Abstract
Background/Objective:
Postprocedural infections are a significant cause of morbidity after spinal interventions.
Methods:
Literature review. An extensive literature review was conducted on postprocedural spinal infections. Relevant articles were reviewed in detail and additional case images were included.
Results:
Clinical findings, laboratory markers, and imaging modalities play important roles in the detection of postprocedural spinal infections. Treatment may range from biopsy and antibiotics to multiple operations with complex strategies for soft tissue management.
Conclusions:
Early detection and aggressive treatment are paramount in managing postprocedural spinal infections and limiting their long-term sequelae.
Keywords: Postprocedural spinal infection, Osteomyelitis, Epidural abscess, Complications of spinal fusion, Surgical site infection
INTRODUCTION
Spinal surgery continues to become more sophisticated. With an expanding number of techniques and technologies, the number of invasive procedures continues to increase. Some procedures are diagnostic, such as diskography, myelography, and lumbar puncture, whereas others are therapeutic. Regardless of the type of intervention, all invasive modalities carry an associated risk of postprocedural infection. The rate of infection is proportional to the magnitude of intervention (1). Despite undeniable improvement in diagnostic modalities, antibiotic therapy, surgical technique, and postoperative care, infectious complications result in significant morbidity and occasional mortality (2). Infection rates vary with the type of surgery and the anatomical site. The incidence of surgical site infection after decompressive laminectomy, diskectomy, and fusion is quoted to be 3% or even lower, but the incidence increases to as high as 12% with the addition of instrumentation (3–7). As efforts to contain health care costs continue, many procedures are done with same-day or brief acute hospital stays. Even after extensive procedures, patients are often transferred to inpatient rehabilitation facilities or discharged home within the first few days. As a result, physiatrists, internists, and visiting advanced practice nurses are often the providers that are in direct contact with these patients during the time frame that postprocedural infections become manifest. The focus of this article is on the pathogenesis, diagnosis, and treatment of postprocedural infections, because early identification and appropriate management are paramount for limiting the scope of residual morbidity.
PATHOGENESIS
The development of a surgical site infection after spine surgery is multifactorial. However, the various risk factors contributing to the development of infection in this patient population can be classified into 3 broad categories: microbiological, patient/host, and surgical. Understanding the basic science behind the pathogenesis of surgical site infections fosters efforts at prevention and management of this complication.
Microbiology
For a clinical infection to occur at the surgical site, bacteria must be present at the operative/procedural site in substantial quantity (>105 organisms) (8). Three possible sources of bacteria are direct inoculation at the time of surgery (9–11), soiling of the incision in the fresh postoperative phase (12), or through hematogenous seeding (13). Most postprocedural infections are a consequence of direct inoculation and thus meticulous operative technique is paramount.
Review of the literature indicates a fairly consistent source for postoperative infection. The primary pathogens in acute infections are the gram-positive cocci, specifically Staphylococcus aureus, Staphylococcus epidermidis, and β-hemolytic Streptococci. S aureus is the most common organism cultured from acute postoperative infections (2,6,14). Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Proteus species are the common gram-negative isolates from post-operative wounds (1). Delayed or chronic infections are usually caused by skin flora of low virulence such as Propionibacterium acnes (10) and diphtheroids (2). Patients who abuse intravenous drugs have a higher incidence of infections with gram-negative rods (1). Infections with nosocomial organisms are more common in patients with a protracted hospital course or ICU stay (14).
Prophylactic antibiotics have been clearly established as an effective adjunct to decrease the chance of a surgical site infection. Keller and Pappas (3) reported a dramatic decrease in infection rates from 2.7% to 0% with the use of preoperative prophylactic antibiotics. Infection rates after lumbar diskectomies dropped from 9.3% to 1% with the use of preoperative antibiotics (15). Another study showed that infection rates were significantly lower (4.3%) in patients treated with preoperative antibiotics vs those treated with placebo (12.7%) before undergoing clean lumbar surgery (16). A laboratory study by Guiboux et al (17) showed that postoperative disk space infection in a rabbit model was effectively prevented by a single preoperative dose of intravenous cefazolin or vancomycin given within 1 hour before surgery, and no benefit was rendered in giving postoperative doses. Most spine surgeons recommend administering a first-generation cephalosporin (or clindamycin/vancomycin in patients with penicillin allergy) within 1 hour before the surgery. Because antibiotic levels have been shown to decrease with operative time (18,19), some surgeons recommend redosing patients after 4 hours of operative time. However, clinical studies comparing a single preoperative dose to multiple intraoperative doses of antibiotics fail to show any statistical difference (16,20). Because antibiotic-resistant infections have become an increasingly prevalent problem, many hospitals have increased efforts to monitor emerging patterns of antibiotic resistance and to regulate the use and duration of antibiotics for prophylactic purposes. Methicillin-resistant S aureus (MRSA) is the most common resistant organism isolated. Studies have shown that infections caused by resistant organisms may be associated with increased morbidity, mortality, and costs. Some risk factors associated with MRSA infections include previous hospitalization, intensive care unit (ICU) stay, indwelling catheters, before prolonged antibiotic therapy, advanced age, and exposure to patients colonized or infected with MRSA (21).
Patient/Host Factors
Several patient-related factors play a role in the pathogenesis of infection; some are modifiable, whereas others are nonmodifiable. Advanced age, developmental delay, immunosuppression, spinal trauma, and diabetes mellitus are some nonmodifiable risk factors. Obesity, smoking, indwelling catheters, malnutrition, and extended hospitalization are modifiable risk factors. High rates of infection have been observed in elderly patients and spine trauma patients (13,22,23). Insensate patients with spinal dysraphism and developmental delay undergoing scoliosis surgery have also been found to have higher rates of postoperative infection (12).
Obesity has also been quoted as a risk factor by many (8,14,24). This may be related to both biophysical alterations in obesity and technical issues with surgery. Tissue dissection in these patients can be extensive. Wide retraction and use of electrocautery can lead to fat necrosis and large devitalized areas promoting bacterial proliferation (8). Malnutrition, objectively defined by a serum albumin level <3.5 g/dL, a total lymphocyte count less than 1,500 to 2,000 cells/mm3 and a serum transferrin level of 150 μg/dL is another documented risk factor. Klein and Garfin (25) reported preoperative malnourishment in 25% of 114 patients undergoing elective lumbar fusion and 11 of 13 reported complications in this series occurred in this malnourished group. Paradoxically, obesity also can be a risk factor for malnutrition, especially in those patients undergoing rapid weight reduction (1).
Malignancy, chemotherapy, immune suppression, and diabetes mellitus have also been implicated as risk factors for postprocedural infections (2,24–26). Diabetes mellitus, especially when it is uncontrolled with blood glucose levels greater than 200 mg/dL is shown to impede chemotaxis and phagocytosis, hence diminishing immune response to bacteria/infections (2,27–32). Other causes of immunosuppression, such as rheumatoid arthritis (particularly when requiring corticosteroid treatment), cancer patients on chemotherapy, and patients infected with human immunodeficiency virus who have low CD4 counts, all can increase the potential for postoperative infectious complications. Operating through previously irradiated tissue or previously operated tissue has also shown an increased risk for surgical site infection (2,14).
Most surgeons will attempt to address modifiable factors preoperatively to minimize the risk of postoperative infection. Patients should be counseled to quit smoking both for reducing risk of infection and preventing pseudarthrosis. All active infections should be identified and treated before undertaking elective spine surgery (1,2,27,28,30). Systemic conditions such as diabetes, rheumatoid arthritis, HIV, and malnutrition should be optimized preoperatively. Furthermore, patients with a prolonged hospital course may be considered for discharge and return as a same-day admission for elective spinal surgery to minimize nosocomial/iatrogenic infections.
Surgical Factors
In general, the invasiveness or complexity of the procedure is directly related to the infectious complication rate. Postprocedural diskitis has been reported after a plethora of spinal interventions including less invasive procedures such as diskography (33), chemonucleosis (34,35), myelography (36), paraspinal injections, lumbar punctures, and epidural injections. Diskectomy has infection rates ranging from 0.6% to 5% (1,37). Fusion without instrumentation has been associated with infection rates from 0.4% to 4.3%. The use of internal fixation raises the risk significantly (6.6–8.7%) (28,38). Several theories attempt to explain this increase likelihood of infection. It is suggested that instrumentation can cause local soft tissue irritation leading to inflammation and seroma formation with subsequent infection. The implants provide an avascular surface for bacteria to lay down a glycocalyx, which in turn protects them from antibiotic penetration (1). Finally, metallosis from micromotion of the instrumentation leads to granuloma formation and yet another medium for bacterial colonization (9).
Unlike posterior thoracolumbar wounds, anterior cervical surgery generally has a very low infection rate of 0% to 1% (2,6,39). When infection occurs after an anterior surgical procedure, a high index of suspicion must be maintained to rule out pharyngoesophageal injury. Recommendations to decrease the risk of injury include the use of blunt retractors, which are intermittently released; caution should be exercised with deep retractor placement under the longus coli (1). Posterior cervical surgery has a higher rate of infection, comparable to posterior lumbar surgery at approximately 1% for simple decompressions (39) and as high as 4.5% (40) to approximately 9% for instrumented fusions (5). Surgical time correlates with the absolute number of bacteria found in the wound. Some authors have shown an increased risk after 3 hours of surgery (14). Careful preoperative planning and efficient execution can help minimize unnecessary delay in surgical treatment and improve infection rates.
CLASSIFICATION
Surgical site infections are commonly subclassified as superficial or deep. Superficial infections are limited to the skin and subdermal/subcutaneous layer without fascial involvement. Deep infections occur below the lumbodorsal fascia for a posterior lumbar wound or below the ligamentum nuchae and fascial layer for posterior cervical wounds. Anteriorly, deep infections occur below the anterior abdominal fascia or below the platysma layer for the lumbar and cervical wounds, respectively. Deep infections may also involve the disks or the spinal column resulting in diskitis, osteomyelitis, or epidural abscess. These infections can further be classified into acute (within 3 weeks of the procedure) or chronic/delayed (>4 weeks after the procedure) (1).
CLINICAL PRESENTATION
Worsening pain 1 to 4 weeks after a spinal procedure is the most common symptom suggestive of infection (41). Pain may or may not follow a period of initial relief. The patients often confuse this pain as a recurrence of their original back pain, thus confusing the diagnosis with that of a mechanical cause. The back pain is generally out of proportion to the physical findings and may be referred to the buttock, thigh, leg, groin, perineum, or abdomen (37). Constitutional symptoms have also been reported but are less common than pain. A floridly septic picture with high fevers, chills, and sweats is a rare, but dramatic, presentation (37). In the setting of superficial infections, local wound changes, such as erythema or drainage, may be present (1,2,27). Postoperative infections after anterior cervical procedures may present with painful swallowing secondary to a retropharyngeal abscess. Persistent progressive pain is once again the hallmark of a developing infection. Occasional drainage is noted and frank sepsis is uncommon. The patients, however, may complain of malaise and night sweats.
Significant pain with lumbar range of motion is the sine qua non for postprocedural diskitis (37). The incision is usually unremarkable, and in fact, less than 10% of surgical incisions show signs of purulent infection with erythema, drainage, or dehiscence (41). The presence of a neurological deficit (motor/sensory involvement or bowel/bladder changes) should raise the suspicion for an epidural abscess. Sixteen percent of epidural abscess are postoperative complications (1).
Although surgical site infections may present with a benign appearing wound, significant wound drainage beyond the first postoperative week should always be a cause for concern. Because many patients are either discharged home or transferred to inpatient rehabilitation centers within the first few days, this important time frame is often outside the direct supervision of the surgeon. Specific discharge instructions regarding surveillance of the wound are best reinforced by periodic correspondence between the surgical team and visiting nurses or staff at the rehabilitation facility. The surgeon should be alerted of any ongoing drainage, erythema, dehiscence, or fevers.
LABORATORY MARKERS
Several laboratory tests can be used to help establish the diagnosis of postprocedural infection. Initial blood work should consist of a complete blood count (CBC) with a differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood cultures.
The white blood cell (WBC) count may, or may not, be elevated, depending on host and pathogen factors. Thus, this parameter is unreliable, when used in isolation, for the diagnosis of infection. Less than 50% of the cases of infective spondylodiskitis had an elevated WBC count according to a recent report (42).
Similar to the WBC count, the ESR alone is not very specific for infection. However, it is a relatively sensitive marker for an inflammatory process and can be reliably followed during the treatment course of a patient to assist with clinical decision making such as cessation of antibiotics. In the setting of infection, the ESR is 78% to 82% sensitive and 38% to 62% specific as a laboratory marker (43,44). When interpreting ESR values, it is paramount to understand that the ESR is routinely elevated in the first 1 or 2 weeks after a procedure/operation of the spine. Several authors have attempted to delineate the ESR trend in the postoperative period. Kapp and Sybers (45) reported that the ESR was rarely elevated greater than 25 mm/h and that it generally returned to baseline by the third week of an uncomplicated spine surgery. Jonsson et al (46) reported a higher postoperative peak in ESR in more extensive spine procedures (102 mm/h) compared with the limited procedures (75 mm/h). Furthermore, they reported that the ESR peaked on the fourth postoperative day and returned to baseline after 2 weeks in most patients. A study conducted by Thelander and Larsson (47) showed an even longer postoperative period (6 weeks) during which the ESR was elevated. In light of these data, the ESR is not thought to be useful as a definitive marker for infection.
CRP, an acute-phase reactant, is possibly the most sensitive indicator of postoperative infection (43,44). Both, CRP elevation and its return to baseline after surgery are more rapid compared with the ESR values. CRP levels usually peak on the second or third postoperative day and normalize within 2 weeks after surgery/procedure. A rise in CRP values after the aforementioned timeframe correlates highly with the presence of an infection (44). Some authors recommend obtaining preoperative ESR and CRP values as a baseline for comparison with postoperative measurements (43–49). In the future, other laboratory markers such as interleukin-6 (IL-6) may provide us with the most accurate and reliable means of diagnosing surgical site infection.
IMAGING
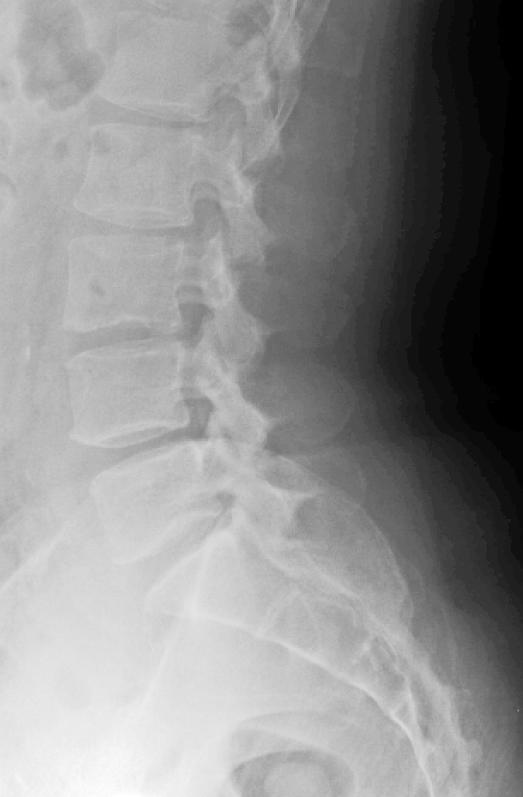
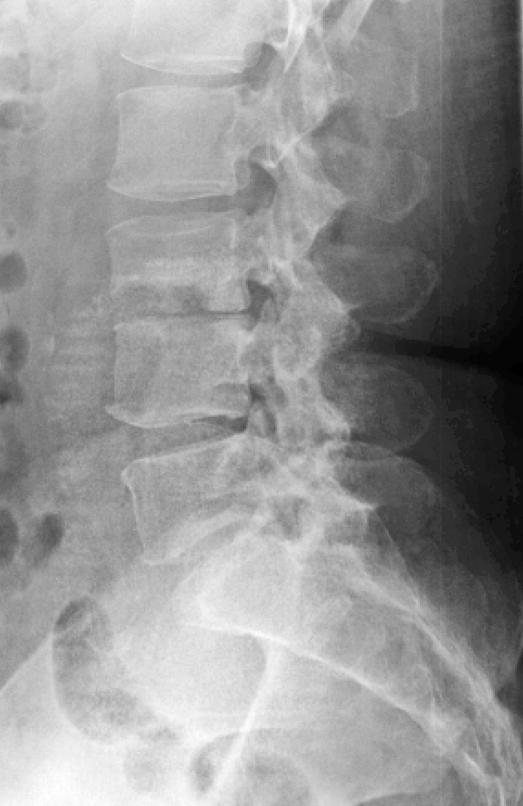
Plain radiographs are the first imaging modality obtained when infection is suspected; however, negative radiographic findings do not exclude infection. Usually, there are no changes in the first 3 weeks (Figure 1), even as clinical and laboratory markers are emerging (37). In cases of diskitis, disk space narrowing and endplate changes are the earliest findings (Figure 2), seen from the fourth to the sixth postoperative week (41). Paravertebral soft tissue shadows may be visible on plain films and might signify a paravertebral abscess. Plain radiographs are also helpful in inspecting surgical implants. Lysis around the implants (suggestive of loosening) and overall alignment can be readily assessed from the x-rays.
Figure 1. Lateral plain radiograph of a patient with recurrent back and leg pain 3 weeks after a percutaneous diskectomy at L3–L4.
Figure 2. Same patient 3 weeks later showing loss of height and endplate rarefaction at L3–L4.
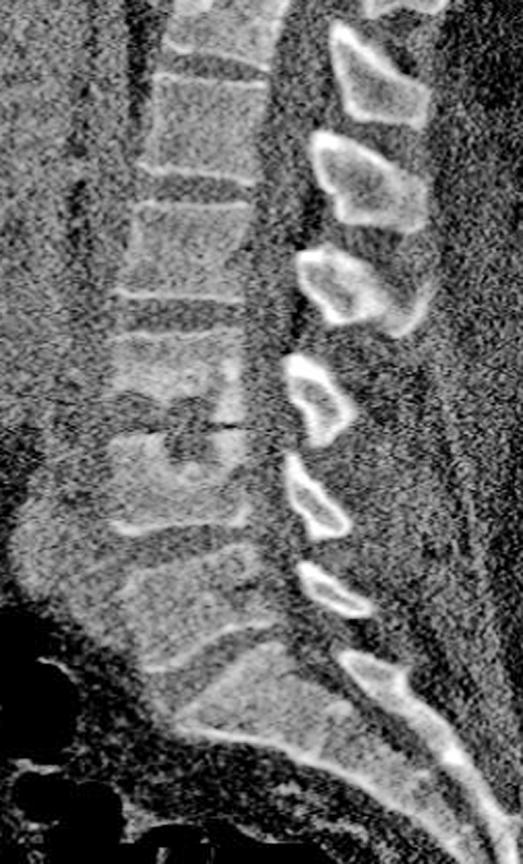
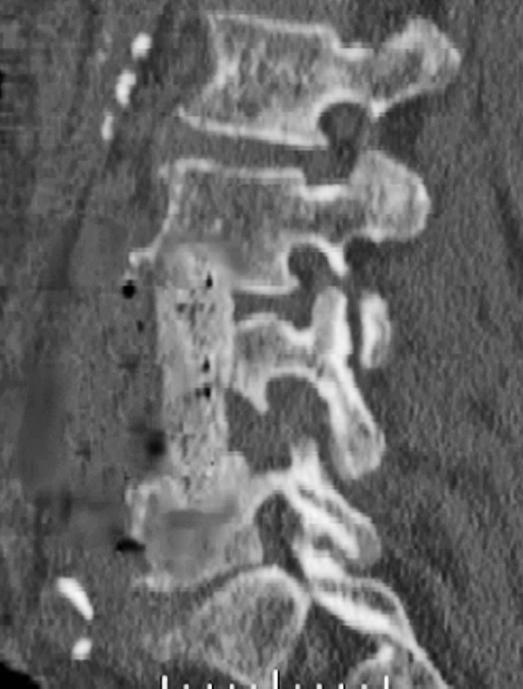
Computed tomography (CT) scans show areas of early bony destruction and soft tissue collections with better anatomical detail than plain radiographs (41). Similar to plain films, early changes include that of erosive and destructive changes at the endplates and disk space narrowing (Figure 3). The image quality and the level of detail may be compromised in the presence of instrumentation-related scatter/artifact, particularly if stainless steel implants were used. CT guidance can also be used to obtain a tissue biopsy of postoperative diskitis/osteomyelitis or needle aspiration of pus from abscess cavities to provide a microbiological diagnosis (50). The specimens should be sent for Gram stain and cultures to identify the organism and its susceptibilities before starting antimicrobial therapy.
Figure 3. Sagittal reformatted CT of the same patient showing the endplate destruction associated with diskitis/osteomyelitis.
Nuclear medicine studies have been used as part of the diagnostic workup of spinal infections, but usually show nonspecific increased uptake in the majority of cases (37). Gallium 67 often identifies the presence of a postoperative disk space infection earlier than techne-tium-99m scans or plain films (51,52). The results are less accurate and predictive than they are for appendicular infections. A sequential technetium-99m study followed by a gallium-67 study increases the cumulative accuracy of these studies (1). Indium 111-labeled WBC scans may also be useful in establishing the diagnosis but are infrequently used secondary to suboptimal specificity (11).
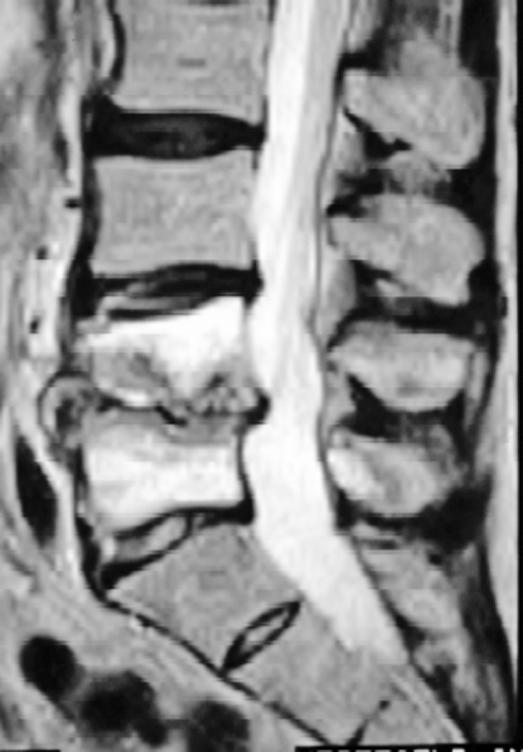
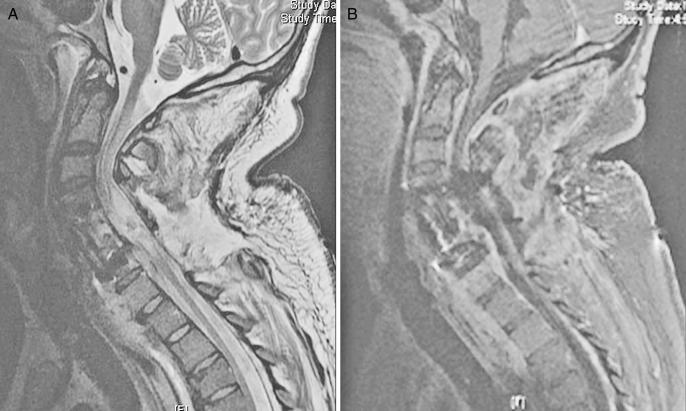
Magnetic resonance imaging (MRI) with gadolinium enhancement is the imaging modality of choice to delineate postprocedural spine infections. MRI is shown to have the highest sensitivity and specificity in the diagnosis of postprocedural diskitis, superior to both technetium-99 and gallium-67 scans (53), with a reported sensitivity of 93% and a specificity of 97%. MRI findings suggestive of postprocedural diskitis include a decreased signal on T1 and an increased signal on T2-weighted images in the disk space, secondary to edema from the inflammation and infection (Modic I changes). There may also be edema of the adjacent endplates seen as increased signal intensity on T2-weighted images (54) (Figure 4). According to a study by Van Goethem et al (55), contrast enhancement of the disk space/paraspinal tissues in the absence of Modic I changes is suggestive of infection when comparing MRI scans of asymptomatic individuals with those with biopsy proven diskitis. An epidural abscess is seen as an area isointense with the cord or cauda equina on T1-weighted images that may cause effacement of the neural structures, and on the T2-weighted images, these lesions reveal a high signal expression. Gadolinium-enhanced T1-weighted images provide additional evidence of infection, as enhancement of the collection is typically quite apparent (Figure 5A and B).
Figure 4. MRI of a patient who developed diskitis after L4–L5 microdiskectomy. Note the increased signal intensity of the endplates and disk on this T2-weighted image.
Figure 5. Sagittal MRIs of a patient who developed osteomyelitis and epidural abscess after anterior-posterior spinal fusion with corpectomy. T2-weighted MRI (A) and sagittal T1 after administration of gadolinium (B). Note the epidural abscess that is well delineated on the post-gadolinium image.
As previously discussed, infection after an anterior cervical procedure is rare and may be caused by an injury to the pharynx or esophagus during the surgery (56). Delay in presentation of 2 to 4 months has been reported (57). In addition to the tests described above, an otolaryngology consultation and evaluation should be requested. A contrast esophagogram can show a leak in 75 % to 100% of such cases (58).
TREATMENT
Treatment of postprocedural spine infections should be initiated in a timely fashion. Both nonsurgical and surgical management have a role in selected cases. Identification of a microbial pathogen and administration of specific antibiotics are essential. In cases of diskitis after microdiskectomy or spinal injections (diskograms), CT-guided biopsy and aspiration may be sufficient to establish the diagnosis and start antibiotics. Blood cultures may also be obtained to help guide the antimicrobial therapy. While cultures are pending, broad-spectrum antibiotics with antistaphylococcal coverage may be instituted. The cornerstones of nonoperative management of postprocedural diskitis are immobilization/bracing and organism-specific antibiotic therapy (37). The exact length of the antimicrobial treatment is based on clinical, laboratory, and radiographic responses. In the nonoperatively managed patients, serial clinical examination and laboratory surveillance tests with WBC count, ESR, and CRP are performed. Special considerations for the management of a surgical site infection involving antibiotic-resistant organisms include minimizing patient contact and reduction of the spread of the organism by adhering to universal precautions (59) and the use of organism-specific antimicrobials. However, with the increasing prevalence of resistant organisms, standard antibiotic regimens are becoming less effective. Fortunately, new classes of antibiotics with activity against MRSA and vancomycin-resistant enterococci (VRE) are available. Quinupristin/dalfopristin (streptogramin class) and line-zolid are examples of new antimicrobials that are effective in treating approximately 80% of the VRE and other gram-positive infections (21). In addition to systemic parenteral antibiotic therapy, local wound care along with the use of topical antimicrobial agents may also be of benefit in eradicating resistant organisms. Wright et al (60) showed that silver in the form of silver-coated dressing is a useful prophylactic or therapeutic agent for the prevention of wound colonization by organisms that impede healing, including antibiotic-resistant bacteria.
Absence of improvement after 6 weeks of antibiotics and bracing may necessitate an open biopsy, surgical debridement, and stabilization (27). Other indications for open surgical debridement and management of post-procedural spine infections are as follows: drainage from or dehiscence of the incision, clinical sepsis, neurological deficits secondary to fluid collection or mass effect, a spinal or epidural abscess, and instability from bone destruction or implant/fixation failure (27,42). The goal of surgery is to debride all necrotic and nonviable tissue followed by stabilization of the spine to prevent deformity and/or neurologic injury.
In certain cases of refractory diskitis/osteomyelitis after less invasive procedures (diskograms, IDET, etc), minimally invasive techniques entailing percutaneous disk debridement and fusion have been described (42,61,62). This approach is appropriate only in the absence of a significant superficial or deep collection and would not be used for an open draining wound. Minimally invasive techniques have the advantages of less postoperative pain, shorter hospital stay, and possibly an accelerated healing response (42,61,62). The success in the lumbar spine is overshadowed by the high complication rate of 33% in the thoracic spine. Complications include the need for conversion to open procedures to address inadequately drained epidural abscesses (42).
The principles of open surgical treatment are exploration of the wound to define the extent of the infection (deep vs superficial), followed by debridement of necrotic and infected tissue and bone graft. Removal of instrumentation has long been debated in the literature. Some recommend removing all bone graft and spinal implants (63), whereas others support leaving well-fixed posterior instrumentation (particularly titanium) and bone grafts in place, even in the face of active infection (3,5,6,30,64,65). Radical debridement of the involved disks and vertebral bodies usually entails an anterior approach with structural bone grafting (Figure 6), followed by posterior instrumentation and fusion. The procedure may be staged if the patient can not tolerate a prolonged operation.
Figure 6. Sagittal reformatted CT after anterior debridement and strut grafting for the patient with postoperative osteomyelitis shown in Figure 4. The patient later underwent a staged posterior fusion with instrumentation.
Some recent reports using anterior instrumentation in the cervical spine in the setting of gross infection have cited good results, including eradication of the infection (66–68). Usually surgical site infections after an anterior instrumented fusion are managed with removal of all spinal hardware and necrotic tissue with autograft reconstruction of the defect (37). Recently, allografts have gained favor because they are readily available, but the literature indicates a higher incidence of infection after the use of allograft bone (12). Displacement of the vertebral column, neural compression, and paralysis are potential complications associated with loss of spinal stability when implants are removed before adequate spinal fusion (2,69,70). In addition, in vivo studies have shown less adhesion of bacterial-produced biofilm to titanium surfaces compared with stainless steel (71). Therefore, we advocate retaining well-fixed titanium spinal implants whenever feasible while treating with a combination of operative debridement and intravenous antibiotics to eradicate infection. Removal of instrumentation may be indicated, however, if the patient presents with a late infection after the spine segments have been fused (3–9 months) or if the implants are grossly loose. The latter scenario poses a challenge to the treating physician as removal of spinal instruments in the absence of fusion requires debridement with re-instrumentation incorporating more levels and or external brace stabilization.
Deep tissue specimens should be sent for cultures in the setting of suspected infection because of the high yield of this tissue (100%) (72). Thorough lavage with saline is recommended after debridement. Addition of an antibiotic to the irrigant has been shown to decrease the bacterial load (73). The wound may be closed over drains (1,6), which are removed when the output decreases to less than 20 mL/12 h. Thalgott et al (74) reported that immunocompetent patients who had a single organism needed only 1 operation with a thorough irrigation and debridement followed by regular closure over closed suction drains. Those with multiple organisms needed up to 3 surgeries and inflow–outflow irrigation drains, whereas those with multiple organism and myonecrosis had wounds that were left open to heal. Weinstein et al (2) left all their deep infections open and packed them with gauze until the wound bed appeared healthy. This was followed by wound closure over drains in a delayed fashion. Glassman et al (27) studied the use of antibiotic-impregnated cement beads as an adjunct to serial irrigation and debridements. Tobramycin or a combination of tobramycin and vancomycin were used in the beads, and they reported good results with no recurrence in 19 of 22 patients with deep infections at a mean follow-up of 23 months. Recorded local levels of antibiotics are high and have been shown to be effective in the infected spine injury rabbit model (75).
In the case of an epidural abscess, the surgical approach is decided by the location of the abscess (1). An anterior abscess in the cervical or thoracic spine is best approached ventrally and a dorsal abscess is approached from a posterior approach. Anterior approaches are also effective for debriding vertebral osteomyelitis and for reconstructing the anterior spinal column. For anterior cervical abscesses, an intraoperative evaluation of the pharynx and esophagus by a head and neck surgeon may be indicated. In the case of pharyngoesophageal fistulae, a diversion and/or a local muscle flap may be needed (1).
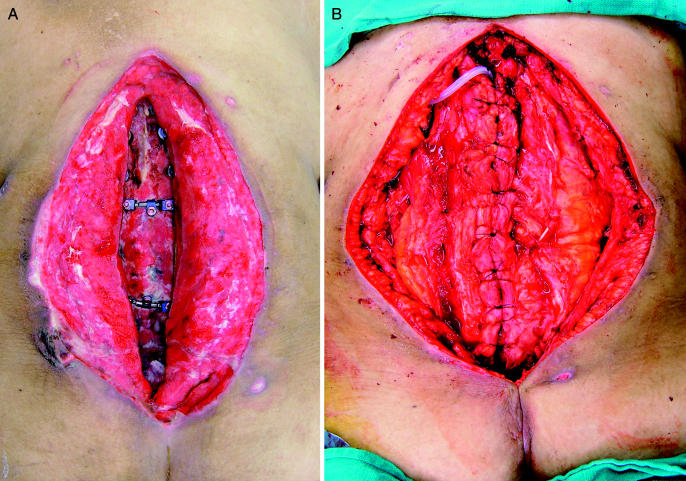
In the authors' opinion, large posterior wounds, left open for delayed closure, should be evaluated by a plastic surgeon. The objectives of closure are wound coverage, obliteration of dead space, padding of exposed structures, and enhancing local vascularity, thus improving antibiotic transport and leukocyte function. A relatively newer technique of wound management is the use of a vacuum-assisted closure device (VAC; KCI Medical, San Antonio, TX) to assist in granulating open wounds over hardware and bone (76,77). The VAC sponge introduces negative pressure to the wound, resulting in removal of interstitial edema, improvement of blood supply, and stimulation of cellular proliferation of granulations tissue (Figure 7A and B). Bacterial cell counts from the tissues have been shown to decrease compared with control wounds without the VAC. Potential contraindications to use of the VAC include the presence of fistulae, neoplasms, bleeding diatheses, and anticoagulation.
Figure 7. Intraoperative photograph of a patient who developed a severe surgical site infection after an extensive posterior thoracolumbar fusion. Note the extensive granulation tissue that was induced by the use of the VAC system (A). (B) Rotational muscle flap that was later done by the plastic surgery service for definitive closure.
COMPLICATIONS
Complications of surgical site infections are related to the morbidity of the infection and the treatment rendered. Often, multiple surgeries and long-term antibiotic administration are needed. Some of the acute and major complications include neurologic injury, sepsis, Clostridium difficile colitis, multiorgan failure, and death (2). The long-term sequelae are frequently associated with pseudarthrosis (2,78,79) and generally result in chronic pain and poor function.
SUMMARY
Postprocedural infections are an uncommon yet significant cause of morbidity after spinal interventions. The key to a successful outcome is clinical diligence to establish a timely diagnosis and institute an expedient treatment. Laboratory markers and advanced imaging modalities may help identify subtle cases of deep infections with a benign appearing wound. Certain cases of diskitis and osteomyelitis that are not accompanied by a large fluid collection, draining wound, or spinal instrumentation can often be treated by a CT-guided biopsy/aspiration to identify the pathogen followed by antimicrobial treatment. If operative management is needed, one or more visits to the operating room may be required for adequate management. Infections add morbidity to the patients' care and recovery and portend a decreased fusion rate with potentially diminished functional outcome. Meticulous operative technique and vigilant postoperative follow-up are imperative in minimizing this complication.
REFERENCES
- Bassewitz HL, Fishgrund JS, Herkowitz HN. Postoperative spine infections. Semin Spine Surg. 2000;12:203–211. [Google Scholar]
- Weinstein MA, McCabe JP, Cammisa FP. Postoperative spinal wound infection: a review of 2391 consecutive index procedures. J Spinal Disord. 2000;13:422–426. doi: 10.1097/00002517-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthop Clin North Am. 1972;3:99–111. [PubMed] [Google Scholar]
- Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD. Treatment of postoperative wound infections following spinal fusion and instrumentation. J Spinal Disord. 1995;8:278–283. doi: 10.1097/00002517-199508040-00003. [DOI] [PubMed] [Google Scholar]
- Lonstein J, Winter R, Moe J, Gaines D. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop. 1973;96:222–233. [PubMed] [Google Scholar]
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975–980. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- Roberts FJ, Walsh A, Wing P, Dvorak M, Schweigel J. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366–370. doi: 10.1097/00007632-199802010-00016. [DOI] [PubMed] [Google Scholar]
- Cruse PJ, Foord R. A five year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206–210. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- Dubousset J, Shufflebarger H, Wenger D. Late infection with CD instrumentation. Orthop Trans. 1994;18:121. [Google Scholar]
- Richards BS, Herring JA, Johnston CE, Birch JG, Roach JW. Treatment of adolescent idiopathic scoliosis using Texas Scottish Rite Hospital instrumentation. Spine. 1994;19:1598–1605. doi: 10.1097/00007632-199407001-00008. [DOI] [PubMed] [Google Scholar]
- Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 1997;22:2444–2451. doi: 10.1097/00007632-199710150-00023. [DOI] [PubMed] [Google Scholar]
- Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KW, Lenke LG. Deep wound infection after neuromuscular scoliosis surgery. Spine. 2000;25:2461–2466. doi: 10.1097/00007632-200010010-00007. [DOI] [PubMed] [Google Scholar]
- Heggeness MH, Esses SI, Errico T, Yuan HA. Late infection of spinal instrumentation by hematogenous seeding. Spine. 1993;18:492–496. [PubMed] [Google Scholar]
- Wimmer C, Gluch H, Frannzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–128. [PubMed] [Google Scholar]
- Horowitz NH, Curtin JA. Prophylactic antibiotics and wound infections following laminectomy for lumbar disc herniation. J Neurosurg. 1975;43:727–731. doi: 10.3171/jns.1975.43.6.0727. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Findler G, Amit P, Shaked I. Perioperative prophylactic cefazolin in spinal surgery. A double blind placebo-controlled trial. J Bone Joint Surg Br. 1994;76:99–102. [PubMed] [Google Scholar]
- Guiboux JP, Cantor JB, Small SD, Zervos M, Herkowitz HN. The effect of prophylactic antibiotics on iatrogenic intervertebral disc infections: a rabbit model. Spine. 1995;20:685–688. doi: 10.1097/00007632-199503150-00009. [DOI] [PubMed] [Google Scholar]
- Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA. Does intraoperative blood loss affect antibiotic serum and tissue concentrations. Arch Surg. 1996;131:1165–1171. doi: 10.1001/archsurg.1996.01430230047009. [DOI] [PubMed] [Google Scholar]
- Polly DW, Meter JJ, Brueckner R, Asplund A, van Dam BE. The effect of blood loss on intraoperative serum cefazolin levels in patients undergoing instrumented spinal fusion. A prospective controlled study. Spine. 1996;21:2363–2367. doi: 10.1097/00007632-199610150-00011. [DOI] [PubMed] [Google Scholar]
- Dobzyniak M, Fischgrund JS, Hankins S. The effect of single versus multiple dose antibiotic prophylaxis on the rate of wound infection in lumbar disc surgery. Annual Meeting of the International Society for Study of the Lumbar Spine, April 9–13, 2000, Adelaide, Australia.
- Clark NM, Hershberger E, Zervos MJ, Lynch JP., III Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr Opin Crit Care. 2003;9:403–412. doi: 10.1097/00075198-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Swank S, Lonstein JE, Moe JH, Winter RB, Bradford DS. Surgical treatment of adult scoliosis. A review of two hundred and twenty-two cases. J Bone Joint Surg Am. 1981;63:268–287. [PubMed] [Google Scholar]
- Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566–569. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27:83–86. [PubMed] [Google Scholar]
- Klein JD, Garfin SR. Nutritional status in the patient with spinal infection. Orthop Clin North Am. 1996;27:33–36. [PubMed] [Google Scholar]
- Jensen JE, Jensen TG, Smith TK, Johnston DA, Dudrick SJ. Nutrition in orthopaedic surgery. J Bone Joint Surg Am. 1982;64:1263–1272. [PubMed] [Google Scholar]
- Glassman SD, Dimar JR, Puno RM, Johnson JR. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine. 1996;21:2163–2169. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop. 1992;284:99–108. [PubMed] [Google Scholar]
- Simpson JM, Silveri CP, Balderston RA, Simeone FA, An HS. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75:1823–1829. doi: 10.2106/00004623-199312000-00013. [DOI] [PubMed] [Google Scholar]
- Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277–285. doi: 10.1097/00002517-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Nolan CM, Beaty HN, Bagdade JD. Further characterization of the impaired bactericidal function of granulocytes in patients with poorly controlled diabetes. Diabetes. 1978;27:889–894. doi: 10.2337/diab.27.9.889. [DOI] [PubMed] [Google Scholar]
- Bagdade JD, Root RK, Bluger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69:26–35. doi: 10.1302/0301-620X.69B1.3818728. [DOI] [PubMed] [Google Scholar]
- Zeiger HE, Jr, Zampella EJ. Intervertebral disc infection after lumbar chemonucleolysis: report of a case. Neurosurgery. 1986;18:616–621. doi: 10.1227/00006123-198605000-00017. [DOI] [PubMed] [Google Scholar]
- Brian JE, Jr, Westerman GR, Chadduck WM. Septic complications of chemonucleolysis. Neurosurgery. 1984;15:730–734. doi: 10.1227/00006123-198411000-00020. [DOI] [PubMed] [Google Scholar]
- Scherbel A, Gardner W. Infections involving the intervertebral disks: diagnosis and management. JAMA. 1960;174:370–374. doi: 10.1001/jama.1960.03030040024007. [DOI] [PubMed] [Google Scholar]
- Silber JS, Anderson G, Vaccaro AR, Anderson PA, McCormick P. Management of postprocedural diskitis. Spine J. 2002;2:279–287. doi: 10.1016/s1529-9430(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Roberts FJ, Walsh A, Wing P, et al. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366–370. doi: 10.1097/00007632-199802010-00016. [DOI] [PubMed] [Google Scholar]
- Zeidman SM, Ducker TB, Raycroft J. Trends and complications in cervical spine surgery: 1989–1993. J Spinal Disord. 1997;10:523–526. [PubMed] [Google Scholar]
- Fehlings MG, Cooper PR, Errico TJ. Posterior plates in the management of cervical instability: long-term results in 44 patients. J Neurosurg. 1994;8:341–349. doi: 10.3171/jns.1994.81.3.0341. [DOI] [PubMed] [Google Scholar]
- Rawlings CE, III, Wilkins RH, Gallis HA, Goldner JL, Francis R. Postoperative intervertebral disc space infection. Neurosurgery. 1983;13:371–376. doi: 10.1227/00006123-198310000-00004. [DOI] [PubMed] [Google Scholar]
- Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Goupille P, Jattiot F, et al. Discitis after lumbar disc surgery. Features of “aseptic” and “septic” forms. Spine. 1992;17:356–358. doi: 10.1097/00007632-199203000-00019. [DOI] [PubMed] [Google Scholar]
- Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wein) 1995;136:145–150. doi: 10.1007/BF01410617. [DOI] [PubMed] [Google Scholar]
- Kapp JP, Sybers WA. Erythrocyte sedimentation rate following uncomplicated lumbar disc operations. Surg Neurol. 1979;12:329–330. [PubMed] [Google Scholar]
- Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine. 1991;16:1049–1050. doi: 10.1097/00007632-199109000-00006. [DOI] [PubMed] [Google Scholar]
- Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 1992;17:400–404. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Bircher MD, Tasker T, Crawshaw C, Mulholland RC. Discitis following lumbar surgery. Spine. 1988;13:98–102. doi: 10.1097/00007632-198801000-00023. [DOI] [PubMed] [Google Scholar]
- Schulitz KP, Assheuer J. Discitis after procedures on the intervertebral disc. Spine. 1994;19:1172–1177. doi: 10.1097/00007632-199405001-00016. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mejias ME, deDios Colmenero J, Sanchez-Lora FJ, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339–345. doi: 10.1086/520212. [DOI] [PubMed] [Google Scholar]
- Bruschwein DA, Brown ML, McLeod RA. Gallium scintigraphy in the evaluation of disk space infections: concise communication. J Nucl Med. 1980;21:925–927. [PubMed] [Google Scholar]
- Norris S, Ehrlich MG, Keim DE, Guiterman H, McKusick KA. Early diagnosis of disc space infection using Gallium-67. J Nucl Med. 1978;19:384–386. [PubMed] [Google Scholar]
- Szypryt EP, Hardy JG, Hinton CE, Worthington BS, Mulholland RC. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disc space infection in an animal model. Spine. 1988;13:1042–1048. doi: 10.1097/00007632-198809000-00012. [DOI] [PubMed] [Google Scholar]
- Boden SD, Davis DO, Dina TS, Sunner JL, Wiesel SW. Postoperative diskitis: distinguishing early MRI findings from normal postoperative disk space changes. Radiology. 1992;184:765–771. doi: 10.1148/radiology.184.3.1509065. [DOI] [PubMed] [Google Scholar]
- Van Goethem JW, Parizel PM, van den Hauwe , Van deKelft E, Verlooy J. The value of MRI in the diagnosis of postoperative spondylodiscitis. Neuroradiology. 2000;42:580–585. doi: 10.1007/s002340000361. [DOI] [PubMed] [Google Scholar]
- Anderson PA, Bohlman HH. Anterior decompression and arthrodesis of the cervical spine: long term motor improvement. Part II—improvement in complete traumatic quadriplegia. J Bone Joint Surg Am. 1992;74:683–692. [PubMed] [Google Scholar]
- Kuriloff DB, Blaugrand S, Ryan J, O'Leary P. Delayed neck infection following anterior spine surgery. Laryngoscope. 1987;97:939–941. doi: 10.1288/00005537-198709000-00017. [DOI] [PubMed] [Google Scholar]
- Shockley WW, Tate JL, Stucker FJ. Management of perforations of the hypopharynx and cervical esophagus. Laryngoscope. 1985;95:939–941. doi: 10.1288/00005537-198508000-00011. [DOI] [PubMed] [Google Scholar]
- Mylotte JM, Kahler L, Graham R, Young L, Goodnough S. Prosepective surveillance for antibiotic resistant organisms in patients with spinal cord injury admitted to an acute rehabilitation unit. Am J Infect Control. 2000;28:291–297. doi: 10.1067/mic.2000.107424. [DOI] [PubMed] [Google Scholar]
- Wright JB, Lam K, Burrell R. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control. 1998;26:572–577. doi: 10.1053/ic.1998.v26.a93527. [DOI] [PubMed] [Google Scholar]
- Gebhard JS, Brugman JL. Percutaneous discectomy for the treatment of bacterial discitis. Spine. 1994;19:855–857. doi: 10.1097/00007632-199404000-00025. [DOI] [PubMed] [Google Scholar]
- Crow WN, Borowski AM, Hadjipavlou AG, et al. Percutaneous transpedicular automated nucleotomy for debridement of infected discs. J Vasc Interv Radiol. 1998;9:161–165. doi: 10.1016/s1051-0443(98)70500-7. [DOI] [PubMed] [Google Scholar]
- Devlin VJ, Boachie-Adjei O, Bradford DS, Ogilvie JW, Transfeldt EE. Treatment of the adult spinal deformity with fusion to the sacrum using CD instrumentation. J Spinal Disord. 1991;4:1–14. [PubMed] [Google Scholar]
- Moe JH. Complications of scoliosis treatment. Clin Orthop. 1967;53:21–30. [PubMed] [Google Scholar]
- Picada R, Winter RB, Lonstein JE, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord. 2000;13:42–45. doi: 10.1097/00002517-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018–1025. doi: 10.1097/00006123-199905000-00047. [DOI] [PubMed] [Google Scholar]
- Vaccaro AR, Harris BM. Presentation and treatment of pyogenic vertebral osteomyelitis. Semin Spine Surg. 2000;12:183–191. [Google Scholar]
- Nakase H, Tamaki R, Matsuda R, Tei R, Park YS, Sakaki T. Delayed reconstruction by titanium mesh-bone graft composite in pyogenic spinal infection: a long term follow-up study. J Spinal Disord Tech. 2006;19:48–54. doi: 10.1097/01.bsd.0000179134.53997.2a. [DOI] [PubMed] [Google Scholar]
- Levi AD, Dickman CA, Sonntag VKH. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975–980. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD. Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Disord. 1995;8:278–283. doi: 10.1097/00002517-199508040-00003. [DOI] [PubMed] [Google Scholar]
- Sheehan E, McKenna J, Mulhall KJ, McCormack D. Adhesion of staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004;22:39–43. doi: 10.1016/S0736-0266(03)00152-9. [DOI] [PubMed] [Google Scholar]
- El-Gindi S, Aref S, Salama M, et al. Infection of intervertebral discs after operation. J Bone Joint Surg Br. 1976;58:114–116. doi: 10.1302/0301-620X.58B1.1270487. [DOI] [PubMed] [Google Scholar]
- Rosenstein BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to prevent infection in postoperative skeletal wounds. An experimental study. J Bone Joint Surg Am. 1989;71:427–430. [PubMed] [Google Scholar]
- Thalgott JS, Cotler HB, Sasso RS. Postoperative infections in spinal implants. Classification and analysis—a multicenter study. Spine. 1991;16:981–984. doi: 10.1097/00007632-199108000-00020. [DOI] [PubMed] [Google Scholar]
- Seligson D, Mehta S, Voos K, Henry SL, Johnson JR. The use of antibiotic impregnated polymethylmethacrylate beads to prevent the evolution of localized infection. J Orthop Trauma. 1992;6:401–406. doi: 10.1097/00005131-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Yuan-Innes MJ, Temple CL, Lacey MS. Vacuum-assisted wound closure: a new approach to spinal wounds with exposed hardware. Spine. 2001;26:E30–E33. doi: 10.1097/00007632-200102010-00006. [DOI] [PubMed] [Google Scholar]
- Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14–17. doi: 10.1097/01.bsd.0000133493.32503.d3. [DOI] [PubMed] [Google Scholar]
- Weiss LE, Vaccaro AR, Scuderi G, McGuire M, Garfin SR. Pseudarthrosis after postoperative wound infections in the lumbar spine. J Spinal Disord. 1997;10:482–487. [PubMed] [Google Scholar]
- Calderone RR, Thomas JC, Haye W, Abeles D. Outcome assessment in spinal infections. Orthop Clin North Am. 1996;27:201–205. [PubMed] [Google Scholar]