Abstract
A Saccharomyces cerevisiae mutant in cell division cycle gene CDC48 shows typical markers of apoptosis: membrane staining with annexin V, indicating an exposure of phosphatidylserine at the outer layer of the cytoplasmic membrane; intense staining, using the terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling method, indicating DNA fragmentation; and chromatin condensation and fragmentation. The coordinate occurrence of these events at different locations in the cell, which have no obvious connection except their relation to apoptosis, implies the presence of the molecular machinery performing the basic steps of apoptosis already in yeast. Saccharomyces cerevisiae may prove a suitable model to trace the roots of apoptosis.
Apoptosis is a form of programmed cell death with an important role in development and homeostasis of metazoan organisms. Apoptosis allows the rapid removal of unwanted or damaged cells that could otherwise inflame the surrounding cells with their cytoplasmic contents. In contrast, during necrosis, a form of cell death that results from overwhelming cellular injury, cells lyse and release cytoplasmic material. The apoptotic program is switched on in irreparably damaged or potentially dangerous cells such as self-reactive lymphocytes or cells that have been infected by viruses. Furthermore, it is involved in tumor suppression and in a wide range of diseases such as AIDS, neurodegenerative processes, and ischemic stroke (Steller, 1995).
Apoptotic cells are characterized by a set of distinct morphological changes (Kerr et al., 1972; Wyllie, 1980; Wyllie et al., 1980). An early marker of apoptosis is the exposition of phosphatidylserine on the cell surface, whereas it is normally concentrated in the luminal layer of the cytoplasmic membrane (Martin et al., 1995). DNA is cleaved between nucleosomes (Wyllie, 1980) and the chromatin condenses (Kerr et al., 1972), typically starting as a ring at the inner side of the nuclear envelope (Clifford et al., 1996). Finally, cells break up into membrane-enclosed fragments, the apoptotic bodies (Kerr et al., 1972), which are rapidly phagocytosed and digested by macrophages.
The initiation of apoptosis is a highly coordinated and regulated process. It can be induced or suppressed by a lot of different intracellular and extracellular signals such as Bcl-2 family proteins (Bax, Bak, and Bcl-2), IL-1β–converting enzyme (ICE)1 proteases (caspases), and p53. p53 is the most frequently altered gene in human cancers. DNA damage induces p53 expression, which leads to cell cycle arrest at G1 or to induction of apoptosis (Donehower and Bradley, 1993). Bax induces apoptosis in mammalian cells by the activation of ICE proteases (Chinnaiyan et al., 1996), which mediate the cleavage of several proteins including those of the nuclear matrix and nuclear envelope, finally leading to DNA fragmentation.
Though no proteins homologous to any of these apoptotic regulators have been detected in the yeasts Schizosaccharomyces pombe and Saccharomyces cerevisiae, the expression of Bax or p53 causes a severe effect on cell proliferation. Overexpression of human p53 inhibits cell growth of S. pombe and S. cerevisiae as in higher eukaryotes, however it does not result in an apoptotic phenotype (Bischoff et al., 1992). Expression of Bax in the yeast S. pombe is lethal, coexpression of the anti-apoptotic protein Bcl-2 suppresses this effect. As in mammals, Bcl-2 appears to inactivate Bax by forming mixed dimers: a mutant of Bcl-2 that fails to heterodimerize with Bax does not rescue yeast cell growth (Jürgensmeier et al., 1997). However, Bax-induced cell death in S. pombe is not accompanied by any classical morphological feature of apoptosis. Neither evidence of nuclear fragmentation nor of chromatin margination against the nuclear envelope, nor internucleosomal DNA fragmentation was observed. Jürgensmeier et al. (1997), therefore labeled the Bax-induced effect “cytotoxicity,” to distinguish it from apoptosis.
We investigated a mutant of baker's yeast in cell division cycle gene CDC48 for markers of apoptosis. Cdc48p plays an important role in the homotypic fusion of the endoplasmic reticulum; in vitro, Cdc48p is the only soluble protein necessary for the fusion of ER-derived vesicles (Latterich et al., 1995). A defect in CDC48 results in a cell cycle arrest as a large budded cell with the nucleus located in the neck between the mother and the daughter cell at 16°C (Moir et al., 1982; Fröhlich et al., 1991).
Cdc48p is a member of the AAA family, which is characterized by a highly conserved element of ∼230 amino acid residues, containing an ATP binding consensus sequence that can be present singly or in two copies. A database of the AAA family is available on the World Wide Web at http://yeamob.pci.chemie.uni-tuebingen.de/.
We describe a cdc48 mutant of baker's yeast displaying several of the characteristic morphological markers of apoptosis. This is the first indication that the basic machinery of apoptosis is already present in unicellular lower eukaryotes.
Materials and Methods
Yeast Plasmids and Strains
YEp52/CDC48 was constructed by adding HindIII sites to CDC48 (directly before start ATG and 205 bp 3′ of the stop codon) and cloning the gene into the HindIII site of YEp52 (Broach et al., 1983). The ChameleonTM site directed mutagenesis kit (Stratagene, Heidelberg, Germany) was used for site-directed mutagenesis of plasmid YEp52/CDC48. All mutations were confirmed by DNA sequencing. The codon of serine 565 of CDC48 was mutagenized to a glycine codon (allele cdc48 S565G, plasmid YEP52/cdc48S565G) with oligonucleotide 5′-GGTATGGTGAAGGGGAATCTAACATCC-3′. For selection of mutant plasmids, a unique BglI site in the bla gene of the vector was destroyed with oligonucleotide 5′-CCCTTCCAGCCGGCTGGTTTATTGC-3′.
YEp52/CDC48 or YEP52/cdc48S565G were transformed into strain KFY247 (MAT a/MAT α CDC48/cdc48::URA3 his4-619/his4-619 leu3-3,112/leu2-3,112 ura3-52/ura3-52) and the transformants were sporulated; segregants containing the cdc48::URA3 disruption were recognized by their ability to grow without external uracil. Segregant KFY437 (MAT a cdc48::URA3 his4-619 leu3-3,112 ura3-52 YEp52/cdc48S565G) was used for all cytologic experiments. Segregant KFY417 (MAT a cdc48::URA3 his4-619 leu3-3,112 ura3-52 YEp52/CDC48) with a wild-type CDC48 on YEp52, and isogenic strain KFY439 with intact CDC48 (MAT a CDC48 his4-619 leu3-3,112 ura3-52) were used as wild-type controls. Cell division cycle mutants used as controls were Hartwell (1973) strains LH12021 (cdc31 ts), arresting with an elongated nucleus in a budded cell; LH369 (cdc1 ts), LH395 (cdc19 ts), LH321 (cdc25 ts), and LH17048 (cdc29 ts), all arresting unbudded; LH370 (cdc2 ts), LH244 (cdc9 ts), and LH386 (cdc20 ts), and strains DBY2028 (MAT a ade2 lys2 leu2 ura3 cdc46 ts), and rE24-15 (MAT α his4-619 cdc48-3ts; Moir et al., 1982), all arresting with a large bud. Strains with a disrupted chromosomal copy of CDC48 and with cdc48 alleles, cdc48 S565T (strain KFY438), cdc48 Y834E (strain KFY415), or cdc48 Y834F (strain KFY416), expressed from vector YEp52 were used as controls. Strains KFY438 and KFY415 grow like wild type, KFY416 shows a doubled generation time on glucose-containing media, resembling cdc48 S565G strain KFY437 in this respect.
Cells were grown on YEP (1% yeast extract, 2% Bacto peptone) containing 4% glucose or 4% galactose, respectively. Cultures were inoculated to reach the stationary phase after 36–48 h of fermentation.
For nuclear staining, cells were washed with PBS, incubated with 1 μg/ ml diaminophenylindole (DAPI) in PBS for 10 min, and then rinsed three times with PBS.
Electron Microscopy
Yeast cells were fixed with phosphate-buffered glutardialdehyde, cell walls were removed, and the cells were postfixed with osmium tetroxide and uranyl acetate, and then dehydrated as described by Byers and Goetsch (1991) for stationary phase cells. After the 100% ethanol washes, cells were washed with 100% acetone, infiltrated with 50% acetone/50% Epon for 30 min and with 100% Epon for 20 h. Cells were transferred to fresh 100% Epon and incubated at 56°C for 48 h before cutting thin sections and staining with lead acetate.
Terminal Deoxynucleotidyl Transferase–mediated dUTP Nick End Labeling (TUNEL) Staining
DNA strand breaks were demonstrated by labeling free 3′-OH termini with FITC-labeled deoxyuridine, which was detected with alkaline phosphatase–coupled, anti-fluorescein antibody, and the formation of a dye precipitate with a phosphatase substrate (In Situ Cell Death Detection Kit, AP; Boehringer Mannheim, Mannheim, Germany). Yeast cells were fixed with 3.7% formaldehyde, digested with lyticase, and applied to a polylysine-coated slide as described for immunofluorescence (Adams and Pringle, 1984). The slides were rinsed with PBS, incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 min on ice, rinsed twice with PBS, incubated with 10 μl TUNEL reaction mixture (200 U/ml terminal deoxynucleotidyl transferase, 10 mM FITC-labeled dUTP, 25 mM Tris/HCl, 200 mM sodium cacodylate, 5 mM cobalt chloride; Boehringer Mannheim) for 60 min at 37°C, rinsed three times with PBS, incubated with 50 μl Converter AP solution (alkaline phosphatase– labeled, anti-FITC antibody; Boehringer Mannheim) for 30 min at 37°C, rinsed three times with PBS, and stained by incubation with 50 μl naphthol) AS-MX phosphate (Sigma Chemical Co., Munich, Germany), 0.8 mg/ml, fast red TR salt (Sigma Chemical Co.), 1 mg/ml, 2% dimethylformamide, 1 mM levamisole in 100 mM Tris/HCl, pH 8.2, for 30 min at room temperature. A coverslip was mounted with a drop of Kaiser's glycerol gelatin (Merck, Darmstadt, Germany).
Annexin V Staining
Exposed phosphatidylserine was detected by reaction with FITC-coupled annexin V (ApoAlert Annexin V Apoptosis Kit; CLONETECH Laboratories, Inc., Palo Alto, CA). Yeast cells were washed in sorbitol buffer (1.2 M sorbitol, 0.5 mM MgCl2, 35 mM potassium phosphate, pH 6.8), digested with 5.5% glusulase (Boehringer Mannheim) and 15 U/ml lyticase (Sigma Chemical Co.) in sorbitol buffer for 2 h at 28°C, harvested, washed in binding buffer (10 mM Hepes/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2; CLONETECH Laboratories, Inc.) containing 1.2 M sorbitol buffer, harvested and resuspended in binding buffer/sorbitol. 2 μl annexin-FITC (CLONETECH Laboratories, Inc.) and 2 μl propidium iodide (500 μg/ml) were added to 38 μl cell suspension, and then incubated for 20 min at room temperature. The cells were harvested, suspended in binding buffer/sorbitol, and applied to a microscopic slide.
Results
A Yeast Mutant in Cell Division Cycle Gene CDC48 Accumulates Cells with Disrupted Chromatin
To evaluate the importance of various conserved residues of Cdc48p, we changed a serine residue in the COOH-proximal AAA box of Cdc48p to a glycine residue (allele cdc48 S565G) by oligonucleotide-directed mutagenesis, and put the mutated gene under the control of a GAL1 promoter. (The GAL1 promoter is induced by galactose in the growth medium and repressed by glucose. CDC48 however, expressed from a glucose-repressed GAL1 promoter, enables cell proliferation, indicating a leaky repression.) The plasmid was transformed into a diploid yeast (KFY247), heterozygously disrupted in CDC48, and transformants were sporulated. Spores containing the disrupted cdc48::URA3 do not germinate on glucose medium and need 7 d to form visible colonies on galactose medium. Control spores containing the same vector carrying a wild-type allele of CDC48 in addition to a disrupted chromosomal copy, form visible colonies within 2–3 d on both glucose and galactose media. On glucose medium, cdc48 S565G segregants grow slowly (generation time 185 min instead of 90 min for the control), and cease proliferating at 37°C (ts phenotype). On galactose medium the segregants grow like a CDC48 wild-type control.
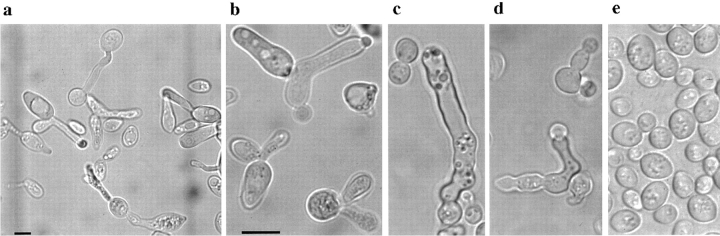
Microscopic observation of stationary phase cultures of these segregants shows abnormally elongated and misformed cells (Fig. 1) with the nuclear chromatin disrupted into several fragments, reminiscent of apoptotic metazoan cells. We systematically investigated the cdc48 S565G strain, KFY437, under different growth conditions for diagnostic markers of apoptosis.
Figure 1.
Morphology of yeast mutant strain KFY437. Stationary phase cells of KFY437 (a–d) and of control strain KFY417 (e) grown on YEP/glucose for 5 d after inoculation (phase contrast). Bars: (a) 10 μm; (b–e) 10 μm.
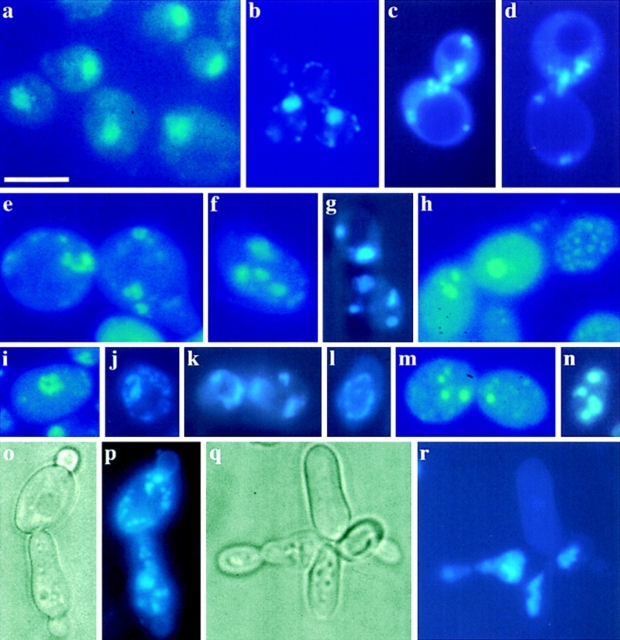
Chromatin condensation and fragmentation is a typical marker of apoptosis. Often, fragments are aligned as a ring close to the nuclear envelope (Clifford et al., 1996). DAPI-stained KFY437 cells from exponentially growing as well as from stationary cultures on glucose medium show a continuous DNA ring within the nucleus in 1–10% of the cells, DNA fragments arranged as a ring at the inner side of the nuclear envelope in 1–20% of the cells, and several randomly distributed nuclear fragments in 10–50% of the cells (Fig. 2). Fragmentation increases with incubation time and after 7 d on glucose medium, 80% of the cells have an abnormal chromatin distribution. In KFY437 cells grown exponentially on galactose, <10% of the cells show chromatin fragmentation. In stationary cultures from galactose medium, 40% of the cells have a fragmented nucleus. Stationary wild-type strains show the nucleus as a single round spot in all cells, their morphology does not change markedly during prolonged incubation (Fig. 2 a). cdc48 point mutants, KFY415, KFY416, and KFY438, show unfragmented nuclei both during exponential growth and in the stationary phase. cdc48-3ts strain rE24-15 arrested at 37°C shows an unfragmented nucleus in the neck between mother and daughter cells (Fig. 2 b). Cell division cycle mutants in genes cdc1, cdc19, cdc20, cdc25, cdc29, and cdc31 that were arrested at 37°C show unfragmented nuclei. Mitochondria appear as dots of far less brightness and size than the nuclei or most nuclear fragments, and are predominantly located near the border of the cells.
Figure 2.
Chromatin fragmentation. DAPI stain (c–n, p, and r) and phase contrast representation (o, and q) of KFY437 cells, and DAPI stain of KFY417 (a) and of cdc48 mutant rE24-15 (b) as controls. i–k were cells grown exponentially on YEP/glucose; a, c–h, and l–n are stationary cells from glucose medium, harvested 2 d after inoculation; o/p and q/r are stationary cells from glucose medium, harvested 5 d after inoculation; b shows exponentially growing cells of rE24-15 that were cell division cycle arrested by a 3-h incubation at 37°C. Bar, 10 μm.
Electron microscopic investigation of exponentially grown KFY437 cultures shows extensive chromatin condensation along the nuclear envelope (Fig. 3, b–d), as well as cells containing nuclear fragments (Fig. 3, e and f). Endoplasmic reticulum is more prominent and its lumen appears wider than in wild-type controls (Fig. 3 a).
Figure 3.
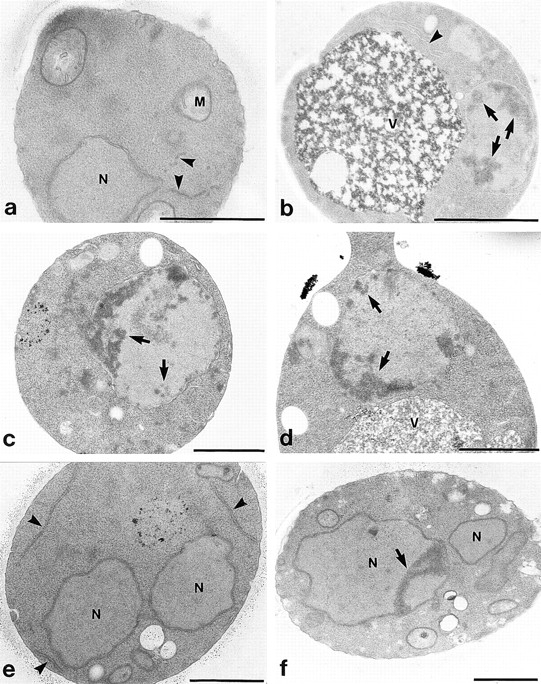
Electron micrographs of yeast mutant strain KFY437. Wild-type control KFY417 (a) and mutant KFY437 (b–f) grown exponentially on YEP/glucose for 24 h. N, nucleus; V, vacuole; and M, mitochondria. Endoplasmic reticulum is marked by arrowheads; chromatin condensation is marked by arrows. Bars, 1 μm.
DNA Is Fragmented in cdc48 Mutant KFY437 after Prolonged Growth
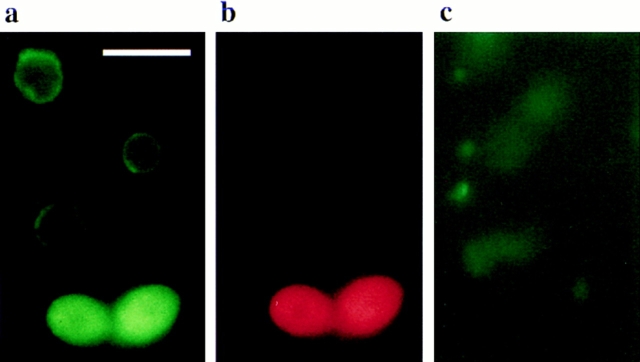
Apoptotic DNA cleavage produces free 3′-OH termini, which can be detected by labeling with modified nucleotides (e.g., DIG-dUTP) catalyzed by terminal deoxynucleotidyl transferase. The TUNEL (Gavrieli et al., 1992; Gorczyca et al., 1993) method is a fast and sensitive way to visualize the amount of DNA fragmentation in individual cells. At the end of the exponential growth phase, both on galactose and on glucose containing media, >80% of KFY437 cells have an intensive red nuclear stain with the TUNEL assay, corresponding to a strong fragmentation (Fig. 4 a). Early exponential cultures of KFY437 contain ∼20% TUNEL-positive cells. Control cultures of wild-type cells (Fig. 4 b), of cdc48 point mutants KFY415, KFY416, and KFY438, of cdc48-3ts strain rE24-15 arrested at 37°C, and of mutants in cell cycle genes cdc2, cdc9, cdc19, cdc31, and cdc46 arrested at 37°C, show unstained or only slightly red nuclei. An aliquot of the protoblasts used for TUNEL staining was tested for integrity by incubation with 23 μg/ ml propidium iodide. Less than 3% of the protoplasts became stained, proving that the DNA fragmentation is not a result of cell necrosis.
Figure 4.
DNA strand breakage visualized by TUNEL staining. Cells of KFY437 (a) and of control strain KFY417 (b) grown on YEP/glucose to the end of the exponential growth phase (36 h after inoculation) were stained for DNA strand breaks with TUNEL. Bar, 10 μm.
Analysis of the isolated chromosomal DNA by agarose electrophoresis did not show a DNA ladder (not shown), that has been found in many apoptotic systems as the result of DNA cleavage between nucleosomes. This may be caused by the S. cerevisiae chromatin structure with approximately no linker DNA between the nucleosomes (Lowary and Widom, 1989). In addition, a number of cell types undergo apoptosis without the occurrence of a DNA ladder, even when chromatin condensation occurs (Oberhammer et al., 1993).
cdc48 Mutant KFY437 Exposes Phosphatidylserine at the Cytoplasmic Membrane
In mammalian cells, phosphatidylserine is predominantly located on the inner leaflet of the plasma membrane, and is translocated to the outer leaflet when apoptosis is induced (Martin et al., 1995). Phosphatidylserine exposure serves as a sensitive marker for early stages of apoptosis. It can be detected with annexin V, which binds to phosphatidylserine with high affinity in the presence of Ca2+. As in mammalian cells, S. cerevisiae has an asymmetric distribution of phospholipids within the cytoplasmic membrane, 90% of the phosphatidylserine is oriented towards the cytoplasm (Cerbón and Calderón, 1991).
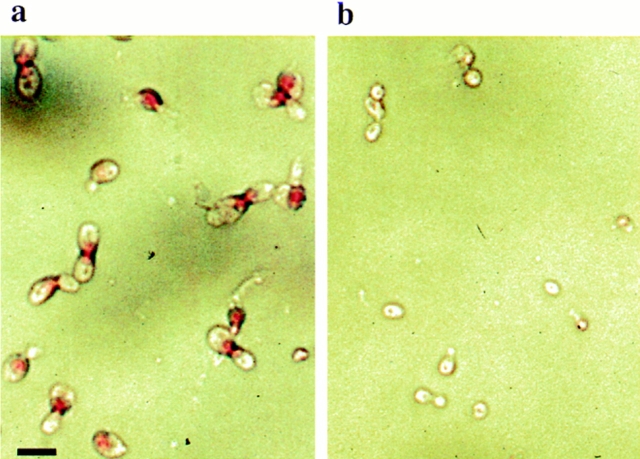
For detection of phosphatidylserine in the outer phase of the cytoplasmic membrane, the cell wall was removed by digestion with lyticase, and the spheroblasts were incubated with FITC-labeled annexin V. More than 70% of the KFY437 cells grown exponentially on glucose medium (Fig. 5 a) and ∼50% of stationary phase cells grown on galactose medium show a strong fluorescence around the whole circumference of the cell. Control cultures of wild-type strains (Fig. 5 c), of cdc48 point mutants KFY415, KFY416, and KFY438, of cdc48-3ts strain rE24-15 arrested at 37°C, and of mutants in cell cycle genes cdc2, cdc19, and cdc31 arrested at 37°C only show weak staining in the cell lumen or no detectable fluorescence. 5–20% of the protoplasts, both from wild-type and mutant strains, take up propidium iodide indicating membrane damage. These cells all show a strong annexin V staining of the whole cell and no peripheral ring (Fig. 5 b).
Figure 5.
Exposition of phosphatidylserine at the cytoplasmic membrane. Cells of KFY437 (a and b) and of control strain KFY417 (c) grown exponentially for 12 h on YEP/glucose were stained with FITC-labeled annexin V for detection of exposed phosphatidylserine (a and c) and propidium iodide for detection of damaged cells (b). Bar, 10 μm.
Discussion
A yeast mutant in cell division cycle gene CDC48 shows a number of morphological and molecular features that are considered typical indicators of apoptosis in metazoan cells: exposure of phosphatidylserine on the outer leaflet of the cytoplasmic membrane, DNA breakage, chromatin condensation and fragmentation, and even the abnormal cell morphology with a series of tiny buds, most of them lacking chromatin (Fig. 2 o/p, and q/r), can be considered the equivalent of apoptotic bodies. The coordinate occurrence of these markers caused by a single point mutation implies their connection even in yeast. None of the other alleles of CDC48, and none of the cell division cycle–arrested mutants investigated shows any of these phenomena, indicating that they are not the inevitable consequence of any cell division cycle arrest.
Apoptosis serves the purpose of removing unused or potentially harmful cells in higher organisms (Raff, 1992). Even in a unicellular organism like yeast, such a mechanism would be of evolutionary advantage. If a cell sensing impending fatal damage stopped proliferating and consuming resources, it would improve the chances for its clonal relatives. However, the fact that similar observations with yeast have not been published before suggests that this is a rare event. In addition, a database search of the complete S. cerevisiae genome shows no potential homologue for genes involved in the triggering of apoptosis in metazoans: yeast contains neither members of the ICE protease family (caspases), nor p53, nor genes with similarity to ced-4, bcl-2, or bax.
It appears more likely that the connection between nuclear condensation, DNA fragmentation, the inversion of the cytoplasmic membrane, and possibly the formation of cell fragments is evolutionarily old, and was used for the development of apoptosis by linking it to signaling pathways in metazoan organisms.
In mammals, exposition of phosphatidylserine serves as bait for macrophages that then deal with the disposal of the apoptotic bodies (Fadok et al., 1992; Martin et al., 1995). Our observation in yeast indicates a more fundamental connection between phosphatidylserine exposure and other events of apoptosis. Therefore, the exposure probably has not been developed to attract macrophages, but rather phagocytic cells throughout evolution have learned to interpret the phenomenon as a signal of attraction.
CDC48 participates in processes ensuring the integrity of the endoplasmic reticulum and the nuclear envelope. Aberrations in these structures caused by cdc48 S565G might trigger the processes observed. In wild-type cells of S. cerevisiae, endoplasmic reticulum is only scarcely seen in electron micrographs (Schwencke, 1991). An electron microscopic observation of the mutant shows that endoplasmic reticulum accumulates and that its lumen appears dilated as compared to wild-type endoplasmic reticulum. This could be a direct effect of the mutation, ultimately leading to the apoptotic phenotype. The phenotype is allele specific; none of the other cdc48 mutant alleles investigated cause any of the apoptotic effects.
The tools of yeast genetics and molecular biology are a powerful means to identify the components of complex physiological pathways. There is no clear picture of the events following the action of the ICE proteases in apoptosis yet. Yeast might be a suitable model for clarification.
Acknowledgments
We thank D. Mecke for discussion and support, H. Schwarz for generous advice, and S. Sigrist for help in preparing the figures. We are grateful to J. Gatfield for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations used in this paper
- ICE
IL-1β–converting enzyme
- TUNEL
terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling
Footnotes
Address all correspondence to Kai-Uwe Fröhlich, Physiologisch-Chemisches Institut, Hoppe-Seyler-Strasse 4, Universität Tübingen, 72076 Tübingen, Germany. Tel.: 49-7071-297-3360. Fax: 49-7071-296-6390. E-mail: kaifr@uni-tuebingen.de
References
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. . J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Casso D, Beach D. Human p53 inhibits growth in Schizosaccharomyces pombe. . Mol Cell Biol. 1992;12:1405–1411. doi: 10.1128/mcb.12.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach, J.R., Y.-Y. Li, L.-C.C. Wu, and M. Jayaram. 1983. Vectors for high-level, inducible expression of cloned genes in yeast. In Experimental Manipulation of Gene Expression. M. Inouye, editor. Academic Press, London. 83–117.
- Byers B, Goetsch L. Preparation of yeast cells for thin-section electron microscopy. Methods Enzymol. 1991;194:602–608. doi: 10.1016/0076-6879(91)94044-d. [DOI] [PubMed] [Google Scholar]
- Cerbón J, Calderón V. Changes of the compositional asymmetry of phospholipids associated to the increment in the membrane surface potential. Biochim Biophys Acta. 1991;1067:139–144. doi: 10.1016/0005-2736(91)90035-7. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. J Biol Chem. 1996;171:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- Clifford J, Chiba H, Sobieszczuk D, Metzger D, Chambon P. RXRα-null F9 embryonal carcinoma cells are resistant to the differentiation, anti-proliferative and apoptotic effects of retinoids. EMBO (Eur Mol Biol Organ) J. 1996;15:4142–4155. [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Bradley A. The tumor suppressor p53. Biochim Biophys Acta. 1993;1155:181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fröhlich K-U, Fries H-W, Rüdiger M, Erdmann R, Botstein D, Mecke D. Yeast cell cycle protein CDC48p shows full length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J Cell Biol. 1991;114:443–453. doi: 10.1083/jcb.114.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situterminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell-division cycle in yeast: V. Genetic analysis of cdcmutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgensmeier JM, Krajewski S, Armstrong RC, Wilson GM, Oltersdorf T, Fritz LC, Reed JC, Ottilie S. Bax- and Bak-induced cell death in the fission yeast Schizosaccharomyces pombe. . Mol Biol Cell. 1997;8:325–339. doi: 10.1091/mbc.8.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latterich M, Fröhlich K-U, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Lowary PT, Widom J. Higher-order structure of Saccharomyces cerevisiaechromatin. Proc Natl Acad Sci USA. 1989;86:8266–8270. doi: 10.1073/pnas.86.21.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D, Steward SE, Osmond BC, Botstein D. Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics. 1982;100:547–563. doi: 10.1093/genetics/100.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO (Eur Mol Biol Organ) J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature (Lond) 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Schwencke, J. 1991. Vacuoles, internal membraneous systems and vesicles. In The Yeasts. Vol. 4. A.H. Rose, and J.S. Harrison, editors. Academic Press, London. 347–432.
- Steller H. Mechanisms and genes of cellular suicide. Science (Wash DC) 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature (Lond) 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]