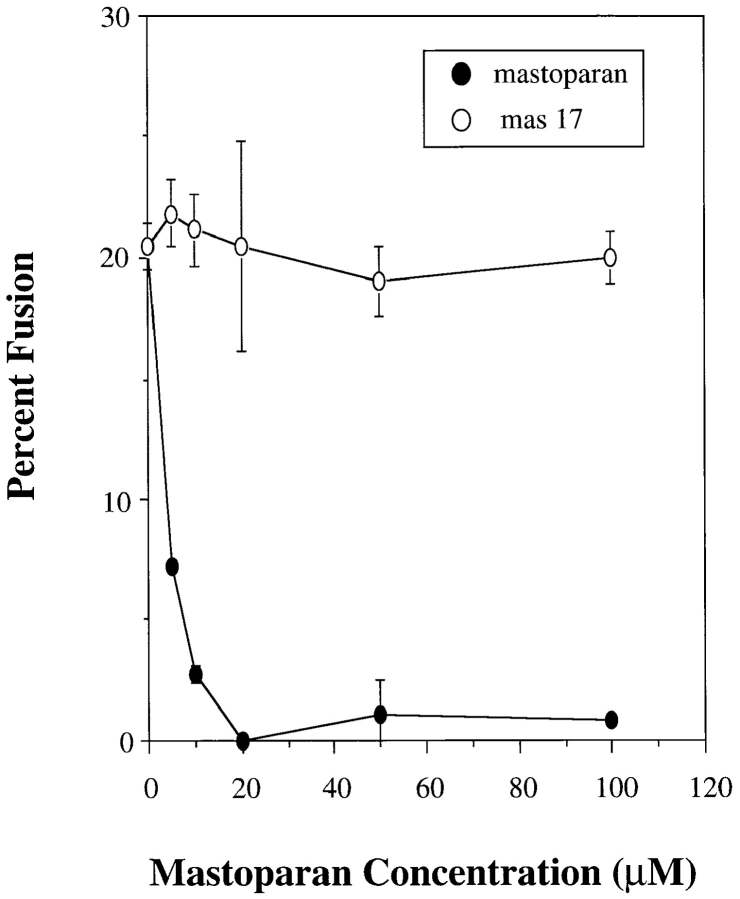
Previous studies have also demonstrated the involvement of heterotrimeric G proteins in vesicle fusion (Colombo et al., 1992b ; Pimplikar and Simon, 1993; Haas et al., 1994). Mastoparan, a heterotrimeric, G protein activator (Fig. 7) inhibited in vitro lysosome fusion, whereas addition of the inactive analogue Mas-17 had no inhibitory effect on in vitro lysosome fusion. To define the heterotrimeric G protein involved in homotypic lysosome fusion, Cholera toxin (Chtx), a Gαs activator and Pertussis toxin (Ptx), a Gαi activator were added to the fusion reaction. Cholera toxin has been shown to specifically activate Gαs and studies on endosome–endosome fusion have demonstrated the role of a heterotrimeric Gαs in early endosome fusion (Colombo et al., 1992b ). Ptx, on the other hand, has been shown to specifically activate Gαi and studies have shown a Gαi to be involved in regulation of apical transport in epithelial cells (Pimplikar and Simon, 1993). As demonstrated in Table III Ptx inhibited in vitro lysosome fusion while Chtx did not inhibit fusion. The inhibitory activity was dependent on the presence of NAD, which activates the toxin.
Figure 7.
Effects of mastoparan and Mas-17 on in vitro lysosome–lysosome fusion. Lysosomes from alveolar macrophages loaded with avidin or b-HRP were treated plus or minus various concentrations of mastoparan or Mas-17. Lysosomes were resuspended in filtered cytosol, combined in the presence of an ATP-regenerating system and in vitro fusion reactions performed as described in Materials and Methods.
Table III.
Effect of Gα Activators on In Vitro Lysosome–Lysosome Fusion
| Sample | Percent fusion | |
|---|---|---|
| + ATP | 33.0 ± 0.5 | |
| − ATP | 2.0 ± 1.0 | |
| 10 μg/ml Ptx | 10.9 ± 0.7 | |
| 50 μg/ml Ptx | 0.5 ± 0.0 | |
| 50 μg/ml Ptx, − NAD | 26.2 ± 0.3 | |
| 10 μg/ml Chtx | 30.9 ± 0.8 | |
| 50 μg/ml Chtx | 32.5 ± 0.4 | |
| 50 μg/ml Chtx, − NAD | 26.9 ± 0.6 |
In vitro fusion was carried out as described in Materials and Methods using purified lysosomes. Toxins were pretreated with DTT preincubation as described previously (Colombo et al., 1992b ). Treated toxin and NAD were added to the vesicles and the complete fusion reaction was added subsequently.
Role of Microtubules in Lysosome Fusion
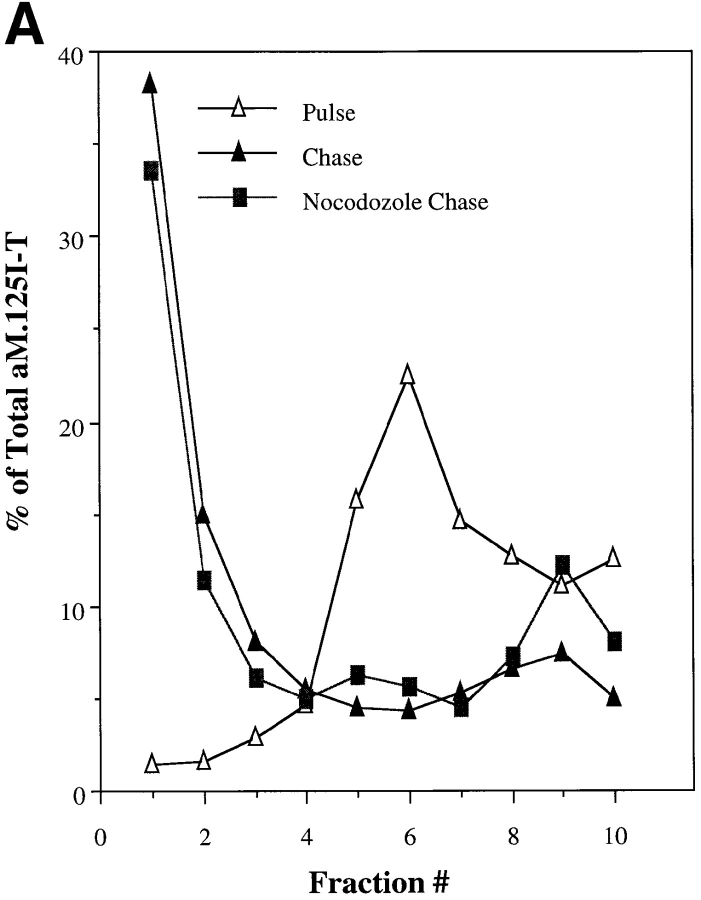
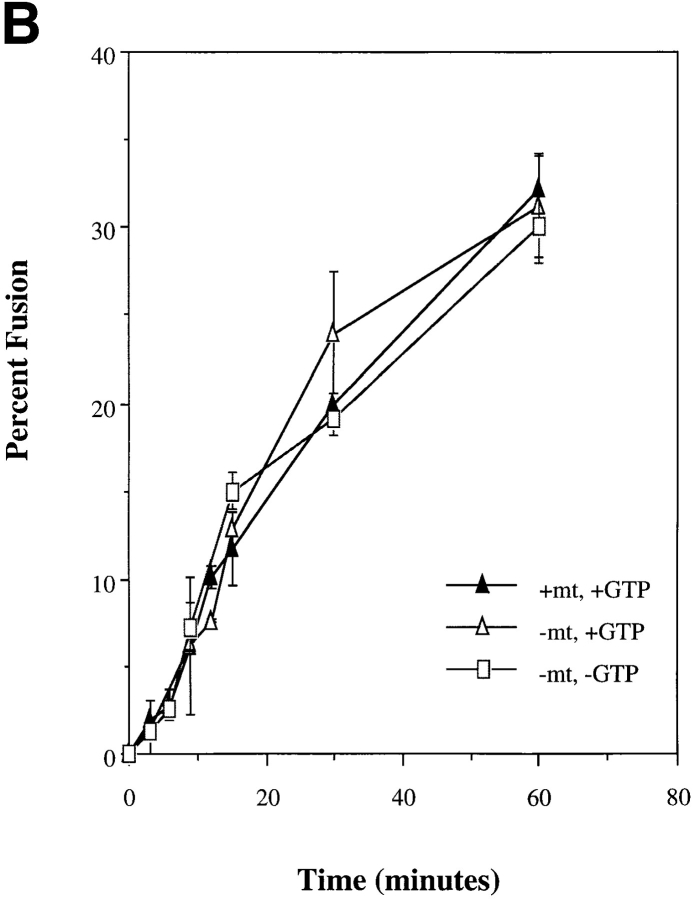
Studies on in vitro endosome–endosome fusion and late endosome–lysosome fusion have demonstrated the need for polymerized microtubules (Gruenberg et al., 1989; Aniento et al., 1993; Mullock et al., 1989, 1994). It is clear that microtubules can affect lysosome distribution and shape in macrophages (Heuser, 1989; Hollenbeck and Swanson, 1990; Knapp and Swanson, 1990; Lin and Collins, 1992; Swanson et al., 1992; Perou and Kaplan, 1993a ; Oh and Swanson, 1996). Their role, however, in affecting lysosomal fusion events is less clear. Incubation of alveolar macrophages with either nocodozole or colchicine resulted in the disruption of microtubules (data not shown) but had minimal effects on movement of internalized ligands through the endocytic system to the lysosome (Fig. 8 A). Similarly, microtubule disruption (data not shown), or addition of taxol-stabilized microtubules did not alter the rate or extent of in vitro lysosome fusion (Fig. 8 B). The presented experiment was performed using a microtubule concentration of 0.033 mg/ml, similar to previous studies (Gruenberg et al., 1989). A fivefold increase or decrease in microtubule concentration had no effect on the extent or kinetics of homotypic lysosome fusion (data not shown). Based on these results we conclude that neither fusion of endocytic vesicles with lysosomes nor homotypic lysosome fusion in alveolar macrophages is dependent upon microtubules.
Figure 8.
(A) Movement of ligand from endosomes to lysosomes in nocodozole-treated alveolar macrophages. Cells were incubated at 37°C for 120 min with or without 10 μM nocodozole. Cells were then pulsed with 10−8 M α macroglobulin (M)125I-trypsin (T) at 37°C for 3 min in the presence or absence of nocodozole. Cells were placed at 0°C, washed, and then placed back at 37°C in the presence or absence of nocodozole for 45 min. Cells were washed, homogenized, and then subcellular fractionation was performed on Percoll gradients. Fractions were assayed for hexoseaminidase and radioactivity. The data are expressed as the percent of αM.125I-T within each fraction. The inset represents the percent movement to lysosomes (coincident with hexoseaminidase or the bottom two fractions) where “pulse” is the amount of radioactivity coincident with lysosomes in cells only pulsed with ligand for 3 min (<5%). “Chase” is the amount of radioactivity coincident with lysosomes in cells incubated for extended times without nocodozole (54%) and nocodozole-treated cells (46%). (B) Kinetics of in vitro lysosome– lysosome fusion in the presence or absence of polymerized microtubules. Isolated lysosomes from alveolar macrophages loaded with avidin or b-HRP were combined for in vitro fusion in the presence or absence of polymerized microtubules as described in Materials and Methods. At various times samples were removed and the amount of fusion determined as described.
Discussion
A variety of studies have defined molecules responsible for both internalization of plasma membrane vesicles, homotypic endosome fusion, and fusion of Golgi vesicles to late endosomes (Lombardi et al., 1993; Pfeffer, 1994; Gruenberg and Maxfield, 1995; Robinson et al., 1996). Similar studies have been carried out in the secretory pathway (Rothman and Wieland, 1996). These studies have used in vitro assays as well as molecular biology techniques to define the machinery for vesicle recognition, fusion, and fission. Such experiments have been buttressed by genetic studies in yeast. In many respects, the machinery for vesicle transport in yeast is homologous with those in mammalian cells, facilitating the identification of putative genes and gene products (Riezman, 1993; Stack and Emr, 1993). The biochemical requirements for yeast vacuolar fusion in vitro, have recently been defined. Vacuolar fusion is involved in vacuolar inheritance (Conradt et al., 1992, 1994; Haas et al., 1994, 1995; Mayer et al., 1996). Less is known, however, about the mechanisms that regulate lysosome fusion and fission in mammalian cells.
Lysosomes are known to obtain membrane and vacuolar contents from endocytosis and de novo–synthesized enzymes from the Golgi apparatus through the late endosome via vesicle fusion events. Membrane fusion was suggested by DeDuve (1963) to explain the relationship between lysosomes, phagosomes, digestive vacuoles, and residual bodies. In general, however, it has not been well appreciated that lysosomes exhibit extensive rounds of self- or homotypic fusion. Studies by Oates and Touster (1980) demonstrated fusion of lysosomes (termed phagolysosomes) in vitro in homogenates obtained from Acanthamoeba castellanii. Storrie and colleagues first observed lysosome fusion in mammalian cells using an assay based on somatic cell fusion (Ferris et al., 1987; Deng and Storrie, 1988). Their studies demonstrated that both the contents and membranes of lysosomes underwent mixing. Perou and Kaplan (1993b) took advantage of the cell fusion approach to devise an assay for the Chediak/beige gene product. Cultured Chediak cells when fused with wild-type cells undergo lysosomal mixing and the fused lysosomes then showed the phenotype of the normal parent. This observation formed the basis of a complementation assay that resulted in the identification of the Chediak/beige gene (Nagle et al., 1996; Perou et al., 1996). These studies also demonstrate that lysosomal mixing occurs and further, that mechanisms exist which regulate the size of lysosomes.
The studies presented here extend these in vivo observations. The in vivo experiments, as demonstrated in Fig. 5, showed that lysosomal fusion is both extensive and rapid. Intermixing of the contents of lysosomes began as early as 15 min of heterokaryon formation where maximum fusion was observed in 90–120 min. It is possible that the observed mixing in vivo may be explained either by unidirectional movement between lysosomes, or by bidirectional movement between late endosomes and lysosomes where late endosomes may also be fusing. The in vitro assay, however, recapitulates the rapidity and extent of lysosomal fusion seen in vivo. Using PNSs as a source of lysosomes, we observed a maximum extent of fusion ranging from 7 to 20% of input b-HRP. When purified lysosomes were used the degree of fusion reached as high as 30% of input b-HRP. Since our system does not detect self-fusion of HRP vesicles or self-fusion of avidin vesicles, we feel that we might be underestimating the actual degree of fusion. There are two explanations why the degree of fusion is higher in the purified lysosome preparation. (a) Lysosomes may aggregate through the isolation procedure and thus be in closer physical proximity; and (b) the purification of lysosomes results in lowered levels of other membranous systems (Golgi, ER, early and late endosomes) that compete for soluble fusion proteins and/or nucleotides. These explanations are not mutually exclusive.
Lysosome fusion relies on cytosolic factors and nucleotides as do most but not all fusion systems. The source of cytosol was irrelevant. Liver, alveolar macrophage, and cultured cell J774E cytosol supported fusion to the same extent. NSF is required for most fusion systems (for review see Rothman and Wieland, 1996) and may well be rate limiting in an in vitro system due to competition by different vesicle systems. Addition of wild-type NSF to the in vitro lysosome–lysosome fusion reaction, in the absence of cytosol, resulted in reconstitution of fusion activity. Addition of an NSF analogue, which is unable to hydrolyze ATP, did not result in lysosome–lysosome fusion. This NSF analogue also acts as a dominant-negative component that inhibited in vitro fusion in the presence of cytosol. The fact that recombinant NSF could replace cytosol in the fusion suggests that all other required macromolecular factors are membrane bound.
Whereas the source of cytosol was irrelevant, what appeared to be relevant was the source of lysosomes and the physiological state of the cells before fractionation. The cultured line J774E gave variable results, with different degrees of fusion depending on the culture conditions. PNSs obtained from cells cultured in 5% serum were consistently less fusogenic then were lysosomes obtained from cells in 10% serum (data not shown). (This applied to in vitro endosome fusion as well.) Lysosomes from alveolar macrophages gave higher degrees of fusion than lysosomes from cultured mouse bone marrow macrophages (data not shown). The reasons for the differences in fusion are unknown. Cell-type differences in lysosomal fusion can, however, be seen in vivo. In alveolar macrophage heterokaryons, lysosome fusion occurs within 1–3 h, while a similar degree of fusion takes 4–6 h in cultured human or murine fibroblasts (Perou and Kaplan, 1993b ). The kinetics of lysosomal fusion using PNS from J774 cells appeared to show a slight lag (compare with Fig. 1), whereas in alveolar macrophages no lag was observed using either PNS or purified lysosomes (compare with Fig. 8). These data further demonstrate that there is cell-type difference in fusion in vivo as well as in vitro.
Multiple studies have identified critical roles for GTP-binding proteins in vesicle trafficking pathways in the secretory and endocytic pathways (Novick and Brennwald, 1993; Pfeffer, 1994; Gruenberg and Maxfield, 1995; Rothman and Wieland, 1996). Studies have localized rabs to a number of different endocytic vesicles. For example, rab4 and rab5 are found on early endosomes, rab7 on late endosomes, and rab9 on trans-Golgi elements (Zerial and Stenmark, 1993). Recently Turner et al. (1997) demonstrated that a rab protein is involved in homotypic ER fusion. To date no rabs, ARFs, or trimeric G proteins have been identified being associated with lysosomes. Our data suggests that such molecules must play a role in homotypic lysosome fusion. Addition of the nonhydrolyzable GTPγs analogue, GDI, or mastoparan, an activator of heterotrimeric G proteins (Higashijima et al., 1988, 1990) inhibits homotypic lysosome fusion. We also observed that treatment of lysosomes with Ptx inhibited fusion. Other studies have demonstrated the involvement of heterotrimeric G proteins in vesicle fusion (Colombo et al., 1992b ; Ktistakis et al., 1992; Pimplikar and Simons, 1993; Haas et al., 1994). Since Ptx requires ARF activity to catalyze incorporation of the target trimeric G protein, this result suggests that an ARF activity must also be membrane bound.
What is the need for extensive homotypic lysosome fusion? One reasonable explanation is that fusion events redistribute luminal contents among the population of lysosomes. Fusion of lysosomes with a late endocytic vesicle results in the contents of that vesicle being subject to the action of lysosomal hydrolases. In many respects, particularly as a result of phagocytosis or macropinocytosis, a single lysosome may fuse with a phagocytic vacuole or a large endocytic vesicle (Racoosin and Swanson, 1993; Oh and Swanson, 1996). The lysosome then obtains material as a bolus. Multiple rounds of lysosomal fusion and fission may guarantee that the amount of substrate in a given lysosome does not overwhelm the amount of lysosomal enzymes available for degradation. Thus, lysosomal fusion may ensure that substrates are always exposed to conditions in which there is an excess of degradative enzymes.
The data in Fig. 2, on alveolar macrophages, gives an indication that in vitro fusion may result in a slight change in buoyant density of lysosomes. The avidin–b-HRP peak and hexoseaminidase peaks do not completely coincide. One explanation of this result is that the fusion products may be larger and consequently less dense.
Whereas our current in vitro assay does not demonstrate it, membrane economics suggest that there must be a corresponding fission event. This is demonstrated by the heterokaryon studies in which extensive lysosome fusion occurs, yet the resulting size distribution reflects the original population (Deng and Storrie, 1988; Deng et al., 1991). Fusion between Chediak and normal cells results in the normal cell phenotype predominating (Perou and Kaplan, 1993b ). Even when fusion occurs between Chediak cells, the resulting lysosome size is not a multiple of the original lysosome but reflects the size distribution of the parental cells. The inescapable conclusion is that once fused, lysosomal fission must occur. Lysosome fusion and fission may not only redistribute the contents of vesicles within cells but generate new lysosomes. Studies on vacuolar inheritance in yeast have shown that fission is required for specific vesicles to be inherited by daughter cells (Warren and Wickner, 1996). Studies are currently underway to determine if lysosome fission is also occurring in vitro and what components are required for lysosome fission.
Lysosomes, as terminal organelles in the endocytic pathway, are continually obtaining solute and membrane from both the endocytic apparatus and the Golgi apparatus. Clearly, increased membrane influx must be balanced by a corresponding loss of membrane (Duncan and Pratten, 1977). There are several possible fates for maintaining membrane homeostasis: recycling to the cell surface, increased degradation (i.e., as seen in multivesicular bodies), and membrane fission. Our data suggests that membrane fission may be a way of dealing with this excess membrane, particularly in conjunction with fusion events. A dynamic fusion/fission system would allow for the generation of new vesicles of a defined size class, providing a uniform size population. Further studies are underway to identify the biochemical requirements for lysosome fusion as well as fission.
Abbreviations used in this paper
- ARF
ADP ribosylation factor
- b-HRP
biotinylated horseradish peroxidase
- b-insulin
biotinylated insulin
- Chtx
Cholera toxin
- GDI
guanine nucleotide–dissociation inhibitor
- HB
homogenization buffer
- HMEM
Hanks' minimal essential medium
- 125I-Tf(Fe)2
125I-transferrin
- αM125I-T
αmacroglobulin-125I-trypsin
- PNS
postnuclear supernatant
- Ptx
Pertussis toxin
Footnotes
Address all correspondence to J. Kaplan, Department of Pathology, Division of Cell Biology and Immunology, University of Utah Health Science Center, Salt Lake City, UT 84132. Tel.: (801) 581-7427. Fax: (801) 581-4517. E-mail: kaplan@bioscience.bilogy.utah.edu
References
- Acharya U, Jacobs R, Peters J-M, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Ajioka RS, Kaplan J. Characterization of endocytic compartments using the horseradish peroxidase/diaminobenzidine density shift technique. J Cell Biol. 1987;104:77–85. doi: 10.1083/jcb.104.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Milligan G, Evans WH. Distribution of G-proteins in rat liver plasma-membrane domains and endocytic pathways. Biochem J. 1989;261:905–912. doi: 10.1042/bj2610905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Balch WE. Timing is everything. Nature (Lond) 1996;383:220–221. doi: 10.1038/383220a0. [DOI] [PubMed] [Google Scholar]
- Balch WE, Kahn RA, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053–13061. [PubMed] [Google Scholar]
- Barbosa MDFS, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RCE, et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature (Lond) 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beron W, Colombo MI, Mayorga LS, Stahl PD. In vitro reconstitution of phagosome-endosome fusion: evidence for regulation by heterotrimeric GTPases. Arch Biochem Biophys. 1995;317:337–342. doi: 10.1006/abbi.1995.1172. [DOI] [PubMed] [Google Scholar]
- Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci USA. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA. Detection of endocytic vesicle fusion in vitro, using assay based on avidin-biotin association reaction. Methods Enzymol. 1992;219:12–21. doi: 10.1016/0076-6879(92)19005-q. [DOI] [PubMed] [Google Scholar]
- Colombo MI, Lenhard JM, Mayorga LS, Stahl PD. Reconstitution of endosome fusion: identification of factors necessary for fusion competency. Methods Enzymol. 1992a;219:32–44. doi: 10.1016/0076-6879(92)19007-s. [DOI] [PubMed] [Google Scholar]
- Colombo MI, Mayorga LS, Casey PJ, Stahl PD. Evidence of a role of heterotrimeric GTP-binding proteins in endosome fusion. Science (Wash DC) 1992b;255:1695–1697. doi: 10.1126/science.1348148. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B, Haas A, Wickner W. Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J Cell Biol. 1994;126:99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JE, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- DeDuve, C. 1963. General properties of lysosomes. In Lysosomes. Ciba Foundation Symposium. A.V.S. de Reuck, and M.P. Cameron, editors. J. and A. Churchill, Ltd. London. 1–35.
- Deng Y, Storrie B. Animal cell lysosomes rapidly exchange membrane proteins. Proc Natl Acad Sci USA. 1988;85:3860–3864. doi: 10.1073/pnas.85.11.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Griffiths G, Storrie B. Comparative behavior of lysosomes and the pre-lysosome compartment (PLC) in in vivo cell fusion experiments. J Cell Sci. 1991;99:571–582. doi: 10.1242/jcs.99.3.571. [DOI] [PubMed] [Google Scholar]
- Diaz R, Mayorga L, Stahl PD. In vitro fusion of endosomes following receptor-mediated endocytosis. J Biol Chem. 1988;263:6093–6100. [PubMed] [Google Scholar]
- Dirac-Svejstrup AB, Soldata T, Shapiro AD, Pfeffer SR. Rab-GDI presents functional Rab9 to the intracellular transport machinery and contributes selectivity to Rab9 membrane recruitment. J Biol Chem. 1994;269:15427–15430. [PubMed] [Google Scholar]
- Duncan R, Pratten MK. Membrane economics in endocytic systems. J Theor Biol. 1977;66:727–735. doi: 10.1016/0022-5193(77)90241-7. [DOI] [PubMed] [Google Scholar]
- Elazar Z, Mayer T, Rothman JE. Removal of rab GTP-binding proteins from Golgi membranes by GDP dissociation inhibitor inhibits inter-cisternal transport in the Golgi stacks. J Biol Chem. 1994;269:794–797. [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: An important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris AL, Brown JC, Park RD, Storrie B. Chinese hamster ovary cell lysosomes rapidly exchange contents. J Cell Biol. 1987;105:2703–2712. doi: 10.1083/jcb.105.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characteristics of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle function in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglamann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiaeis required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO (Eur Mol Biol Organ) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989;108:855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima T, Uzu S, Nakajiama T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins) J Biol Chem. 1988;263:6191–6194. [PubMed] [Google Scholar]
- Higashijima T, Burneir J, Ross EM. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. J Biol Chem. 1990;265:14176–14186. [PubMed] [Google Scholar]
- Hollenbeck PJ, Swanson JA. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature (Lond) 1990;346:864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Evidence for reutilization of surface receptors for alpha-macroglobulin protease complexes in rabbit alveolar macrophages. Cell. 1980;19:197–205. doi: 10.1016/0092-8674(80)90401-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J, Nielson ML. Analysis of macrophage surface receptor. I. Binding of α-macroglobulin-protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979;254:7329–7335. [PubMed] [Google Scholar]
- Kjeken R, Brech A, Lovdal T, Roos N, Berg T. Involvement of early and late lysosomes in the degradation of mannosylated ligands by rat liver endothelial cells. Exp Cell Res. 1995;216:290–298. doi: 10.1006/excr.1995.1037. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Swanson JA. Plasticity of the tubular lysosomal compartment in macrophages. J Cell Sci. 1990;95:433–439. doi: 10.1242/jcs.95.3.433. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Linder ME, Roth MG. Action of brefeldin A blocked by activation of a pertussis-toxin-sensitive G protein. Nature (Lond) 1992;356:344–346. doi: 10.1038/356344a0. [DOI] [PubMed] [Google Scholar]
- Lenhard JM, Kahn RA, Stahl PD. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992;267:13047–13052. [PubMed] [Google Scholar]
- Leyte A, Barr FA, Kehlenbach RH, Huttner WB. Multiple trimeric G-proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO (Eur Mol Biol Organ) J. 1992;11:4795–4804. doi: 10.1002/j.1460-2075.1992.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SXH, Collins CA. Immunolocalization of cytoplasmic dynein to lysosomes in cultured cells. J Cell Sci. 1992;101:125–137. doi: 10.1242/jcs.101.1.125. [DOI] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Riedere MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO (Eur Mol Biol Organ) J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of sec17p (a-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mullock BM, Branch WJ, van Schaidk M, Gilbert LK, Luzio JP. Reconstitution of an endosome-lysosome interaction in a cell-free system. J Cell Biol. 1989;108:2093–2099. doi: 10.1083/jcb.108.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM, Perez JH, Kuwana T, Gray SR, Luzio JP. Lysosomes can fuse with a late endosomal compartment in a cell-free system from rat liver. J Cell Biol. 1994;126:1173–1182. doi: 10.1083/jcb.126.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik QM, Leak ES, Fariss B. Studies on alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961;86:133–136. [PubMed] [Google Scholar]
- Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ, Perou CM, Boissy RE, et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14:307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- Novick P, Brennwald P. Friends and family: the role of the rab GTPases in vesicular traffic. Cell. 1993;75:597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Novick P, Garret MD. No exchange without receipt. Nature (Lond) 1994;269:18–19. doi: 10.1038/369018a0. [DOI] [PubMed] [Google Scholar]
- Nurnberg B, Ahnert-Hilger G. Potential roles of heterotrimeric G proteins of the endomembrane system. FEBS (Fed Eur Biol Soc) Lett. 1996;389:61–65. doi: 10.1016/0014-5793(96)00584-4. [DOI] [PubMed] [Google Scholar]
- Oates PJ, Touster O. In vitro fusion of Acanthamoebaphagolysosomes. I. Demonstration and quantitation of vacuole fusion in acanthamoeba homogenates. J Cell Biol. 1980;68:319–338. doi: 10.1083/jcb.68.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YK, Swanson JA. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol. 1996;132:585–593. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou C, Kaplan J. Chediak-Higashi syndrome is not due to a defect in microtubule based lysosomal mobility. J Cell Sci. 1993a;106:99–107. doi: 10.1242/jcs.106.1.99. [DOI] [PubMed] [Google Scholar]
- Perou C, Kaplan J. Complementation analyses of the Chediak- Higashi syndrome: the same gene may be responsible for the defect in all patients and species. Somatic Cell Genet. 1993b;19:459–468. doi: 10.1007/BF01233251. [DOI] [PubMed] [Google Scholar]
- Perou CM, Moore KJ, Hagle DL, Misumi DJ, Woolf EA, McGrail SJ, Holmgran L, Brody TH, Dussault BJ, Monroe CA, et al. Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet. 1996;13:303–308. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- Peter F, Nueoffer C, Pind SM, Balch WE. Guanine nucleotide dissociation inhibitor is essential for rab1 function in budding from the endoplasmic reticulum and transport through the Golgi stack. J Cell Biol. 1994;126:1393–1406. doi: 10.1083/jcb.126.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, Simons K. Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature (Lond) 1993;362:456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Yeast endocytosis. Trends Cell Biol. 1993;3:273–276. doi: 10.1016/0962-8924(93)90056-7. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature (Lond) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science (Wash DC) 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- Stack JH, Emr SD. Genetic and biochemical studies of protein sorting to the yeast vacuole. Curr Opin Cell Biol. 1993;5:641–646. doi: 10.1016/0955-0674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? . Bioessays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Locke A, Ansel P, Hollenbeck PJ. Radial movement of lysosomes along microtubules in permeabilized macrophages. J Cell Sci. 1992;103:201–209. doi: 10.1242/jcs.103.1.201. [DOI] [PubMed] [Google Scholar]
- Turner MD, Plutner H, Balch WE. A Rab GTPase is required for homotypic assembly of the endoplasmic reticulum. J Biol Chem. 1997;272:13479–13483. doi: 10.1074/jbc.272.21.13479. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T, Koenig JH, Ikeda K. Synchronized endocytosis studied in the oocyte of a temperature-sensitive mutant of Drosophila melanogaster. Cell Tissue Res. 1990;259:119–207. doi: 10.1007/BF00318441. [DOI] [PubMed] [Google Scholar]
- Ward JH, Kushner JP, Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982;257:10817–10323. [PubMed] [Google Scholar]
- Ward DM, Hackenyos DP, Kaplan J. Fusion of sequentially internalized vesicles in alveolar macrophages. J Cell Biol. 1990a;110:1013–1022. doi: 10.1083/jcb.110.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Hackenyos DP, Kaplan SD, Kaplan J. Inhibition of late endosomes-lysosome fusion: studies on the mechanism by which isotonic-K+buffers alter intracellular ligand movement. J Cell Physiol. 1990b;145:522–530. doi: 10.1002/jcp.1041450319. [DOI] [PubMed] [Google Scholar]
- Ward DM, Perou CM, Lloyd M, Kaplan J. ‘Synchronized' endocytosis and intracellular sorting in alveolar macrophages: The early sorting endosome is a transient organelle. J Cell Biol. 1995;129:1229–1240. doi: 10.1083/jcb.129.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Vitale N, Mukai H, Rouot B, Thierse D, Aunis O, Bade MF. Exocytosis in chromaffin cells: possible involvement of the heterotrimeric GTP-binding protein Go . J Biol Chem. 1993;268:14715–14723. [PubMed] [Google Scholar]
- Zerial M, Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]