Abstract
Background/Objectives:
To evaluate the relationship between the severity of cervical spinal cord injury (SCI) (American Spinal Injury Association [ASIA] grade), presence of neurogenic shock, and timing of surgical intervention. This is a post-hoc analysis from the Sygen multicenter randomized controlled trial.
Methods:
Blood pressure (BP) and heart rate (HR) data were collected when patients were first assessed in the emergency room (Time A) and at the time of randomization (Time B). Individuals were subdivided by ASIA grade and by the level of the systolic BP (SBP).
Results:
Only individuals with cervical SCI from the Sygen trial (n = 577) were evaluated. Severe complete SCI (ASIA grade =A) was established in 57% of these patients. A total o f 74 (13%) patients with neurogenic shock (SBP < 90 mmHg) at Time A were identified. The SBP increased significantly from Time A to Time B (P < 0.0001). The median time from SCI to surgical intervention, for ASIA A, was 80.9 hours for patients with initial SBP < 90 mmHg and 58 hours for patients with initial SBP ≥ 90 mmHg (P = 0.025). Multivariable analysis after adjusting for confounders revealed a statistically significant difference in the time to surgical intervention based on SBP for ASIA A (P = 0.026), yet not for ASIA B or C/D.
Conclusions:
The presence of neurogenic shock was associated with a delay in the timing of surgical intervention in patients with cervical SCI. Detailed evaluation of autonomic dysfunctions following SCI including cardiovascular instability could improve our understanding of the complexities of clinical presentations and possible neurological outcomes.
Keywords: Randomized controlled trial; Sygen; Blood pressure; Neurogenic shock; Spinal cord injuries, cervical
INTRODUCTION
Acute injury to the spinal cord in humans, especially at the cervical level, results in severe hypotension and persistent bradycardia that are common components of the phenomenon known as neurogenic shock (1,2). This event is more profound and long lasting in humans after spinal cord injury (SCI) than in experimental animals (3). In addition to neurogenic shock, the acute phase of SCI is also associated with “spinal shock” (4,5). Some authors use these terms interchangeably; however, it is important to recognize that these are 2 clinically important and distinct conditions. Neurogenic shock is characterized by changes occurring in blood pressure control following SCI, whereas spinal shock is characterized by a marked reduction or abolition of sensory, motor, or reflex function of the spinal cord below the level of injury (4).
Low arterial blood pressure (BP) and the presence of neurogenic shock (BP < 90 mmHg) after SCI may result in ischemia of the spinal cord and are potential contributing factors to the cascade of the secondary mechanisms involved in further damage of fragile neuronal tissue (6,7). Hypoperfusion of the spinal cord could result both from low systolic BP (SBP) and from mechanical compression of the spinal cord (8–10). Increases in systemic blood pressure may improve perfusion to the injured, distorted spinal cord (11–13). Several contemporary series of spinal cord injured patients treated with aggressive medical management with maintenance of mean arterial blood pressure in the high normal ranges (85–90 mmHg) have suggested improved neurological outcomes with this management plan (13–15). Presently, there is no consensus as to whether it is better to achieve surgical decompression of the spinal cord early or to postpone the procedure until patients are more stable (16,17).
Many clinical and experimental studies in the area of SCI have been predominantly focused on evaluation of motor and sensory consequences of this devastating injury. The present analysis was performed to examine the association between the timing of surgery and the severity of hemodynamic instability in the acute period following cervical SCI (18). First, we evaluated whether the hemodynamic parameters varied according to the severity of the injury. Second, we examined the change in the hemodynamic parameters over time between the time of the first emergency room assessment to the time of randomization. Finally, we evaluated whether the time from injury to surgical intervention was affected by the presence or absence of neurogenic shock in patients with different severities of cervical SCI.
METHODS
A post-hoc analysis from the Sygen multicenter randomized controlled trial (18) was performed to evaluate the relationship between the severity of SCI (American Spinal Injury Association [ASIA] grade) and cardiovascular parameters in patients with cervical SCI. The ASIA grades were categorized into motor and sensory complete ASIA A, motor complete ASIA B, and motor incomplete ASIA C/D (19). Similar to previous analyses of Sygen trial patients, ASIA C/D were combined in 1 group due to the very small numbers in ASIA D (20). The Sygen trial excluded patients who had severe thoraco-abdominal injuries that precluded the ability to determine the ASIA grade (21).
For this study, we obtained descriptive statistics regarding the systolic BP, diastolic BP (DBP), and heart rate (HR) recorded at different times in the Sygen study. The data were obtained at the time of the first emergency room admission, at the Sygen center (which may have been the first emergency room), and at the time of randomization. The hemodynamic parameters obtained at each time point were recorded and used for analysis. Data regarding management of hemodynamic parameters by either volume substitution or vasopressors were not evaluated in this study. Demographic data such as the age and gender of the patient along with the cause of injury (categorized into automobile accident, fall, gunshot wound, water-related injury, and other) were collected. The severity of SCI, utilizing the ASIA scale, was determined at different times during the Sygen study. For purposes of this study, we used the first reliable ASIA grade obtained for randomization (within 72 hours post injury). Features regarding the spinal column injury and subsequent treatment included presence of a spine fracture, need for traction, anterior vs posterior surgical approach, and the time from initial spine injury to surgical intervention.
The analysis consisted of 3 components. The first part evaluated whether the hemodynamic parameters varied according to the severity of cervical SCI at different times. The second aspect of the analysis evaluated the change in the SBP, DBP, and HR over time. Lastly, the study evaluated whether the time from injury to surgical intervention was affected by the level of SBP (ie, presence or absence of neurogenic shock, SBP < or ≥ 90 mmHg) in different severities of cervical SCI (22).
Hemodynamic Parameters and ASIA Grade
The first part of this study endeavored to determine whether each of the hemodynamic parameters (SBP, DBP, and HR) varied according to the severity of spinal cord injury (ASIA grade).
The means and standard deviations for each of the parameters were obtained for each of the ASIA grades and at each of the time points. Hemodynamic parameters in this study were measured at different time periods from the time of initial evaluation after the injury to the time of randomization. Time A reflected the time point of measurements of BP and HR at the initial emergency room, which may have been the Sygen center. Time B was denoted as the time of evaluation of hemodynamic parameters at randomization. One-way analysis of variance (ANOVA) between group designs was used to analyze the differences in the means of each parameter for the ASIA grades. The Tukey-Kramer HSD (honestly significant difference) was used to perform the multiple comparisons between the groups (ASIA grade) for the hemodynamic parameters.
Change in Hemodynamic Parameters Over Time
The second aspect of this study determined whether there was a significant change in each of the hemodynamic parameters over time. The univariate one-way ANOVA (repeated measures design) was used to evaluate the SBP, DBP, and HR.
Neurogenic Shock and Timing of Surgical Intervention
The last component of the analysis involved subdividing the data into cases with SBP of <90 mmHg and cases with SBP of ≥90 mmHg, at the time of the first emergency room visit (Time A). Medians and standard deviations were obtained for the hemodynamic parameters and for the hours from the time of injury to surgical intervention. Data were also obtained on other variables that may affect the time from injury to surgical intervention. The distribution of the age of the patients, gender of the patients, presence of spinal fracture, type of surgical intervention (anterior vs posterior), requirement for traction, and presence of an intermediate stopover prior to arrival at the surgical center were also obtained. The above was performed for each of the ASIA grades. Cases were deleted for this aspect of the analysis if the SBP at Time A was missing. The semiparametric log-rank statistic or the Wald statistic was performed for the time to surgery from SBP and each of the potential confounding variables for each of the ASIA grades (23).
All covariates that reached a statistical significance of less than 0.2 or those that posed a clinical significance were entered into the analysis. The analysis was performed separately for each of the ASIA grades using the nonparametric Cox proportional hazard model to determine the significance of SBP in predicting time from injury to surgery, after adjusting for the potential confounding variables. The hazard ratios for the regression coefficients and their 95% confidence intervals (CI) were obtained. The proportional hazard assumption was assessed by determining the significance of an interaction term of each variable of interest with time. The statistical analysis was performed using the JMP (software by Statistical Analysis System, Inc. Cary, NC).
RESULTS
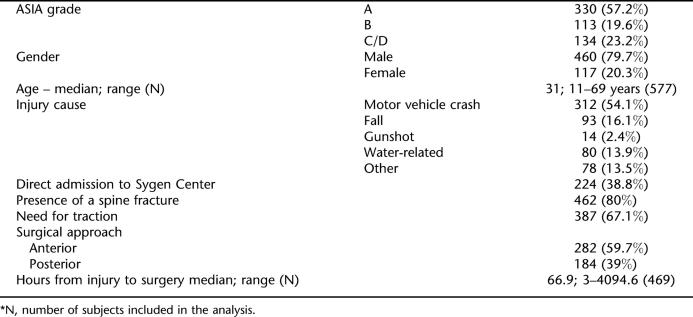
The Sygen trial randomized a total of 760 patients from 28 centers in North America (18). The majority of the individuals sustained cervical SCI (577, 75.9%) and were included in the present study. The median age of the cervical SCI sample population was 31 years (range 11–69 y), with a predominance of males (79.7%) (Table 1). Severe complete SCI (ASIA A) was diagnosed in 330 (57.2%) of these individuals. Incomplete SCI, ASIA B or C/D, was present in 113 (19.6%) and 134 (23.2%) patients, respectively. The major cause of injury was motor vehicle crashes (MVCs), seen in 54.1% (312/577) of cases. Sixty-one percent of patients stopped at an intervening emergency room prior to transfer to the Sygen center. Spine fractures were seen in 80% of patients, and traction was required in 67.1%. Eighty-two percent of the patients required surgical intervention, with the median time from initial admission to surgical intervention estimated at 66.9 hours.
Table 1.
Demographic and Clinical Data for Individuals With Cervical Spinal Cord Injury in the Sygen Study *
Hemodynamic Parameters and Severity of SCI
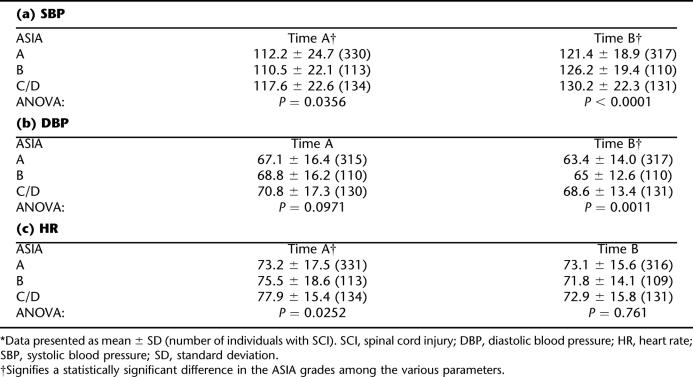
At the time of arrival at the first emergency room (Time A), the mean SBP was 112.2 mmHg for ASIA A, 110.5 mmHg for ASIA B, and 117.6 mmHg for ASIA C/D patients (Table 2a). One-way ANOVA revealed a statistically significant (P = 0.036) difference in the mean SBP for the different ASIA grades. At time A, the SBP was significantly higher for the ASIA C/D group than the ASIA B group (P = 0.0356).
Table 2.
Changes in Hemodynamic Parameters Between Time A (Initial Time) and Time B (Randomization) in Individuals With Different Severity of Cervical SCI in the Sygen Study *
The DBP did not vary significantly according to the severity of SCI at Time A (P = 0.097). There was a statistically significant difference in the DBP according to severity of SCI at Time B (P = 0.0011, Table 2b). Tukey's HSD test revealed that ASIA C/D had a significantly higher DBP than ASIA A at Time B. There was a significantly lower HR for ASIA A than ASIA C/D at Time A (P = 0.025), yet this difference was not observed at Time B (P = 0.761, Table 2c).
Changes in Hemodynamic Parameters Over Time
From Time A to Time B, the hemodynamic parameters showed improvement in all groups of individuals with cervical SCI. Repeated measures analysis revealed a statistically significant increase in SBP over time after injury (univariate G-G epsilon, P < 0.0001). For example, SBP in individuals with ASIA A SCI increased by 8.2% at the time of randomization (from 112.2 ± 24.7 mmHg to 121.4 ± 18.9 mmHg, Table 2). However, HR decreased significantly over time (univariate G-G epsilon, P = 0.0086). There was no significant change in the DBP over time (P = 0.15).
Neurogenic Shock and Timing of Surgical Intervention
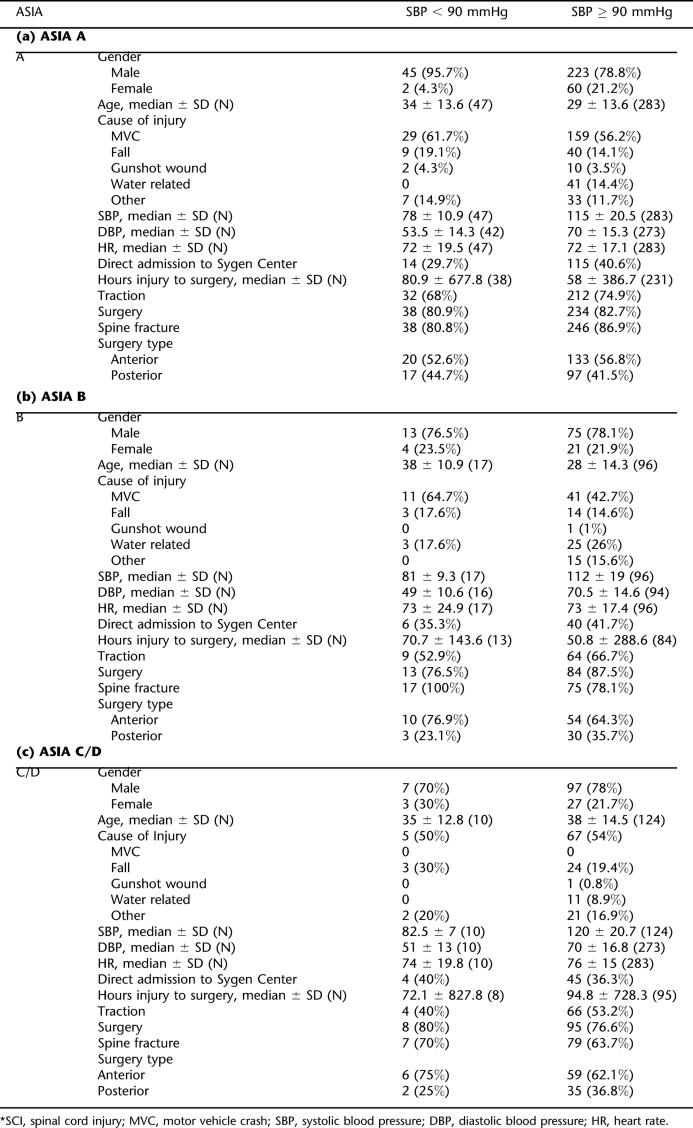
The last aspect of the analysis evaluated the effect of SBP at admission on the timing of surgical intervention. A total of 74 (13%) patients with neurogenic shock (SBP < 90 mmHg) at Time A were identified (Table 3). There was a significantly higher number of patients with neurogenic shock in the ASIA A (17%) and B (18%) groups than in ASIA C/D (7%) (Table 3). A predominant cause of the SCI in these individuals was MVCs. Spine fractures were seen in 80% of the patients, traction was required in 56%, and surgical interventions in 82%.
Table 3.
Demographic and Clinical Data for Individuals With Different Severities of SCI at Time A *
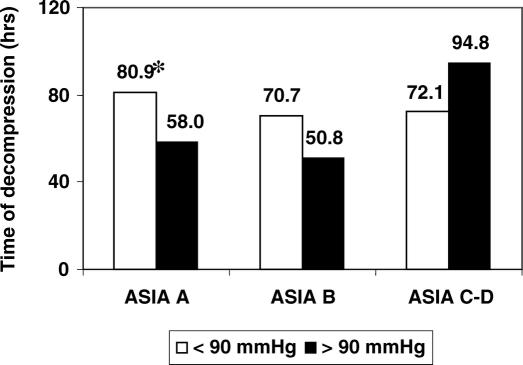
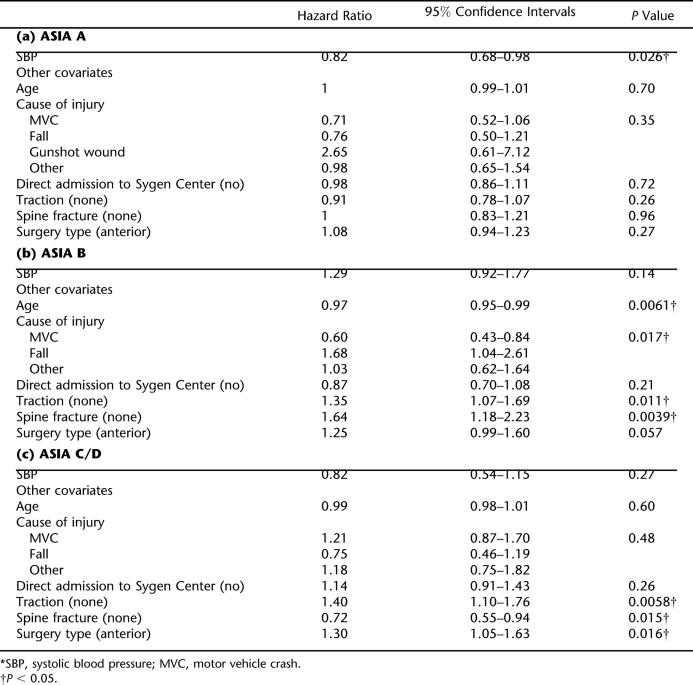
The median time from initial injury to surgery was 66.9 hours for patients sustaining a cervical SCI. The median time from initial injury to surgery varied according to the ASIA grade at randomization and SBP at Time A. The median time to surgery for ASIA A patients with SBP < 90 mmHg was 80.9 hours, which was significantly greater than the 58-hour time to surgery for ASIA A patients with a SBP ≥ 90 mmHg (P = 0.025; Table 3a, and Figure 1). The time to surgery did not vary significantly for ASIA B (P = 0.82) and ASIA C/D (P = 0.65) patients according to SBP, based on bivariate analysis (Table 3b and c and Figure 1). These results were confirmed by multivariate analysis (Table 4). Some of the covariates revealed to be statistically significant (P < 0.2) included the age of the patient (P = 0.14), cause of injury (P = 0.093), presence of traction (P = 0.026), presence of a spine fracture (P = 0.092), and type of surgery (P = 0.093) for ASIA B cases, while cause of injury (P = 0.099), presence of a spine fracture (P = 0.065) and type of surgery (P = 0.037) were also significant for ASIA C/D cases. The clinical significance of these covariates in predicting time to surgery is unclear in this post-hoc analysis.
Figure 1. Effect of presence of neurogenic shock on time of surgical decompression in patients with cervical SCI. * represents a statistically significant difference (P = 0.025).
Table 4.
Multivariate Analysis
DISCUSSION
Spinal cord injury results in injury to the descending pathways that are involved in autonomic control (24–26). In particular, the loss of descending tonic sympathetic control has been implicated as a major cause of the cardiovascular instability during the acute posttraumatic stage (27–29). Low arterial blood pressure and persistent bradycardia are common features of the neurogenic shock that occurs after injury to the spinal cord.
Previously, numerous investigators have examined the effect of acute SCI on hemodynamic parameters in humans. However, either the number of patients was limited or the authors failed to evaluate the association between the severity of SCI and cardiovascular abnormalities. Lehmann et al examined 48 consecutive patients with cervical SCI and demonstrated that 68% of patients with severe SCI were hypotensive (30). Levi et al examined cardiovascular parameters in the acute period of cervical SCI in 50 patients (13). Hypotension was present in 23.6% (7 of 31) of patients with severe SCI (Frankel A), and 9.1% (1 of 11) of patients with less severe injuries (Frankel C/D). Recently, Bilello et al evaluated 83 individuals with cervical SCI and indicated that 29% had experienced neurogenic shock in the acute period of SCI. These authors also showed that there was no significant difference in the presence of neurogenic shock between patients with upper cervical SCI (C1–C5) and lower cervical SCI (C6–C7), 31% vs 24%, respectively (31). However, patients with upper cervical SCI had a greater need for cardiovascular interventions (24%) than did patients with lower cervical SCI (5%). Unfortunately, this study did not comment on the severity of cervical SCI and presence of neurogenic shock. In our study the total number of individuals with neurogenic shock was 13%; however, individuals with severe cervical SCI had a frequency of neurogenic shock comparable to previous reports. In our study, neurogenic shock was observed in 17% of individuals with ASIA A and in 18% of individuals with ASIA B.
We have shown that the severity of cervical SCI correlates with the severity of abnormal cardiovascular control. We found that SBP measured at the first emergency room assessment in individuals with ASIA B was significantly lower than in individuals with less severe SCI (ASIA C/D) (P = 0.0356). We also found that bradycardia was more pronounced in the early period of SCI in individuals with ASIA A than in individuals with ASIA B and C/D (P = 0.0252). Although we do not have the information on fluid management or vasopressive drug use in these patients, our data showed that hemodynamic parameters were improved significantly in all patients by the time of randomization (Table 2). Previously, we reported that baseline physiologic measurements taken at the time of randomization were improved from these study center admission values, implying early treatment of hypovolemia and restoration of blood pressure. This is presumably important in preventing secondary physiologic injury early after SCI. The EMT and SCI study centers have protocols for SCI that call for early mechanical stabilization and resuscitation (18). There were no significant differences in SBP, DBP, and HR between the groups at the time of initiation of drug therapy.
With respect to neurogenic shock, our study indicated that total of 13% (74 of 577) patients with cervical SCI suffered this condition. The majority of these patients sustained severe (14.5%, ASIA A) or moderate (15%, ASIA B) injuries (Tables 3 and 4).
The authors of this study were aware that hypotension following SCI could be attributed not only to the injury to the spinal cord, but also to associated traumatic injuries resulting in hypovolemia. However, patients with significant injuries to the chest, abdomen, or extremities were excluded from the study (18).
Finally, we examined the association between the presence of neurogenic shock and the timing of surgical decompression in patients with cervical SCI in the Sygen clinical trial. The rationale for this part of our study was based on previous animal experimental data and ongoing clinical discussion on value of acute decompression following SCI. Although no prospective clinical studies have been conducted to assess the effect of hypotension on acute SCI in humans, numerous experimental studies in animals suggested that hypotension contributes to the secondary injury after SCI (7,10). Hypotension results in decrease of blood flow in the spinal cord and consequent ischemia. Moreover, hypotension in the acute period of SCI in animals is also associated with worsening of neurological recovery (32). Our data showed that the median time from injury to surgical decompression varied according to the severity of SCI and presence of neurogenic shock. Patients with severe SCI (ASIA A) and neurogenic shock on average were operated on 30 hours later than patients with similar injuries but without hypotension. A previous study by the Sygen group had also shown that injury severity affected timing to surgical decompression; ASIA B had significantly earlier surgery than ASIA A and C/D (33). The current study did not compare timing to surgery based on injury severity, but compared timing based on SBP for each of the ASIA grades separately. We also acknowledge that other factors (differing physician philosophies on optimal surgical timing) could also influence the timing of surgical intervention (18). We also acknowledge that these facts could contribute to possible error in our analysis. However, this is not random, but systematic to factors determining the care of the patient and arising from the individual physicians' judgment. It is potentially a source of bias, not random error. A number of other covariates were also noted to be statistically significant (such as age of the patient, cause of injury, presence of traction and of a spine fracture). The significance of these is unclear in this post-hoc analysis.
CONCLUSION
This study presents a temporal evaluation of the hemodynamic parameters in the acute period following cervical SCI in the large group of patients involved in the Sygen clinical trial. Hypotension and bradycardia were predominant in the initial postinjury period in individuals with severe SCI (ASIA A and B). Although significant hypotension was initially present in all groups of patients, the presence of neurogenic shock was significantly higher in patients with ASIA A and B. Finally, our study showed that presence of neurogenic shock was associated with delay in the time of surgical intervention in patients with SCI. In future studies, detailed documentation and evaluation of autonomic dysfunctions following SCI including cardiovascular instability could improve our understanding of the complexities of clinical presentations and possible outcomes following SCI.
REFERENCES
- Atkinson PP, Atkinson JLD. Subject review. Spinal shock. Mayo Clin Proc. 1996;71:384–389. doi: 10.4065/71.4.384. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res. 2006;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma. 2001;18:727–736. doi: 10.1089/089771501750357663. [DOI] [PubMed] [Google Scholar]
- Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- Nacimiento W, Noth J. What, if anything, is spinal shock. Arch Neurol. 1999;56:1033–1035. doi: 10.1001/archneur.56.8.1033. [DOI] [PubMed] [Google Scholar]
- Wallace MC, Tator CH. Successful improvement of blood pressure, cardiac output, and spinal cord blood flow after experimental spinal cord injury. Neurosurgery. 1987;20:710–715. doi: 10.1227/00006123-198705000-00006. [DOI] [PubMed] [Google Scholar]
- Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Hitchon PW, Dyste GN, Osenbach RK, Todd MM, Yamada T, Jensen AE. Spinal cord blood flow in response to focal compression. J Spinal Disord. 1990;3:210–219. [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG, Thorpe K, Taylor W. Current use and timing of spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J Neurosurg. 1999;91:12–18. doi: 10.3171/spi.1999.91.1.0012. [DOI] [PubMed] [Google Scholar]
- Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: description, intervention, and prediction of outcome. Neurosurgery. 1993;33:1007–1017. [PubMed] [Google Scholar]
- Hadley MN, Walters BC, Grabb PA, et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery. 2002;50(Suppl 3):S1–S199. [PubMed] [Google Scholar]
- King BS, Gupta R, Narayan RK. The early assessment and intensive care unit management of patients with severe traumatic brain and spinal cord injuries. Surg Clin North Am. 2000;80:855–870. viii–ix. doi: 10.1016/s0039-6109(05)70100-6. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Sekhon LH, Tator C. The role and timing of decompression in acute spinal cord injury: what do we know? What should we do. Spine. 2001;26(Suppl 24):S101–S110. doi: 10.1097/00007632-200112151-00017. [DOI] [PubMed] [Google Scholar]
- Chen TY, Dickman CA, Eleraky M, Sonntag WKH. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine. 1998;23:2398–2403. doi: 10.1097/00007632-199811150-00007. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine. 2001;26(Suppl 24):S58–S67. doi: 10.1097/00007632-200112151-00013. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine. 2001;26(Suppl 24):S68–S86. doi: 10.1097/00007632-200112151-00014. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine. 2001;26(Suppl 24):S87–S98. doi: 10.1097/00007632-200112151-00015. [DOI] [PubMed] [Google Scholar]
- Bilello JF, Davis JW, Cunningham MA, Groom TF, Lawrence D, Sue LP. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127–1129. doi: 10.1001/archsurg.138.10.1127. [DOI] [PubMed] [Google Scholar]
- Whitten CW, Chu Z, Giesecke AH, Ivy R, Allison P, Kaplan H. An analysis of survival in patients with traumatic injuries who received transfusions of 40-units or more. Anesthesiology. 1995;83(3A):A218. Abstract?; [Google Scholar]
- Mathias CJ, Frankel HL. Autonomic disturbances in spinal cord lesions. In: Bannister R, Mathias CJ, editors. Autonomic Failure, A Textbook of Clinical Disorders of the Autonomic Nervous System. London: Oxford Medical Publications; 2002. pp. 839–881. [Google Scholar]
- Furlan JC, Fehlings MG, Halliday W, Krassioukov AV. Autonomic dysreflexia associated with intramedullary astrocytoma of the spinal cord. Lancet Oncol. 2003;4:574–575. doi: 10.1016/s1470-2045(03)01197-5. [DOI] [PubMed] [Google Scholar]
- Teasell R, Arnold AP, Krassioukov AV, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system following spinal cord injuries. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- Lebedev VP, Krasyukov (Krassioukov) AV, Nikitin SA. Electrophysiological study of sympathoexcitatory structures of the bulbar ventrolateral surface as related to vasomotor regulation. Neuroscience. 1986;17:189–203. doi: 10.1016/0306-4522(86)90236-8. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Fehlings MG. Effect of graded spinal cord compression on cardiovascular neurons in the rostroventrolateral medulla. Neuroscience. 1999;88:959–973. doi: 10.1016/s0306-4522(98)00267-x. [DOI] [PubMed] [Google Scholar]
- Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 2006;44:341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- Lehmann KG, Lane JG, Piepmeier JM, Batsford WP. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol. 1987;10:46–52. doi: 10.1016/s0735-1097(87)80158-4. [DOI] [PubMed] [Google Scholar]
- Bilello JF, Davis JW, Cunningham MA, Groom TF, Lemaster D, Sue LP. Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg. 2003;138:1127–1129. doi: 10.1001/archsurg.138.10.1127. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH, Linden RD. The relationships among the severity of spinal cord injury, motor and somatosensory evoked potentials and spinal cord blood flow. Electroencephalogr Clin Neurophysiol. 1989;74:241–259. doi: 10.1016/0168-5597(89)90055-5. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP. Timing of surgical decompression for severe spinal cord injury: retrospective results from a large multi-center clinical trial. J Neurotrauma. 2003;10:1069. [Google Scholar]