Abstract
Two recombinant aequorin isoforms with different Ca2+ affinities, specifically targeted to the endoplasmic reticulum (ER), were used in parallel to investigate free Ca2+ homeostasis in the lumen of this organelle. Here we show that, although identically and homogeneously distributed in the ER system, as revealed by both immunocytochemical and functional evidence, the two aequorins measured apparently very different concentrations of divalent cations ([Ca2+]er or [Sr2+]er). Our data demonstrate that this contradiction is due to the heterogeneity of the [Ca2+] of the aequorin-enclosing endomembrane system. Because of the characteristics of the calibration procedure used to convert aequorin luminescence into Ca2+ concentration, the [Ca2+]er values obtained at steady state tend, in fact, to reflect not the average ER values, but those of one or more subcompartments with lower [Ca2+]. These subcompartments are not generated artefactually during the experiments, as revealed by the dynamic analysis of the ER structure in living cells carried out by means of an ER-targeted green fluorescent protein. When the problem of ER heterogeneity was taken into account (and when Sr2+ was used as a Ca2+ surrogate), the bulk of the organelle was shown to accumulate free [cation2+]er up to a steady state in the millimolar range. A theoretical model, based on the existence of multiple ER subcompartments of high and low [Ca2+], that closely mimics the experimental data obtained in HeLa cells during accumulation of either Ca2+ or Sr2+, is presented. Moreover, a few other key problems concerning the ER Ca2+ homeostasis have been addressed with the following conclusions: (a) the changes induced in the ER subcompartments by receptor generation of InsP3 vary depending on their initial [Ca2+]. In the bulk of the system there is a rapid release whereas in the small subcompartments with low [Ca2+] the cation is simultaneously accumulated; (b) stimulation of Ca2+ release by receptor-generated InsP3 is inhibited when the lumenal level is below a threshold, suggesting a regulation by [cation2+]er of the InsP3 receptor activity (such a phenomenon had already been reported, however, but only in subcellular fractions analyzed in vitro); and (c) the maintenance of a relatively constant level of cytosolic [Ca2+], observed when the cells are incubated in Ca2+-free medium, depends on the continuous release of the cation from the ER, with ensuing activation in the plasma membrane of the channels thereby regulated (capacitative influx).
Transient increases of the cytosolic-free Ca2+ ([Ca2+]c)1 are general processes used by eukaryotic cells to regulate a whole host of functions. The mechanisms responsible for such increases are essentially two: increased Ca2+ influx through the plasma membrane and/or rapid release from intracellular Ca2+ stores. For many years, the cytological nature of these Ca2+ stores has remained an open issue in cell biology. Initially, the InsP3-sensitive store was identified with the ER (Streb et al., 1983, 1984). Successive, detailed studies on the subcellular distribution of the protein components known to have a role in rapidly exchanging stores (i.e., InsP3 and ryanodine receptors; the various sarcoendoplasmic reticulum Ca2+-ATPases [SERCA]; the ER lumenal Ca2+ binding proteins; other ER protein markers [for review see Pozzan et al., 1994]) revealed, however, the heterogeneity of the endomembrane system (Volpe et al., 1988; Sitia and Meldolesi, 1992). Whether, and to what extent, such a molecular heterogeneity of individual ER subcompartments is reflected also in their function remains to be established. The ER is composed, in fact, by a system of lumenally interconnected membrane-bound elements which could function coordinately in larger units, such as those known to give rise to discrete [Ca2+]c dynamic events (e.g., sparks, hot spots, etc.). Another question that remains unanswered is whether the heterogeneity extends to the equilibria of Ca2+ fluxes and thus induces differences in [Ca2+] in the various ER subcompartments.
To start the investigation of the above problems, we have recently developed a low affinity recombinant form of aequorin, the Ca2+ binding photoprotein, which, because of its molecular properties, is synthesized by bound polysomes and then retained within the ER lumen of intact cells (Montero et al., 1995). Using this probe we have demonstrated that the level of Ca2+ within the endomembrane system, [Ca2+]er, is several orders of magnitude higher than [Ca2+]c, too high to be accurately determined by the aequorin technique. In these previous studies we have also shown that Sr2+ can be used as a good Ca2+ surrogate and that conditions can be found in which the lumenal concentrations of this divalent cation can closely mimic those of Ca2+. In particular, the average resting steady state levels of [Sr2+] inside the ER of HeLa cells (∼2–3 mM) were found to rapidly decrease upon addition of an InsP3-producing agonist (Montero et al., 1995). Parallel studies by several other groups, using either recombinant aequorins or intracellularly trapped indicators, yielded [Ca2+]er surprisingly variable and, in general, lower than our [Sr2+]er values ranging from 1–5 (with aequorin: Kendall et al., 1992, 1994, 1996) to 200–1,500 μM (with low affinity Ca2+ dyes: Hofer and Machen, 1993; Tse et al., 1994; Chatton et al., 1995; Hofer et al., 1995; Chen et al., 1996) under steady state conditions. In a recent contribution, Button and Eidsath (1996), using recombinant ER-targeted aequorin, raised the possibility that these low values were due to a calibration artefact dependent, in turn, on the heterogeneity of [Ca2+]er. The problem, however, was not addressed experimentally.
To reinvestigate [Ca2+]er and its possible heterogeneity, we have now expanded our work in HeLa cells by using two ER-targeted chimeric aequorins in parallel. The first (erAEQmut), characterized by low Ca2+ affinity, is the same previously used by Montero et al. (1995); the second (erAEQwt), has the affinity for Ca2+ of wild-type aequorin. By the use of these two probes and loading the ER with either Ca2+ or Sr2+, we demonstrate here that the low values of [Ca2+]er reported previously by others with ER-targeted wild-type aequorin (Kendall et al., 1992, 1994, 1996; Button and Eidsath, 1996) reflect the behavior of only small, heterogeneous subcompartments that reproduce the behavior of the majority of the ER neither quantitatively nor qualitatively. Moreover, using Sr2+ as a Ca2+ surrogate, we reveal new properties of the Ca2+ release induced by receptor-generated InsP3. A mathematical model based on ER heterogeneity, which describes quantitatively and qualitatively the kinetics of the luminescent aequorin signal in HeLa cells, is also presented.
Materials and Methods
The construction strategy of ER-targeted aequorin chimeras containing either erAEQwt or erAEQmut has been described previously (Montero et al., 1995). HeLa cells were grown in DME supplemented with 10% FCS. For generating cell clones stably expressing erAEQwt or erAEQmut, a 10-cm dish of HeLa cells was transfected with 36 μg of erAEQwt/pcDNAI or erAEQmut/pcDNAI and 4 μg of pSV2neo (Southern and Berg, 1982). Selection was carried out with 0.8 mg/ml G418 as described elsewhere (Rizzuto et al., 1994, 1995). 90 clones were isolated and tested for aequorin expression by measuring the Ca2+-dependent light emission of coelenterazine-reconstituted cell lysates. Clones EM26 and EN28, the highest producers of erAEQmut and erAEQwt, respectively, were used in the experiments presented here. Similar data were obtained using either other permanent clones or transiently transfected cells as described elsewhere (Montero et al., 1995; frequency of transfected cells = 40–50%). The ER-targeted green fluorescent protein, erGFP, was generated by substituting the cDNA sequence encoding aequorin with that encoding the S65T mutant (Rizzuto et al., 1996) of GFP in the expression plasmid. Transient transfection with this plasmid was carried out using the calcium-phosphate procedure, using either the erAEQs expressing clones or the wild-type HeLa cells.
Stably and transiently transfected cells were investigated by immunomicroscopy using antibodies against the tag included in the aequorin chimera cDNA. Dual immunofluorescence was also carried out with antibodies against the endogenous ER marker p72 (Montero et al., 1995). For ultrastructural studies, the cells were fixed with 4% paraformaldehyde, 0.2% glutaraldehyde. In addition to conventional Epon-embedded sections, ultrathin cryosections were prepared and labeled with immunogold particles (6 nm in diameter). Details about microscopical techniques used and particle density counting in various subcellular organelles can be found in Villa et al. (1992).
Cell clones were plated onto 13-mm-round coverslips. Before reconstituting aequorin, [Ca2+]er was reduced by incubating the cells for 5 min with the SERCA inhibitor 2,5-di(tert-butyl)-1,4-benzohydroquinone (tBuBHQ) (10 μM) in Krebs-Ringer modified buffer (KRB; 125 mM NaCl, 5 mM KCl, 1 mM Na3PO4, 1 mM MgCl2, 5.5 mM glucose, 20 mM Hepes, pH 7.4, 37°C), and supplemented with 3 mM EGTA. Similar results were obtained when [Ca2+]er was reduced with different protocols (Montero et al., 1995). The cells were then incubated for 45–60 min in KRB containing 0.1 mM EGTA, 10 μM tBuBHQ, and 5 μM coelenterazine to reconstitute the fully functional photoprotein. The coverslip was then washed for 5 min in KRB-containing 0.1 mM EGTA, 5% BSA, and 10 μM tBuBHQ, and finally placed in the perfusion chamber of a purpose-built luminometer (Rizzuto et al., 1995). All experiments were performed in KRB medium at 37°C. Aequorin light emission was calibrated using a computer algorithm (Brini et al., 1995) that uses the calibration curve (luminescence vs. [cation2+]) obtained experimentally. The calibration curve of erAEQwt exposed to Ca2+ (Brini et al., 1995), as well as that of erAEQmut exposed to Ca2+ or Sr2+ (Montero et al., 1995) have been shown previously. The calibration curve of erAEQwt exposed to Sr2+ was determined similarly.
The fluorescence of living cells transiently transfected with erGFP was analyzed using a confocal microscope (RCM800; Nikon, Inc., Tokyo, Japan). The 488-nm line of the crypton-argon laser was selected by an interference filter on the exciting pathway, and the emitted light was collected through an interference filter centered at 510 nm. 0.5-μM optical slices were analyzed through the use of a small pinhole. In the experiment of photobleaching recovery, control samples and cells that underwent the Ca2+-depletion protocol were first imaged under standard conditions; a mask was then introduced in the light path of the exciting beam so that only part of the field was illuminated. The effective protection of the masked field from the exciting beam was confirmed by the lack of photobleaching in neighboring cells.
All experiments have been carried out at 37°C. Unless otherwise specified, all materials were from Sigma Chemical Co. (St. Louis, MO).
Results
What Is the Ca2+ Concentration within the ER Lumen?
The first series of experiments was carried out to establish why the [Ca2+]er obtained in our laboratory by the use of ER-targeted aequorin was so profoundly different from that reported by other groups (Montero et al., 1995; Kendall et al., 1992, 1994, 1996; Button and Eidsath, 1996). A possibility to be considered was a different subcellular localization of the protein probe. In the previous studies, such a distribution had been shown to coincide with that of various endogenous ER lumenal proteins investigated by immunocytochemistry, only at the light microscope level, however. To establish this point definitely, the distribution of exogenous aequorin has now been investigated by EM, using immunogold labeling of ultrathin cryosections of both stably and transiently transfected HeLa cells. Table I shows that, in these cells, intense antichimeric aequorin immunogold labeling was localized within the lumen of recognizable ER elements, including the perinuclear cisternae. Among the other organelles some, including the Golgi complex and the nucleus, exhibited a labeling at the background level, a result that excludes harboring of the photoprotein within these structures. In mitochondria, on the other hand, a higher labeling was appreciated (∼12% of the ER). To establish whether this result was specific, due to a moderate degree of missorted aequorin, functional experiments were carried out. In particular, the effect of the mitochondrial uncoupler, carbonylcyanide p-(trifluoromethoxy) phenylhydrazone (FCCP), on the erAEQ luminescence response was investigated. The uncoupler did not affect the erAEQ light output with either Sr2+ or Ca2+ (see also below), indicating that the immunolabeling of mitochondria is probably unspecific, due to cross-reactivity of the anti-tag antibody.
Table I.
Aequorin-Tag Immunogold Labeling of Intracellular Structures in Transfected HeLa Cells
| Structure | Total analyzed surface area | Gold particles/ μm2 area | ||
|---|---|---|---|---|
| μm2 | ||||
| ER cisternae | 2.8 | 87.60 ± 29.80 | ||
| Perinuclear cisternae | 1.1 | 81.46 ± 7.86 | ||
| Nucleus | 7.2 | 5.10 ± 2.30 | ||
| Golgi complex | 1.0 | 5.00 ± 3.10 | ||
| Mitochondria | 1.4 | 14.10 ± 5.80 |
Averages ± SD. Values shown were obtained from 10 micrographs of HeLa cells transiently transfected with aequorin, printed at 50,000 × (total analyzed section area = 146 μm2), chosen at random. They were not subtracted from the background (4.5 particles/μm2) assessed over parallel cryosections, but processed with a preimmune serum.
Functional evidence for the concentration of the transfected photoprotein within the ER lumen was also obtained by taking advantage of the well-known specificity of thapsigargin, a classical blocker of the SERCAs. Thapsigargin was added to the cells after reconstitution with coelenterazine in the EGTA medium (see Materials and Methods). When the erAEQmut expressing cells without and with 1 μM thapsigargin pretreatment were switched to medium containing 1 mM CaCl2, only the former exhibited the well-known consequence of Ca2+ binding, the rapid consumption of the photoprotein (95% within 1 min; Montero et al., 1995). In thapsigargin-pretreated cells, on the contrary, only a minor fraction (∼10%) of aequorin was consumed upon 1 mM Ca2+ addition (data not shown). The rest of the luminescence was released only after cell permeabilization with appropriate concentrations of digitonin. This result is consistent with the localization of the photoprotein within the ER lumen, where Ca2+ does not accumulate when SERCAs are blocked. Taken together, the immunolocalization and chemiluminescence data demonstrate that the vast majority of recombinant aequorin is distributed within an endomembrane system not only morphologically indistinguishable from the ER, but also >90% dependent from thapsigargin-sensitive SERCAs for its Ca2+ accumulation.
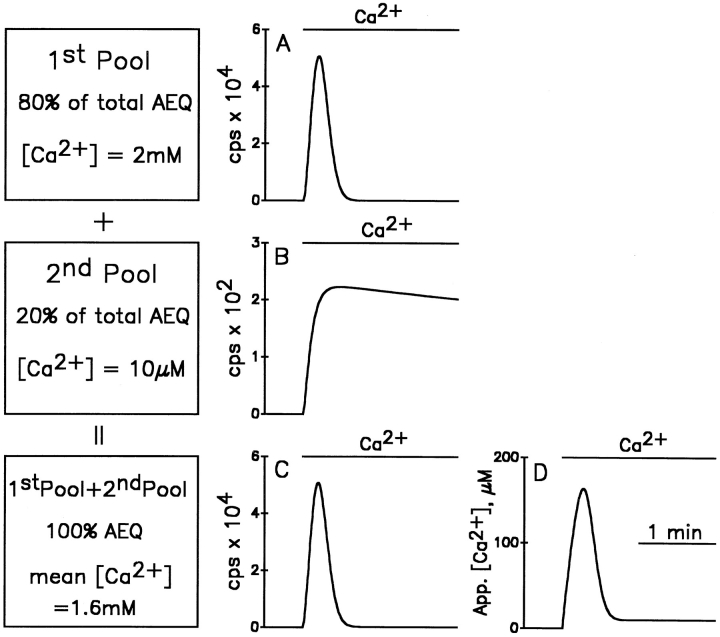
Another possible explanation for the large discrepancies observed among the previous aequorin studies could be heterogeneity of the ER, with subcompartments containing largely different [Ca2+]. This possibility had already been suggested in our previous report on the use of erAEQ (Montero et al., 1995), but no direct experimental evidence was provided to support it. To focus on this possibility, a theoretical model has been developed in two versions (the more refined of the two is illustrated in Fig. A1, see Appendix). In the simple version of the model (Fig. 1, shown for the low Ca2+ affinity erAEQmut only; with the wild-type isoform the results were even more extreme), we simulated the behavior of aequorin luminescence during refilling of two compartments that accumulate Ca2+ exponentially; one to 2 mM (Fig. 1 A, 1st pool), the other to only 10 μM (Fig. 1 B, 2nd pool). In the first, high [Ca2+] compartment, which was assumed to contain 80% of total aequorin, luminescence increases rapidly to a peak and then all aequorin is consumed within 30 s. On the contrary, in the second, low [Ca2+] compartment, which was assumed to contain 20% of the total photoprotein, light emission increases to a plateau that takes several minutes to consume all its aequorin. Because of the different kinetics of aequorin consumption, if the luminescence time course of the two compartments is summed (Fig. 1 C), and the resulting curve calibrated as if all aequorin was in a single compartment, the apparent [Ca2+] kinetics (Fig. 1 D) is not the average of the two pools, but rather a complex curve composed of a rapid peak, followed first by a major drop, and then by a very low and slowly declining plateau. It should be emphasized that none of the values in Fig. 1 D fit the [Ca2+] values assumed to exist within the two compartments of the model. Rather, the peak rise of [Ca2+] in the larger compartment is underestimated by a factor of 20, and its steady state by two orders of magnitude.
Figure A1.
Detailed mathematical model mimicking the effect of the addition of Ca2+ or Sr2+ to either erAEQwt- or erAEQmut-expressing HeLa cells. In this model the ER space is divided into three high [Ca2+]er and three low [Ca2+]er subcompartments in which Ca2+ or Sr2+ were assumed to increase exponentially. Continuous lines represent theoretical Ca2+ and Sr2+ values obtained from the model. Dotted lines represent typical calibrated values obtained experimentally (Fig. 2).
Figure 1.
Simple version of the theoretical model predicting the rates of aequorin consumption and the calibrated [Ca2+] during refilling of the ER, assuming the existence of 1 or 2 compartments. (A) The predicted luminescence results recorded during exponential refilling of a single compartment up to a steady state of 2 mM, with a time constant of 120 s. For simplicity it is assumed that the whole cell population could emit 1,000,000 photons in total, 800,000 from the high Ca2+ compartment and 200,000 from the low Ca2+ compartment. (B) The predicted luminescence recorded during exponential refilling of a single compartment up to a steady state of 10 μM, with a time constant of 5 s. (C) The result of adding the two luminescence records of A and B. (D) The calibrated [Ca2+] levels calculated from the data in C, assuming all aequorin to be in the same compartment.
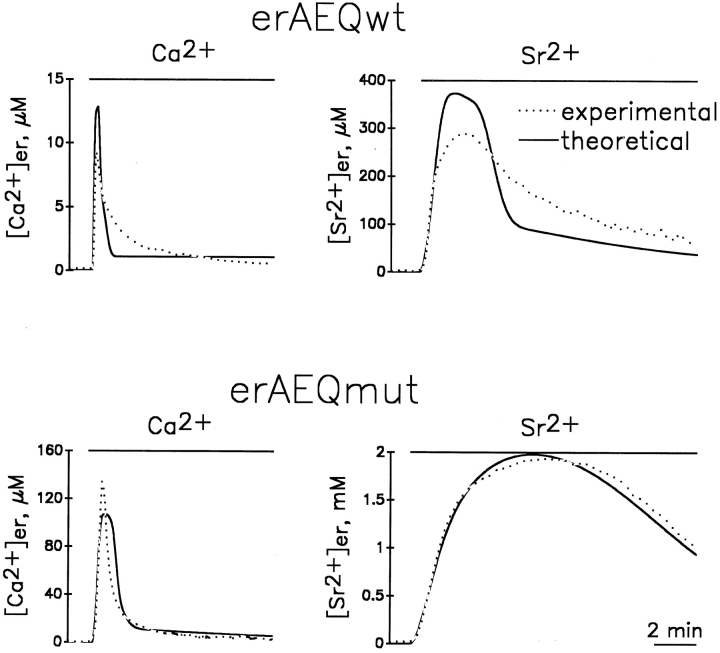
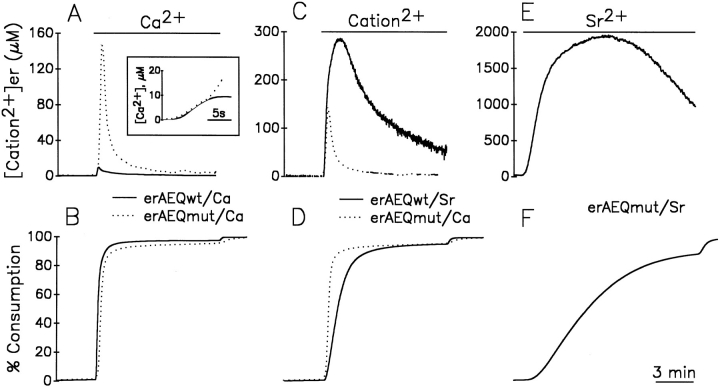
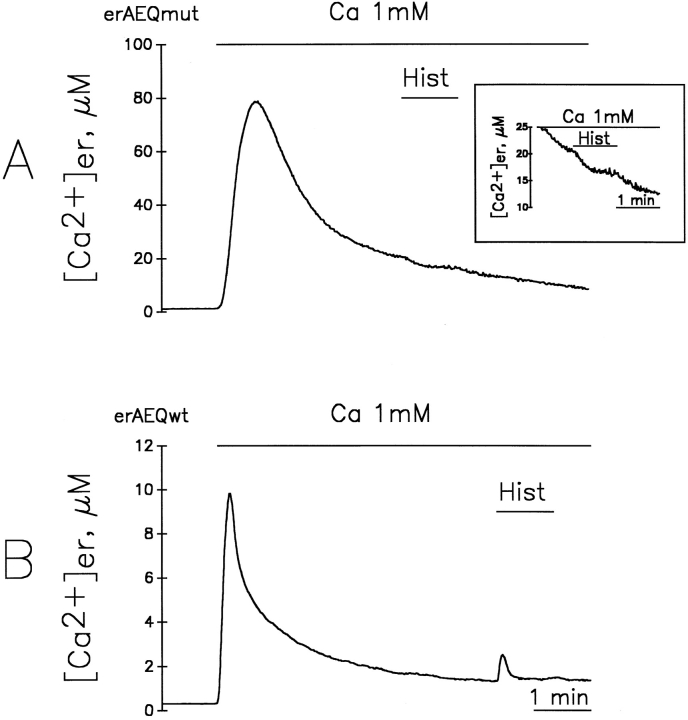
To establish whether the state of the [Ca2+]er in HeLa cells has anything in common with the assumptions underlying the model of Fig. 1, experiments were carried out by the parallel study of cells expressing either one of the two aequorin isoforms available, erAEQwt and erAEQmut. In the experiments described in Fig. 2, A and B, cells expressing erAEQmut and erAEQwt, after the depletion-reconstitution protocol, were exposed to medium containing 1 mM CaCl2. In Fig. 2 A, the calibrated [Ca2+]er kinetics from erAEQmut-expressing cells (dotted trace) was byphasic, with a peak at 140 μM after 20–30 s and a plateau at ∼10 μM. About 90% of total aequorin luminescence was consumed in the first minute (Fig. 2 B; Montero et al., 1995). This apparent [Ca2+]er kinetics is strongly reminiscent of the theoretical curve shown in the model of Fig. 1. Information as to whether these results were indeed due to ER heterogeneity of [Ca2+] throughout the ER, or in contrast, reflected the real time course of the changes occurring kinetically within the organelle, came from the comparison with parallel results obtained using erAEQwt-transfected cells. In fact, if the Ca2+ concentrations were homogeneous within the organelle, the kinetics of photon emission (and thus of aequorin consumption) upon Ca2+ addition were expected to differ according to the different affinities for the cation of the two photoprotein isoforms, whereas the calibrated [Ca2+] was expected to remain identical throughout the entire experiment. The results obtained showed that the rate of photon emission was indeed faster in the cells expressing erAEQwt when compared to their erAEQmut counterparts (data not shown), whereas the calibrated [Ca2+]er (Fig. 2 A) were identical only during the first few seconds and then diverged drastically with time. In particular, the peak [Ca2+]er with erAEQwt was about one order of magnitude lower compared to that of erAEQmut after calibration. This result is clearly incompatible with a model of homogeneous [Ca2+]er, whereas it is easily explained by the heterogeneity model. In Fig. 2 B, the data from A were plotted as percentage of aequorin consumption. Comparison between Fig. 2, A and B demonstrates that the discrepancy among the calibrated values of [Ca2+]er became evident after the consumption of ∼65% of the erAEQwt.
Figure 2.
Apparent kinetics of [cation2+]er during store refilling with Ca2+ and Sr2+ as measured in cells expressing erAEQmut and erAEQwt. (A and B) Continuous lines represent cells expressing erAEQwt. Dotted lines represent cells expressing erAEQmut. Where indicated, the perfusion medium contained 1 mM CaCl2. A shows the calibrated values of [Ca2+]er with both aequorins, with the inset illustrating the first seconds after Ca2+ addition at higher magnification. B shows the data of aequorin luminescence recalculated as an accumulative plot, considering the total amount of photons emitted during the whole experiment (normalized to 100%) and the percentage emitted as a function of time. In this plot it is immediately obvious the residual amount of aequorin available at any given time being compared with the calibrated values of [cation2+]er. (C and D) The erAEQmut-expressing HeLa cells (dotted lines) were challenged where indicated with 1mM Ca2+, whereas the erAEQwt-expressing cells (continuous lines) were challenged with 1 mM Sr2+. (E and F) The erAEQmut HeLa cells were perfused with 1 mM Sr2+ where indicated.
Another simple prediction of the heterogeneous [Ca2+]er model is that the discrepancies among the calibrated values obtained with the two aequorin isoforms should depend only on the rates of aequorin consumption, and not on the type of aequorin or cation accumulated in the ER lumen. Fig. 2, C and D, show that this is indeed the case. Cells expressing erAEQmut were challenged with Ca2+ (dotted traces), and their erAEQwt counterparts with Sr2+ (continuous traces). In these experiments the calibrated [cation2+]er values were almost superimposed for the first 20 s (Fig. 2 C), but became dramatically different at later times. By comparing Fig. 2, C and D, it is evident that the [Sr2+] and [Ca2+] kinetics diverged when the consumption of erAEQmut approached 60% of the total content. The kinetics of photon emission and the calibrated signal of cells expressing erAEQmut challenged with Sr2+ were also measured (Fig. 2, E and F). In this case the calibrated signal overlapped with that of erAEQwt (also challenged with Sr2+) for the first 35 s (Fig. 2, compare C and E). With erAEQmut, however, the [Sr2+] continued to increase, and eventually reached a plateau at ∼2 mM before starting to decline when the consumption of aequorin approached 65–70% of the total (Fig. 2, compare E and F).
Taken together, the data of Fig. 2 provide convincing experimental evidence in favor of our interpretation of the abnormally low [Ca2+]er values measured with recombinant targeted aequorins; i.e., that these values are due to artefacts of the calibration procedure, dependent in turn on the heterogeneity of [Ca2+]er in the compartments containing the aequorins. The initial rates of Ca2+ and Sr2+ uptake into the ER appear very close in fact, no matter whether using the wild-type or mutated erAEQ as the probe. However, when ∼60–65% of the total photoprotein is consumed, the calibrated [cation2+]er apparently starts to decrease. Although at different rates, this apparent decrease was always found to take place independently of the nature of the cation (Ca2+ or Sr2+) and of the aequorin isoform used. The observation that the [cation2+]er apparently decreases after the consumption of the same percentage of the aequorin content, but does so independently of the absolute value of [cation2+]er, suggests this phenomenon does not reflect the characteristics of the process of cation2+ accumulation, but those of the aequorin calibration procedure. This behavior can, in fact, be mimicked closely by the heterogeneity models (Figs. 1 and A1).
Is the Heterogeneity of the [cation2+]er Due to the Unloading–Reloading Protocol?
The question arises as to whether the heterogeneity in [Ca2+]er is indeed a feature of the organelle or is generated rather by the drastic Ca2+-depletion protocol adopted to obtain a successful functional reconstitution of aequorin with coelenterazine. Some evidence argues against the latter hypothesis. In particular: (a) no secretion of erAEQ was observed as a consequence of the depletion, indicating that membrane traffic is not grossly altered by the procedure (Montero et al., 1995); (b) in some experiments, reconstitution with coelenterazine was carried out without prior Ca2+ depletion (under these conditions, although the total amount of emitted photons was drastically reduced, the apparent [Ca2+]er was found to be ∼10 and 1 μM in cells expressing the mutated and wild-type aequorin isoform, respectively; i.e., the same values measured at steady state with the standard depletion-refilling protocol); and (c) to prevent remodelling of the ER during this preincubation period, depletion of Ca2+ and reconstitution with coelenterazine were carried out at 4°C in some experiments. The cells were then rapidly warmed to 37°C and refilling with Sr2+ or Ca2+ initiated. Under these conditions, no qualitative changes in the kinetics of apparent [Ca2+ or Sr2+]er were observed with respect to controls kept at 37°C throughout the whole experiment. Unexpectedly, however, the total light emitted by the cells reconstituted at low temperature was considerably greater (>10-fold), indicating that by this protocol, reconstitution is more efficient (data not shown).
Further evidence indicating that the depletion protocol causes no appreciable alteration of the ER lumenal continuity is provided by the experiments presented in Fig. 3. HeLa cells were transiently transfected with the GFP construct retained in the ER by the same mechanism (binding to BiP) used for the aequorins, and their morphology was monitored in vivo with high spatial and temporal resolution by confocal fluorescence microscopy. Fig. 3 A shows that the delicate reticular structure of the ER was preserved after Ca2+ depletion. The morphological changes taking place in the course of the experiment were due in part to an effect of Ca2+-free medium producing a small shrinkage of the cells, and in part to the dynamic organization of the endomembrane system, since they resembled those observed in control cells maintained in the Ca2+-containing medium.
Figure 3.
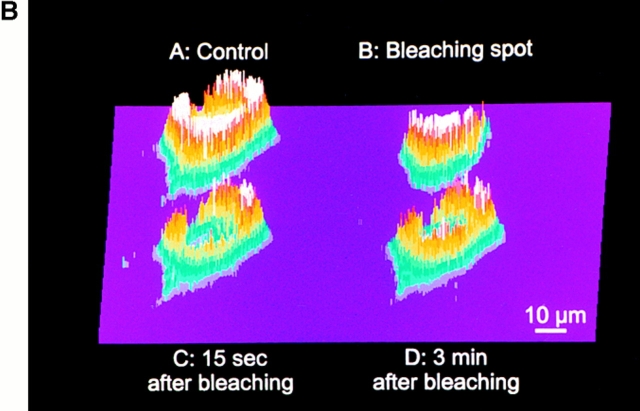
Effects of the Ca2+-depletion protocol on the morphology and lumenal continuity of the ER as measured with erGFP. HeLa cells were transiently transfected with the erGFP construct and analyzed 2 d later. (A) ER morphology as revealed by erGFP fluorescence analyzed by confocal microscopy in live cells before (left) and 1 h after incubation in Ca2+-free, EGTA-containing KRB, and treatment with 30 μM tBuBHQ (right). (B) Photobleaching and recovery. After the depletion protocol was terminated, the image shown in A (control) was taken with the standard illumination protocol. A mask was then introduced in the exciting light path and the second image was taken (B; bleaching spot). The sample was then continuously illuminated with the highest laser power for 3 min before removing the mask and immediately taking the third image (C; 15 s after bleaching) with the standard settings. The last image (D; 3 min after bleaching) was collected under standard conditions, 3 min after the third. The cells were not illuminated during this recovery period. Bar, 9 μm.
In the experiments presented in Fig. 3 B, we took advantage of the photobleaching recovery phenomenon to reveal that, even after the Ca2+-depletion protocol, the luminal continuity of the ER network is maintained. An erGFP- expressing cell (A, upper left) was imaged at the end of the depletion protocol while maintained in the Ca2+-free EGTA-containing medium, and the intensity of the fluorescent signal at each pixel was expressed as a color-coded three-dimensional (3-D) plot. One half of the cell was then protected from the exciting beam by a mask, while the rest (B, upper right) was continuously illuminated for 3 min with maximal laser intensity. At the end of the photobleaching period, the laser intensity was reduced back to normal, and new images of the whole cell were taken immediately after removal of the mask and 3 min later. As can be seen in the lower left panel (C), at the first of these time points the signal was not only drastically reduced in the illuminated half but also attenuated in the protected half. 3 min later (D, lower right), fluorescence appears homogeneously distributed. These findings can only be explained by the diffusion of GFP throughout the lumenally continuous ER network of the cell.
Kinetics of Divalent Cation Release from the ER
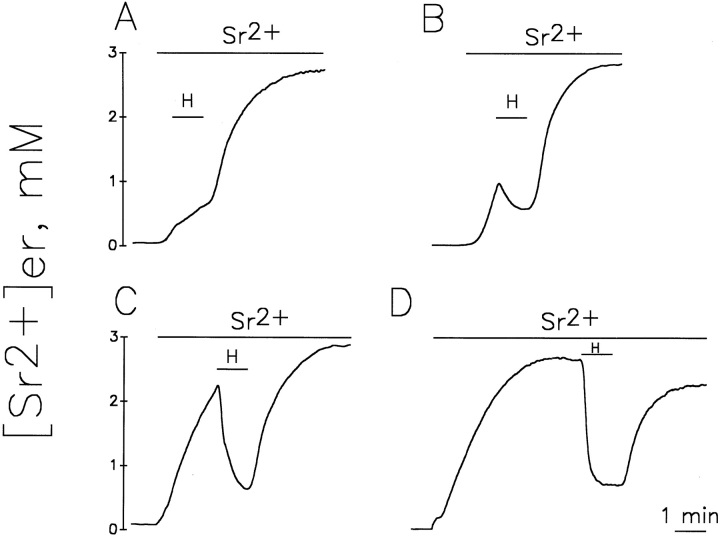
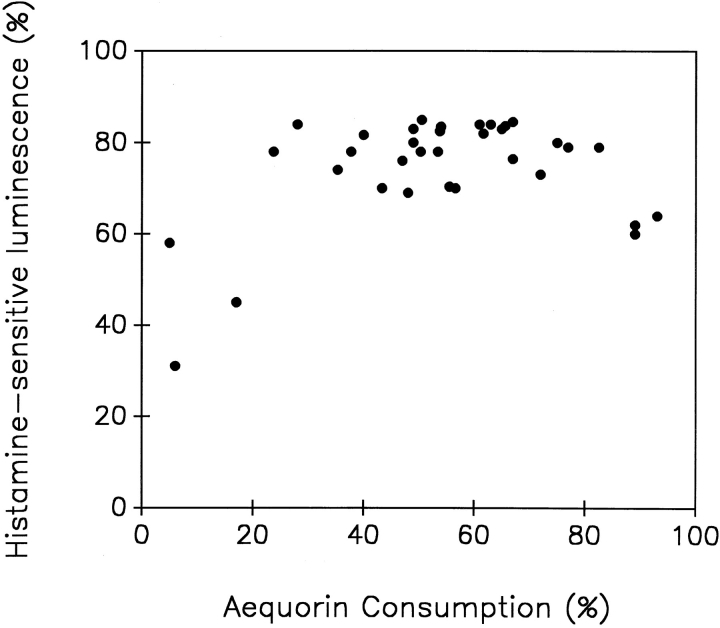
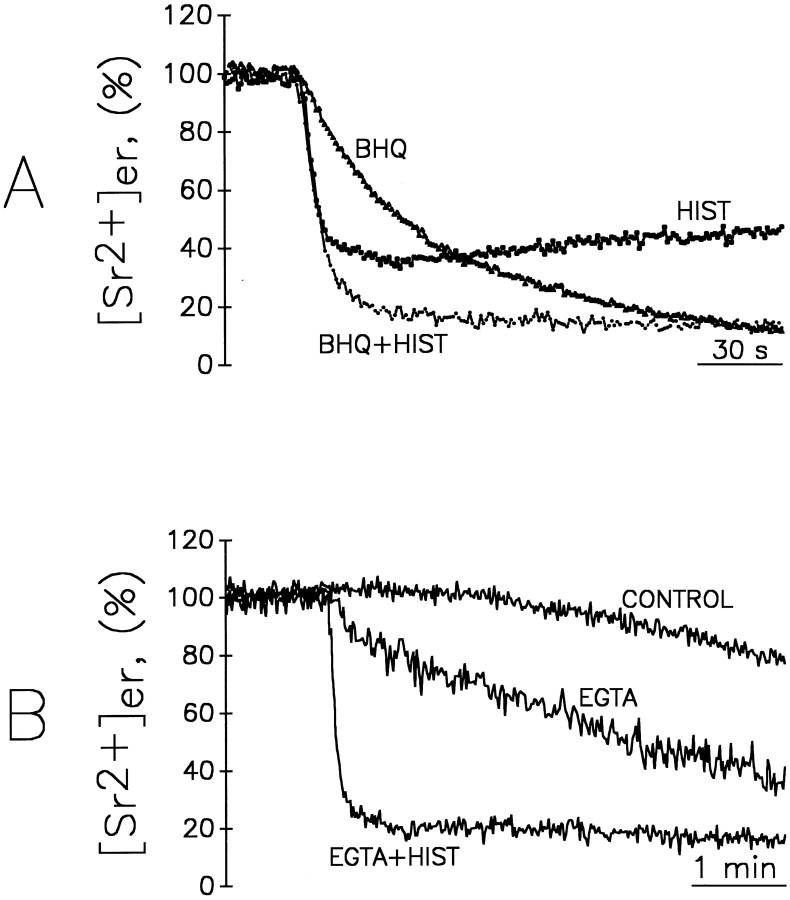
Together with our previous data (Montero et al., 1995) the results presented above indicate that in a large part of the ER, the steady state [Ca2+] is in the millimolar range. Because of these high values, erAEQmut and Sr2+ appear to be the best tools to quantitate, albeit indirectly, Ca2+ homeostasis in the bulk ER with the present methodology. By using this approach we could thus address two key questions concerning ER Ca2+ homeostasis: (a) what is the influence of the [cation2+]er on the rate and extent of the InsP3 dependent release? and (b) does InsP3 generated by receptor stimulation release cation2+ from the whole ER or from one (or more) subcompartment(s) of this endomembrane system only? To answer the first question, cells expressing erAEQmut, after depletion of Ca2+ and reconstitution with coelenterazine (as described in Materials and Methods), were exposed to Sr2+ and challenged at different times with a maximal stimulatory concentration of histamine. Fig. 4 shows that addition of histamine 30 s after Sr2+ (when [Sr2+]er was ∼0.4 mM) caused not a decrease, but only a slowing of the rate of Sr2+ accumulation. On the other hand, 1 min after Sr2+ addition (when [Sr2+]er was ∼1 mM), the addition of histamine induced a net decrease of [Sr2+]er. In the latter two experiments, at the time of histamine addition, aequorin consumption was 0.6 and 5%, respectively. Maximal apparent decreases in [Sr2+]er (−80%) were observed when the stimulus was added 2 and 5 min after Sr2+ (Fig. 4, C and D), when aequorin consumption before histamine addition was between 30–60%. Marked decreases were still observed when histamine was added as late as 10 or 15 min after Sr2+ (not shown). Fig. 5 shows the relationship between the decreases in luminescence and aequorin consumption at the moment of histamine addition, obtained from a series of experiments similar to those shown in Fig. 4. The decrease in luminescence induced by histamine was constant (79 ± 5%, mean ± SD, n = 28) for percentages of aequorin consumption ranging from 20 to >80%. Reductions in the effects of histamine were observed only when the fractions of aequorin consumption were <20 or >85–90%.
Figure 4.
Effects of histamine on [Sr2+]er in erAEQmut-expressing HeLa cells refilled with Sr2+ for different periods of time. Cells were exposed to KRB containing 1 mM Sr2+ for 0.5 (A), 1 (B), 2 (C), or 5 (D) min before shifting to KRB also containing 100 μM histamine. Other conditions are as in Fig. 2.
Figure 5.
Relationship between the drop in aequorin luminescence induced by histamine and the level of aequorin consumption at the moment of histamine addition. Data were obtained from experiments such as those shown in Fig. 4.
To explain the above results it would be important to know whether the drop of [Sr2+]er to ∼0.6 mM (i.e., the maximal decrease, corresponding to nearly an 80% decrease in luminescence, Figs. 4 and 5) reflects the complete unloading of only a subfraction of the ER, or rather the partial unloading of most of it. If the latter interpretation is correct, the steady state would represent an equilibrium between uptake into and release from the ER. The experiments presented in Fig. 6 were carried out to distinguish between these two possibilities. In Fig. 6 A, 4 min after Sr2+ addition, the cells were treated with either histamine, tBuBHQ (a reversible SERCA inhibitor), or a combination of the two. The normalized decrease of [Sr2+]er caused by histamine alone was ∼60%, corresponding to a drop from 1.5 to 0.6 mM in 10 s. tBuBHQ only, on the other hand, caused [Sr2+]er that to decrease to even lower values, ∼10% of the steady state, but with slow kinetics (complete unloading required 5–10 min). The combination of histamine and tBuBHQ resulted in a rapid drop to and below 20% of the initial [Sr2+]er that was not simply the sum of the effects of the two agents. The kinetics of the decay, in fact, approached a monoexponential decay until [Sr2+]er was reduced to <30% of the starting value (corresponding to ∼500 μM). Below this level the kinetics of the decrease clearly deviated from the monoexponential, but were still definitely faster than those of cells treated with tBuBHQ only. These results suggest that the vast majority of the ER is sensitive to InsP3 and that the partial unloading observed under standard conditions results from the balance between the rates of uptake (inhibited by tBuBHQ) and release (stimulated by InsP3). This conclusion is further strengthened by the results presented in Fig. 6 B. In this experiment, removal of the free cation with excess EGTA from the extracellular medium resulted in a net decrease of [Sr2+]er, amounting after 5 min to ∼50% of the initial value. Under these conditions complete unloading required ∼30 min. On the contrary, application of histamine resulted in a rapid decrease of [Sr2+]er (80% of the initial value in 60 s, corresponding to a drop to <300 μM), larger, however, than the decrease caused by histamine added in the Sr2+ containing medium, but similar to that induced in those conditions by the combination of histamine and tBuBHQ.
Figure 6.
Effect of histamine, EGTA, and tBuBHQ on [Sr2+]er. (A) Cells expressing erAEQmut were treated as in Fig. 2 E; i.e., they were allowed to refill the ER with Sr2+ for 4 min. Where indicated, the perfusion medium contained 1 mM Sr2+ and, as indicated, either 100 μM histamine, 10 μM tBuBHQ, or both. (B) Where indicated, KRB contained 1 mM Sr2+ (control), 100 μM EGTA, or 100 μM EGTA + 100 μM histamine. The control shows the artefactual decrease of [Sr2+]er in the absence of additions due to aequorin consumption, as shown in Fig. 2 E. Maximum [Sr2+]er levels were normalized to 100% to facilitate comparison.
As a comparison with the above results from Sr2+, the effects of the InsP3-producing agonist, histamine, were also investigated in cells incubated in the Ca2+ medium, despite the fact that the absolute [Ca2+]er measured in steady state largely reflects the behavior of a small fraction of the organelle not representative of the bulk of the ER. Fig. 7 A shows that, in the case of the erAEQmut-producing clone, addition of histamine 3 min after Ca2+ produced a small decrease of the apparent [Ca2+]er (from 22 to 18 μM, with 92% of aequorin consumed at the moment of histamine addition). If histamine was added later, (i.e., if the apparent [Ca2+]er was allowed to decrease below 10 μM and consumption approached 95%), no effect of histamine was detected, although under these conditions, a clear decrease was observed with the Ca2+ ionophore ionomycin (not shown). With the erAEQwt-expressing clone, significant drops of apparent [Ca2+]er were observed with ionomycin but never upon histamine challenge. In contrast, addition of histamine to the cells expressing erAEQwt when the apparent [Ca2+]er was near 1 μM (percent consumption 97%, Fig. 7 B) produced a clear [Ca2+] peak rise, from 1 to 2.5 μM. This result suggests that a minor fraction of aequorin is retained in a very low Ca2+ compartment which, upon agonist stimulation, accumulates Ca2+ rather than releasing it. The increase of [Ca2+] under these conditions was not abolished by the previous application of tBuBHQ or the mitochondrial uncoupler FCCP, confirming that this unburned aequorin fraction is not trapped within mitochondria. In contrast, addition of ionomycin, instead of histamine, induced a drop in the apparent [Ca2+]er (not shown), indicating that this residual aequorin is also contained within a membrane-enclosed compartment.
Figure 7.
Effects of histamine on erAEQmut- (A) and erAEQwt- (B) expressing HeLa cells after refilling with Ca2+. Ca2+-depleted cells were first incubated with 1 mM Ca2+, and then 100 μM histamine was perfused in the same medium for the times indicated in the figure. The inset in A shows the effect of the addition of histamine to erAEQmut-expressing cells at higher resolution.
Discussion
During the last years, two main experimental tools have been used in an attempt to determine the Ca2+ concentration within the ER of intact cells: low-affinity Ca2+ indicators, taking advantage of the fact that when applied as hydrophobic esters they become trapped not only in the cytosol but also within intracellular organelles (Malgaroli et al., 1987; Hofer and Machen, 1993; Tse et al., 1994; Hirose and Iino, 1994; Chatton et al., 1995; Dawson et al., 1995; Hofer et al., 1995; Chen et al., 1996); and recombinant aequorins specifically targeted to the ER lumen (Kendall et al., 1992, 1994, 1996; Montero et al., 1995; Button and Eidsach, 1996). In contrast to the other cellular compartments (cytosol, nucleus, and mitochondria; Rizzuto et al., 1992; Brini et al., 1993, 1995; Gerasimenko et al., 1995; Stehno-Bittel et al., 1995) where overall results have been consistent both quantitatively and qualitatively, those obtained in the ER by the above experimental approaches were found to differ by as much as three orders of magnitude. As far as the Ca2+ dyes, the reported discrepancies may be explained, at least in part, by the poor specificity of the targeting strategy and by the uncertainties about the effective dissociation constant of the dye once trapped in the ER environment. This last situation differs profoundly from that of recombinant aequorins for which targeting appears highly selective, as revealed by both immunocytochemistry and the sensitivity of the signal to SERCAs inhibitors. Moreover, the Sr2+ affinity in the ER lumen has been directly measured for the erAEQmut isoform and found to be indistinguishable from that measured in vitro (Montero et al., 1995). Other reasons need to be considered therefore, to explain the discrepancies obtained with ER-targeted aequorins. The data presented in this contribution demonstrate that the reported low [Ca2+]er levels measured with aequorin in steady state reflect not the level of the cation in the bulk of ER, but rather that of small subcompartments that behave differently from the rest of the endomembrane system. This artefactual underestimation of the average [Ca2+]er appears to be due, on the one hand, to the characteristics of aequorin chemiluminescence, including the Ca2+-dependent consumption of the probe; and on the other hand, to the heterogeneity in [Ca2+] of the compartments containing aequorin. Our present approach to investigate the problem has been based on the parallel use of two aequorin isoforms differing significantly in their affinity for Ca2+, and of two divalent cations, Ca2+ and its surrogate, Sr2+. Through the use of these four experimental tools we found that the kinetics and the absolute levels of [Ca2+]er obtained after calibration were apparently very different. Given that the intracellular distribution of the two aequorin isoforms is identical, the above findings could only be generated by artefacts of the calibration procedure. In particular, the experimental findings were mimicked by a model (see Appendix and Fig. A1) that assumes the compartments containing aequorin to be heterogeneous in terms of both their rates of Ca2+ accumulation and their [Ca2+] steady state values. In the initial few seconds of cation reaccumulation into cells, the measured [cation2+]er values appeared similar, no matter which probe and cation was used, and at later stages the consumption of aequorin, taking place especially with Ca2+ and with the high-affinity isoform, made the low [cation2+]er subcompartments become progressively more and more predominant in the overall signal. Thus, most aequorin values of [Ca2+]er reported appear to mainly reflect the behavior of subcompartments which, from this point of view, have little (if anything) in common with the bulk of ER. Indeed, when the steady state [Ca2+] values were in the μM level, addition of histamine caused, in the case of erAEQmut, only marginal drops or even no change; in the case of erAEQwt, no decreases, but rather small increases occurred at a time when the real effect of the agonist on the ER consisted of a maximal release of Ca2+.
A major issue of this study was to establish whether, and to what extent, the state of the ER (in particular its heterogeneity revealed by the aequorin measurements), was affected by the experimental conditions used in Ca2+ depletion preceding the Sr2+ loading. The extensive control evidence we have obtained appears to exclude the latter possibility. The idea that the ER is heterogeneous in terms of Ca2+ handling is supported by numerous experimental observations. In particular, the distributions of InsP3 and ryanodine receptors, SERCAs, and as well as those of lumenal Ca2+ binding proteins, have been shown to be quite variable in several cell types (for review see Pozzan et al., 1994). Free [Ca2+], however, had never been investigated from this point of view. The present data demonstrate that the vast majority of the ER lumen contains free Sr2+ at concentrations in the millimolar range. This conclusion also appears valid for [Ca2+]er, although it should be stressed that the [Sr2+]er values reflect only the order of magnitude of the [Ca2+]er and not its exact value. The homogeneous distribution of the cations throughout most of the aequorin-containing compartment appears to be sustained by the extensive lumenal continuity in the ER. How the tubules and cisternae of this system are organized in a network is a classical concept of cell biology; however, the information about the dynamic state of such a network has begun to emerge only recently by the use of various experimental approaches, the most recent being complementary to ours (Terasaki et al., 1994; Cole et al., 1996).
Concerning the aequorin-containing subcompartments characterized by very low (micromolar) [Ca2+], the available information is still insufficient for their cytological nature to be established with certainty. In the Golgi complex, the organelle that continuously receives membranes and proteins from the ER, high resolution ultrastuctural analysis indicates that the signal did not exceed background. Moreover, unpublished data from our group, carried out with a Golgi-targeted aequorin, indicate that the [cation2+] in the Golgi is very similar, although not identical, to that of the ER. The unexpected, moderate labeling of mitochondria is presumably due to the cross-reaction of the antitag antibodies used with a local antigen, and not to a missorting of the photoprotein, inasmuch as the mitochondrial uncoupler FCCP had no effect on the aequorin signals recorded with either Ca2+ or Sr2+. Likewise, no clear explanation is available for results showing that a small aequorin fraction is still consumed even after full blockade of SERCAs with thapsigargin. In fact, Ca2+ pumps insensitive to the inhibitor can be present in the ER (Waldron et al., 1995). An open possibility, therefore, is that the very low [Ca2+] subcompartments consists of specialized ER areas. At the moment, however, this conclusion must be considered with caution, since the very low amount of probe contained in this compartment prevents its detailed investigation. In addition, it should be mentioned that, when the depletion protocol was carried out at a low temperature, the apparent size of the low [Ca2+]er compartment decreased significantly (not shown). This result strongly suggests that the Ca2+ heterogeneity of the ER is sustained by dynamic fusion equilibria that change depending on the conditions of the cells.
Additional important information obtained from our aequorin experiments concerns the dynamic changes of [Ca2+]er in living cells. A problem extensively discussed during recent years is the existence of regions sensitive and insensitive to IP3 in the ER (Berridge and Irvine, 1989; Bootman et al., 1992; Berridge, 1993; Pozzan et al., 1994). If we exclude the very low [Sr2+]/[Ca2+] subcompartments, (which altogether account for only a small fraction (at the most 5–10%) of the environment containing aequorin, the data presented here demonstrate that InsP3 production causes a generalized drop of [Sr2+]er. When the agonist was applied in the Sr2+-free medium, without influx through the plasma membrane, the decrease in [Sr2+]er caused by histamine was almost indistinguishable from that caused by tBuBHQ, a SERCA blocker. In other words, InsP3 production does rapidly result in the reduction of the [cation2+] in most of the ER. In contrast, when InsP3 is generated in the presence of extracellular Sr2+, ER emptying is less pronounced. The reason for this effect most likely depends on at least three factors: (a) activation of Sr2+ influx through the plasmalemma via the capacitative pathway (for review see Fasolato et al., 1994); (b) inactivation of InsP3 receptors by low [Sr2+]er; and (c) increased pumping by the SERCAs once the [Sr2+]er is decreased. In particular, we and others have shown previously (Bootman et al., 1992; Clementi et al., 1992; Brini et al., 1995) that when cells are challenged with histamine, the kinetics of the cytosolic [Ca2+] (or [Sr2+]; Montero et al., 1995) changes are biphasic, composed first by a rapid peak (primarily due to cation2+ release), followed by a prolonged plateau (due to cation2+ influx), during which activation of the SERCAs is (presumably) accompanied by a reduction of the InsP3 level, and/or by partial inactivation of the InsP3-gated channels, with establishment of a new steady state. Our experiments in HeLa cells confirm that the bulk of the ER is either directly sensitive to InsP3, or equilibrates rapidly with the InsP3-sensitive regions of the organelle. In addition, our results provide at least a partial explanation to the well-established observation that the intensity of the Ca2+ current (or the rate of Ca2+ influx) triggered by agonist stimulation is much smaller when cells are maintained in a Ca2+-containing medium, rather than when the cation is reintroduced into the medium after stimulation in Ca2+-free conditions (Ca2+ depletion–Ca2+ readdition protocol). Activation of the capacitative influx is thought, in fact, to be proportional to the reduction of [Ca2+]er (Jacob, 1990; Montero et al., 1992), and, indeed, the decrease of this parameter is substantially greater in the second than in the first of these two conditions. The above conclusions are not in contrast with the recent observation in a few cell lines that prolonged increase in cytosolic [Ca2+] induces a major increase in the Ca2+ content of an as yet unidentified organelle, possibly another ER subcompartment (Pizzo et al., 1997). Our present data suggest, in fact, that if this subcompartment exists in HeLa cells, it probably contains only a minor fraction of the expressed aequorin.
Two other points need to be stressed. The first stems from the observation that, when the InsP3-producing agent was applied during the first period of ER refilling with Sr2+, there was no drop in [Sr2+]er, but only a reduction of the uptake rate. This result appears consistent with a number of indirect in vitro observations and models predicting the release of Ca2+ through InsP3 receptors dependent on [Ca2+]er (Irvine, 1990; Missiaen et al., 1992; Bezprozvanny and Ehrlich, 1994). The lack of a net decrease of [Sr2+]er, despite a major Sr2+ concentration gradient between the ER lumen and the cytosol, documents for the first time that in an intact cell, the opening of (or the flux through) InsP3-gated channels is inefficient up to a threshold level of [Sr2+]er. Such a conclusion may also be valid for [Ca2+]er, although a conclusion appears premature because the affinities of Sr2+ for lumenal sites and/or InsP3 receptors are quite different from those of Ca2+ (Marshall and Taylor, 1994; Hannaert-Merah et al., 1995). Moreover, the incubation in EGTA-containing medium was shown to lead to slow, but substantial, decreases of [cation2+] in the ER. This observation explains why, under these conditions, the decrease in cytosolic [Ca2+] is notoriously slow despite the substantial rate of the plasma membrane Ca2+ pump activity. Our data indicate, in fact, that [Ca2+]c is maintained relatively constant at the expense of a continuous efflux of the cation from the ER.
In conclusion, studies carried out in parallel with two aequorin isoforms and two divalent cations, taking advantage of their different binding properties, on the one hand have provided an explanation to the highly divergent [Ca2+]er results previously obtained with the targeted photoprotein approach; on the other hand, they have provided evidence for, or explanation to, well-known problems of Ca2+ homeostasis: differential activation of capacitative influx; [Ca2+]er regulation of IP3 receptors; and persistence of [Ca2+]c in cells incubated in Ca2+-free media. Moreover, these studies have revealed the existence of a type of heterogeneity within the ER; i.e., subcompartments with high and low [Ca2+], until now considered only on the basis of indirect evidence (Pozzan et al., 1994; Button and Eidsath, 1996). Further investigation of this last issue by other techniques, including the combination of aequorin imaging with high resolution immunocytochemistry and subcellular fractionation with molecular characterization of isolated fractions, might ultimately reveal further aspects not only important for cell Ca2+ homeostasis, but also for other various functions of the ER.
Acknowledgments
We thank G. Ronconi (University of Padova), M. Santato (University of Padova), and J. Fernandez (University of Valladolid) for technical assistance, P. Podini (St. Raffaele Science Institute, Milan, Italy) for the ultrathin cryosection immunogold labeling work, and P. Pinton (University of Padova) for his contribution in confocal microscopy experiments.
Abbreviations used in this paper
- [Ca2+]c
cytosolic-free Ca2+
- [Ca2+]er
Ca2+ level within the endomembrane system
- FCCP
carbonylcyanide p(trifluoromethoxy) phenylhydrazone
- KRB
Krebs-Ringer modified buffer
- SERCA
sarcoendoplasmic reticulum Ca2+ ATPases
- tBuBHQ
2,5-di(tert-butyl)-1,4-benzohydroquinone
Appendix
We report here in more detail about the mathematical model that predicts time-dependent variability of the relationship between aequorin photon emission and apparent [cation2+]er levels, taking into consideration the existence of heterogeneous ER subcompartments with different [Ca2+] ([Sr2+]) (Fig. 1). Ca2+ and Sr2+ are assumed to accumulate at the same rate, and therefore the differences between the calibrated values obtained with either cation, as well as those with the same cation using either erAEQwt or erAEQmut, are considered to be due only to the artefacts introduced by ER subcompartment heterogeneity. The rate of [cation2+] increase in each compartment is defined by two parameters: the maximum steady state level of [cation2+] and the τ of the exponential. Additionally, an exponentially decreasing initial delay was introduced by multiplying the cation values by a monoexponential function going from 0 (at the moment of cation2+ addition) to 1. This initial delay mimics the smooth initial increase in [cation2+]er obtained experimentally, due in part to the time required to reach the steady state [cation2+]c after cation2+ addition to the extracellular medium. An initial fitting of the experimental and theoretical curves was obtained for experiments similar to those in Fig. 2 using a model with two compartments; one accounting for 90% of the ER lumenal space and refilling exponentially up to 2.5 mM, and the other representing 10% of the space and refilling to lower [Ca2+]er levels (1–100 μM). In this first formulation, however, the model predicted rates of aequorin consumption distinctly faster than those measured experimentally with erAEQ-Sr2+, erAEQmut-Ca2+, erAEQwt-Sr2+, and erAEQwt-Ca2+. The fit of all four experimental conditions improved when the high Ca2+/Sr2+ space was divided into several subcompartments with different rates of refilling and slightly different maximal Ca2+/Sr2+ levels: first subcompartment; 35% of space, maximum cation level 2.45 mM, τ = 35 s; second subcompartment; 30% of space, maximum cation level 3.2 mM, τ = 120 s; third subcompartment; 25% of space, maximum cation level 3.1 mM, τ = 310 s. The low Ca2+/Sr2+ subcompartment was assumed to account for the residual 10% of the space. To mimic the experimental results with the four probes, this pool also had to be divided into three subcompartments; fourth subcompartment; 3% of space, maximum cation level 200 μM, τ = 40 s; fifth subcompartment; 3% of space, maximum cation level 20 μM, τ = 8 s; sixth subcompartment; 4% of space, maximum cation level 1 μM, τ = 2 s. The τ of the exponentially decreasing initial delay was set at 156 s. For the calculation of the apparent [cation2+]er, the luminescence counts produced per second in each subcompartment were calculated independently and then added to produce a virtual experiment. This experiment was then recalculated to fit the final mean [cation2+]er values shown in Fig. 2 with the four probes (erAEQwt-Ca2+, erAEQmut-Ca2+, erAEQwt-Sr2+, and erAEQmut-Sr2+), in each case taking into account only their binding affinity according to the calibration curves. Fig. A1 shows the good fit of the calibrated [Ca2+]er and [Sr2+]er theoretical curves obtained by applying the model with those obtained experimentally. The values obtained with this model for the half-times of aequorin consumption after cation addition were 11 s for erAEQwt with Ca2+, 65 s for erAEQwt with Sr2+, 31 s for erAEQmut with Ca2+, and 322 s for erAEQmut with Sr2+. The experimental values (mean ± SD) were 14 ± 3 (n = 13); 68 ± 14 (n = 11); 29 ± 4 (n = 16), and 280 ± 60 s (n = 11), respectively (Fig. 2).
It should be stressed that the values of [cation2+]er and the relative sizes of the different compartments are necessarily simplifications of the modeling, given the very dynamic and probably continuously changing structure of the ER in live cells. Although our evidence clearly indicates that in the vast majority of the ER [Ca2+] is in the ∼1 mM range, the assumption of the model that [Ca2+] is the same as that indicated by [Sr2+], in the 2.45–3.2 mM range, is an oversimplification.
Footnotes
M. Montero is a recipient of an European Union Training and Mobility of Researchers return grant and W.J.J. Scheenen of a European Union Human Capital and Mobility fellowship. This work was supported by grants from the Fondo de Investigaciones Sanitarias from the Spanish Health Ministry (96/0456) to J. Alvarez, from Telethon, from the EU programs Human Capital and Mobility, Biomed 2, and Copernicus, and from Human Frontier Science Program to T. Pozzan, R. Rizzuto, and J. Meldolesi.
Address all correspondence to Mayte Montero, Department of Biochemistry and Molecular Biology and Physiology, Faculty of Medicine, University of Valladolid, E-47005 Valladolid, Spain. Tel.: 34.83.423085. Fax: 34.83.423588. E-mail: mmontero@cpd.uva.es
References
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature (Lond) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature (Lond) 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-triphosphate (InsP3)- gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Taylor CW. All-or-nothing Ca2+mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol (Camb) 1992;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Murgia M, Pasti L, Picard D, Pozzan T, Rizzuto R. Nuclear Ca2+concentration measured with specifically targeted recombinant aequorin. EMBO (Eur Mol Biol Organ) J. 1993;12:4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c) J Biol Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- Button D, Eidsath A. Aequorin targeted to the endoplasmic reticulum reveals heterogeneity in luminal Ca2+ concentration and reports agonist- or IP3-induced release of Ca2+ . Mol Biol Cell. 1996;7:419–434. doi: 10.1091/mbc.7.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton J-Y, Liu H, Stucki JW. Simultaneous measurements of Ca2+ in the intracellular stores and the cytosol of hepatocytes during hormone-induced Ca2+oscillations. FEBS (Fed Eur Biochem Soc) Lett. 1995;368:165–168. doi: 10.1016/0014-5793(95)00632-j. [DOI] [PubMed] [Google Scholar]
- Chen W, Steenbergen C, Levy LA, Vance J, London RE, Murphy E. Measurement of free Ca2+ in sarcoplasmic reticulum in perfused rabbit heart loaded with 1,2-bis(2-amino-5,6-difluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid by 19F NMR. J Biol Chem. 1996;271:7398–7403. [PubMed] [Google Scholar]
- Clementi E, Scheer H, Zacchetti D, Fasolato C, Pozzan T, Meldolesi J. Receptor-activated Ca2+influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science (Wash DC) 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- Dawson AP, Rich GT, Loomis-Husselbee JW. Estimation of the free [Ca2+] gradient across endoplasmic reticulum by a null point method. Biochem J. 1995;310:371–374. doi: 10.1042/bj3100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+influx: how many mechanisms for how many channels? 1994. Trends Pharmacol Sci. 1994;15:77–82. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O, Gerasimenko J, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP- ribose-mediated release of Ca2+from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Hannaert-Merah Z, Combettes L, Coquil JF, Swillens S, Mauger J-P, Claret M, Champeil P. Characterization of the coagonist effects of strontium and calcium on myo-inositol thisphosphate-dependent ion fluxes in cerebellar microsomes. Cell Calcium. 1995;18:390–399. doi: 10.1016/0143-4160(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Hirose K, Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+stores. Nature (Lond) 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Machen TE. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci USA. 1993;90:2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Schlue W-R, Curci S, Machen TE. Spatial distribution and quantitation of free luminal [Ca] within the InsP3-sensitive internal store of individual BHK-21 cells: ion dependence of InsP3-induced Ca release and reloading. FASEB (Fed Am Soc Exp Biol) J. 1995;9:788–798. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- Irvine RF. ‘Quantal' Ca2+ release and the control of Ca2+entry by inositol phosphates — a possible mechanism. FEBS (Fed Eur Biochem Soc) Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol (Camb) 1990;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall JM, Dormer RL, Campbell AK. Targeting aequorin to the endoplasmic reticulum of living cells. Biochem Biophys Res Commun. 1992;189:1008–1016. doi: 10.1016/0006-291x(92)92304-g. [DOI] [PubMed] [Google Scholar]
- Kendall JM, Badminton MN, Dormer RL, Campbell AK. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal Biochem. 1994;221:173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- Kendall JM, Badminton MN, Salanewby GB, Campbell AK, Rembold CM. Recombinant apoaequorin acting as a pseudo-luciferase reports micromolar changes in the endoplasmic reticulum free Ca2+of intact cells. Biochem J. 1996;318:383–387. doi: 10.1042/bj3180383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987;105:2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ICB, Taylor CW. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J. 1994;301:591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady state phenomenon controlled by luminal Ca2+in permeabilized cells. Nature (Lond) 1992;357:599–601. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Montero M, Alvarez J, García-Sancho J. Control of plasma-membrane Ca2+ entry by the intracellular Ca2+-stores. Kinetic evidence for a short-lived mediator. Biochem J. 1992;288:519–525. doi: 10.1042/bj2880519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+concentration in the endoplasmic reticulum of intact cells. EMBO (Eur Mol Biol Organ) J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Fasolato C, Pozzan T. Dynamic properties of an inositol-1,4,5-trisphosphate and thapsigargin-insensitive calcium pool in mammalian cell lines. J Cell Biol. 1997;136:1–13. doi: 10.1083/jcb.136.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–635. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AWM, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+revealed by specifically targeted recombinant aequorin. Nature (Lond) 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Pozzan T. Targeting recombinant aequorin to specific intracellular organelles. Methods Cell Biol. 1994;40:339–358. doi: 10.1016/s0091-679x(08)61121-8. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Bastianutto C, Marsault R, Pozzan T. Photoprotein-mediated measurement of calcium ion concentration in mitochondria of living cells. Methods Enzymol. 1995;260:417–428. doi: 10.1016/0076-6879(95)60155-4. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, De Giorgi F, Rossi R, Tsien RY, Pozzan T. Double labeling of subcellular structures with organelle-targeted GFP mutants in vivo. Curr Biol. 1996;6:183–188. doi: 10.1016/s0960-9822(02)00451-7. [DOI] [PubMed] [Google Scholar]
- Sitia R, Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992;3:1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- Stehno-Bittel LS, Lückhoff A, Clapham DE. Calcium release from the nucleus by InsP3receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature (Lond) 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Streb H, Bayerdorffer E, Haase W, Irvine RF, Schulz I. Effect of inositol 1,4,5-trisphosphate in isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81:241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Tse A, Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+oscillations. Proc Natl Acad Sci USA. 1994;91:9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Sharp AH, Racchetti G, Podini P, Bole DG, Dunn WA, Pozzan T, Snyder SH, Meldolesi J. The endoplasmic reticulum of Purkinje neuron body and dendrites: molecular identity and specialization for Ca2+transport. Neuroscience. 1992;49:467–477. doi: 10.1016/0306-4522(92)90111-e. [DOI] [PubMed] [Google Scholar]
- Volpe P, Krause KH, Hashimoto S, Zorzato F, Pozzan T, Meldolesi J, Lew DP. Calciosome, a cytoplasmic organelle: the inositol trisphosphate-sensitive Ca2+store of nonmuscle cells? Proc Natl Acad Sci USA. 1988;85:1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron RT, Short AD, Gill DL. Thapsigargin-resistant intracellular calcium pumps. Role in calcium pool function and growth of thapsigargin-resistant cells. J Biol Chem. 1995;270:11955–11961. doi: 10.1074/jbc.270.20.11955. [DOI] [PubMed] [Google Scholar]