Abstract
Abstract. Binding of a T cell to an appropriate antigen-presenting cell (APC) induces the rapid reorientation of the T cell cytoskeleton and secretory apparatus towards the cell–cell contact site in a T cell antigen receptor (TCR) and peptide/major histocompatibility complex–dependent process. Such T cell polarization directs the delivery of cytokines and cytotoxic mediators towards the APC and contributes to the highly selective and specific action of effector T cells. To study the signaling pathways that regulate cytoskeletal rearrangements in T lymphocytes, we set up a conjugate formation assay using Jurkat T cells as effectors and cell-sized latex beads coated with various antibodies as artificial APCs. Here, we report that beads coated with antibodies specific for the TCR-CD3 complex were sufficient to induce T cell polarization towards the bead attachment site, as judged by reorientation of the microtubule-organizing center (MTOC) and localized actin polymerization. Thus, these cytoskeletal changes did not depend on activation of additional coreceptors. Moreover, single subunits of the TCR complex, namely TCR-ζ and CD3ε, were equally effective in inducing cytoskeletal polarization. However, mutagenesis of the immunoreceptor tyrosine-based activation motifs (ITAMs), present three times in TCR-ζ and once in CD3ε, revealed that the induction of cytoskeletal rearrangements required the presence of at least one intact ITAM. In agreement with this result, lack of functional Lck, the protein tyrosine kinase responsible for ITAM phosphorylation, abolished both MTOC reorientation and polarized actin polymerization. Both inhibitor and transient overexpression studies demonstrated that MTOC reorientation could occur in the absence of Ras activation. Our results suggest that APC-induced T cell polarization is a TCR-mediated event that is coupled to the TCR by the same signaling motif as TCR-induced gene activation, but diverges in its distal signaling requirements.
Polarization of a T cell response towards a triggering antigen-presenting cell (APC)1 is thought to contribute to the specificity of the immune response. Upon encountering an APC, T cells rapidly undergo cytoskeletal polarization, which includes the formation of a tight collar of polymerized actin at the T cell–APC interface and the reorientation of the microtubule-organizing center (MTOC) towards the bound APC (Geiger et al., 1982; Ryser et al., 1982). Whereas F-actin accumulation at the cell–cell interface was suggested to stabilize and favor continuous T cell antigen receptor (TCR)–antigen interactions (Valitutti et al., 1995b ), MTOC reorientation is thought to position the T cell secretory apparatus into close proximity with the APC. Indeed, several studies showed that repositioning of the MTOC was accompanied by the polarized concentration of cytokines and cytotoxic mediators at the T cell–APC/target interface (Kupfer and Dennert, 1984; Kupfer et al., 1991, 1994). Moreover, Poo et al. (1988) have demonstrated that limited TCR cross-linking in a cloned helper T cell line triggered the release of cytokines preferentially over the area of receptor cross-linking. Thus, the engagement of only a fraction of TCRs on a helper T cell results in the polarized delivery of cytokines towards the site of the stimulus.
Cytoskeletal polarization of a T cell is dependent on the presence of specific antigen (Kupfer and Singer, 1989), suggesting the involvement of a TCR-mediated signaling pathway. A variety of cellular responses, including gene activation, proliferation, and secretion of cytokines and cytotoxic mediators, have been reported to be initiated by TCR engagement. Interestingly, activation of Jurkat T cells with immobilized TCR-specific antibodies could induce actin polymerization and formation of F-actin-rich pseudopods, suggesting that TCR-mediated signaling pathways may indeed regulate cytoskeletal organization (Melamed et al., 1991). Furthermore, a recent report implies a role for the small GTPase CDC42 in APC-mediated T cell polarization (Stowers et al., 1995). Members of the Rho subfamily of small GTPases (Rho, Rac, CDC42) are evolutionary conserved regulators of the actin cytoskeleton in a variety of different cell systems (Hall, 1994). Consequently, TCR-mediated signals might activate small GTPases by an as yet unidentified mechanism. However, T cell–APC conjugate formation involves the clustering and engagement of various other T cell surface receptors besides TCR–antigen interactions, including the CD8/CD4 coreceptors and integrins (Kupfer and Singer, 1989; Kupfer et al., 1987). It is conceivable that triggering of these additional receptors is required for or at least contributes to APC-induced T cell polarization.
The TCR is coupled to intracellular signaling pathways by two noncovalently associated signal-transducing complexes, namely the CD3 (γ, δ, and ε) and the TCR-ζ chains. Signaling through these complexes requires a conserved sequence motif (YxxLx(6–8)YxxL), termed the immunoreceptor tyrosine-based activation motif (ITAM) (Irving and Weiss, 1991; Romeo and Seed, 1991). The CD3 and TCR-ζ chains each contain one and three ITAMs, respectively. Upon TCR engagement, the tyrosine residues in the ITAMs are rapidly phosphorylated by the Src-family protein tyrosine kinase (PTK) Lck (van Oers et al., 1996) and serve as docking sites for src-homology-domain 2 (SH2) containing signaling molecules such as the PTKs of the Syk/zeta-associated protein 70 (ZAP-70) family. After recruitment, Syk/ZAP-70 is activated by phosphorylation and contributes to the initiation of downstream signaling events such as calcium mobilization and activation of the Ras pathway. Single CD3 and TCR-ζ chains can independently activate a variety of qualitatively similar proximal and distal signaling events (Irving and Weiss, 1991; Letourneur and Klausner, 1991; Romeo and Seed, 1991; Shinkai et al., 1995), suggesting that the presence of multiple ITAMs in the TCR mainly serves the purpose of signal amplification. However, amino acids surrounding the ITAMs differ in the CD3/TCR chains and have been shown to bind to distinct SH2-containing signaling molecules (Osman et al., 1995, 1996; Songyang et al., 1993). This suggests that, in addition to signal amplification, distinct ITAMs might couple the receptor to qualitatively different signaling pathways. For example, chimeric proteins containing the cytoplasmic tail of CD3ε or TCR-ζ differ in their ability to induce apoptosis in a murine T cell line (Combadiere et al., 1996). Furthermore, the third TCR-ζ ITAM and the CD3ε ITAM have been implicated in coupling the TCR to the actin cytoskeleton in an activation-dependent manner (Rozdzial et al., 1995). Thus, individual subunits might couple the TCR to effector molecules involved in the regulation of cytoskeletal changes.
To investigate the role of TCR-mediated signaling pathways in APC-induced T cell polarization, we set up a conjugation assay using Jurkat T cells or mutants derived from it as effector cells, with antibody-coated, cell-sized latex beads as APCs. We show here that TCR cross-linking alone is sufficient to induce MTOC polarization and actin polymerization at the T cell–bead interface. Cytoskeletal reorientation required the presence of at least one intact ITAM. In support of this result, no cytoskeletal changes could be observed in the Lck-deficient Jurkat mutant JCaM1.6. Furthermore, our studies demonstrate that MTOC reorientation occurs in the absence of Ras activation. Our results imply that APC-induced T cell polarization is a TCR-mediated event that is coupled to the receptor by the same signaling motif as TCR-mediated gene activation, but diverges in its distal signaling requirements, since Ras is not required. The assay described in this study might be a useful tool to characterize signaling molecules involved in T cell polarization.
Materials and Methods
Cells and Reagents
The Jurkat T cell subclone E6, JCaM1.6 (Goldsmith et al., 1988) and large T antigen (TAg) Jurkat cells (kindly provided by Dr. G. Crabtree, Stanford University, Palo Alto, CA; Clipstone and Crabtree, 1992) were maintained in RPMI 1640 medium supplemented with 5% fetal calf serum (Intergen Co., Purchase, NY), 2 mM glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Jurkat-derived stable clones were cultured in complete medium containing 10% FCS and 2 mg/ml G418 (GIBCO BRL, Gaithersburg, MD). JCaM1.6/Lck cells (Straus and Weiss, 1992) were maintained in complete medium containing 10% FCS and 250 μg/ml hygromycin (GIBCO BRL). Poly-l-lysine was obtained from Sigma Chemical Co. (St. Louis, MO). The MAPKK inhibitor PD 098059 and Wortmannin were purchased from Calbiochem-Novabiochem Corp. (San Diego, CA). Polystyrene latex microspheres (diameter 6 μm) were purchased from Polysciences Inc. (Warrington, PA). Antibodies were absorbed to the beads as previously described (Mescher, 1992). Briefly, 5–10 μg of purified antibody were mixed with 107 polystyrene beads in a final volume of 1 ml PBS, and incubated for 90 min at room temperature with constant tumbling. Beads were then blocked in 1.5 ml of PBS/1% BSA for 30 min. After three washes in PBS, latex beads were resuspended in PBS and stored at 4°C. Efficient antibody absorption was verified by flow cytometry.
Antibodies
Antibodies used for stimulation and immunofluorescence microscopy are as follows: the mAb C305 (IgM) specifically recognizes the Jurkat Ti β chain (Weiss and Stobo, 1984). Leu 4 (IgG1) is directed against the human CD3ε chain. RBC4 (IgM) recognizes the transferrin receptor. The mAb 9.1 (IgG3) is specific for human CD2 (Yang et al., 1986). Mouse mAb OKT8 recognizes an extracellular epitope of human CD8 and was acquired from American Type Culture Collection (Rockville, MD). The mAb 7G7B6 is directed against murine CD25 (Tac) and was obtained from American Type Culture Collection. A mouse mAb to human CD11a (IgG1, SPV-L7) was purchased from Zymed Laboratories, Inc. (S. San Francisco, CA). A rat mAb to α-tubulin (YOL1/34) was obtained from Harlan Sera-Laboratories (Crawley, UK) and was detected with an FITC-conjugated, affinity-purified donkey anti–rat (Fab′)2 antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). F-actin was detected with rhodamin-phalloidin (Molecular Probes, Inc., Menlo Park, CA). Antiphosphotyrosine mAb 4G10 was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). An mAb against human Ha-Ras was purchased from Transduction Laboratories (Lexington, KY).
Plasmids
The dominant-negative ZAP-70 constructs SH2 (N+C) and SH2 (N+*C) are described elsewhere (Qian et al., 1996). The N17Ras plasmid was a kind gift from D. Cantrell (ICRF, London, UK).
Chimera Construction and Clones
Stable clones expressing the TT-ε chimeras were kindly provided by Dr. Nicolai S.C. van Oers. Plasmids encoding for chimeric proteins containing the external and transmembrane portions of CD25 fused to the cytoplasmic tails of either CD3ε (TT-ε) or TCR-ζ (TT-ζ) were a gift from Dr. R.D. Klausner (National Cancer Institute, Bethesda, MD; Letourneur and Klausner, 1991). A truncated version of TT-ε was generated by PCR mutagenesis of the original TT-εTo construct (kindly provided by Dr. R.D. Klausner) using the following primers: 5′ primer (within Tac): 5′ ACAGATCTCCAGGTAGCAGTG 3′; 3′ primer (within CD3ε): 5′ TCGATATCACCTATTCTTGAGCCCACT 3′. Wild-type TT-ε in pCDL SRα was replaced by the BglII/EcoRV-digested PCR fragment. The new truncated TT-ε encompasses the three first amino acids of the CD3ε cytoplasmic tail. All constructs were subcloned into pcDNA3 (Invitrogen Corp., San Diego, CA) and transfected into Jurkat T cells by electroporation. Briefly, 107 cells were transfected with 20 μg of plasmid in a Gene Pulser (Bio-Rad Laboratories, Hercules, CA) at 250 V and a capacitance of 960 μF. After transfection, cells were grown for 2 d in complete RPMI before plating out in medium containing 2 mg/ml of G418 (GIBCO BRL). Clones were obtained by limiting dilution. Generation of the CD8-ζ clone has been described elsewhere (Irving and Weiss, 1991). Stable cell lines expressing the wild-type CD8-ζT76 truncation or the CD8-ζ4F mutant were generated as described elsewhere (Irving and Weiss, 1991). Construction of CD8-ζT76 and CD8-ζ4F vectors was based on modifications of the original CD8-ζ plasmid. The CD8-ζT76 truncation mutant was created by PCR mutagenesis from the CD8-ζ wild type using the following oligonucleotides: 5′ primer (within CD8): 5′ CACCATCGCGTCGCAGC3′; T76 truncation: 5′ GATCTCGAGAGGATCCTATTACATCCCAATCTCACTG 3′.
After PCR amplification, the CD8-ζT76 truncated fragment was digested with EcoRV and BamHI, and substituted for the wild-type CD8-ζ in the ptf neo CD8-ζ expression vector (Irving and Weiss, 1991). The mutation was confirmed by sequencing. The CD8-ζT76 mutant truncated the ζ chain two amino acids after the second ITAM. The CD8-ζ4F mutant was created from the CD8-ζT76 truncation by sequential PCR mutagenesis. The outside primers and fragment substitution were the same as for the creation of the CD8-ζT76 truncation. After PCR mutagenesis, the construct was sequenced to verify that only the intended mutations existed.
To mutate the first tyrosine to phenylalanine of the first ITAM of CD8-ζ, the following primers were used: 5′ CAGCTCTTTAACGAGCTCAA 3′, and 3′ TCTTGGTCGAGAAATTGCTC 5′.
To mutate the second tyrosine to phenylalanine of the first ITAM of CD8-ζ, the following primers were used: 5′ GAGGAGTTCGATGTTTTGGA 3′, and 3′ CTTCTCTCCTCAAGCTACAA 5′.
To mutate the first tyrosine to phenylalanine of the second ITAM of CD8-ζ, the following primers were used: 5′ GGCCTGTTCAATGAACTGCA 3′, and 3′ TCCTTCCGGACAAGTTACTT 5′.
To mutate the second tyrosine to phenylalanine of the second ITAM of CD8-ζ, the following primers were used: 5′ AGGCCTTCAGTGAGATTGG 3′, and 3′ ACCGCCTCCGGAAGTCAC 5′.
Conjugate Formation and Immunofluorescence Microscopy
Jurkat T cells or derived clones and mutants were mixed with antibody-coated latex beads at a 2:1 ratio. After centrifugation for 5 min at 100 g, the cell–bead mixture was incubated for an additional 5–25 min at 37°C. Conjugates were then resuspended, plated onto poly-l-lysine-treated slides, and fixed for 30 min in 3.4% paraformaldehyde at room temperature. Fixed cells were permeabilized for 4 min in PBS/0.1% Triton X-100 and blocked for 10 min in PBS/0.2% BSA. Cells were labeled for 30 min with antibody diluted in PBS/0.2% BSA, followed by two washes of 10 min each. After secondary antibody labeling, cells were incubated for 10 min in 33 nM rhodamin-phalloidin. After three final washes, slides were mounted. Samples were either viewed under a laser scanning microscope (LSM 410; Carl Zeiss, Inc., Thornwood, NY) (1st experiment) or a conventional Microphot-FXA microscope (all other experiments). The MTOC was scored as reoriented if it was located in close proximity to the T cell plasma membrane between the T cell nucleus and the bead contact area. At least 100 conjugates were scored in each experiment.
Transient Transfection Conjugate Formation Assay
Cell (107) were transiently cotransfected by electroporation, as described above, with 30 μg of the indicated TT chimera and 40 μg of a vector containing no insert or expressing dominant-negative mutants of ZAP-70 and Ras. After 36 h, dead cells were eliminated by centrifugation over a Ficoll gradient (Sigma Chemical Co.). After two washes, conjugates were formed and analyzed as described above.
Results
Anti–TCR-coated Latex Beads Induce MTOC Reorientation and Polarized Actin Polymerization in Jurkat T Cells
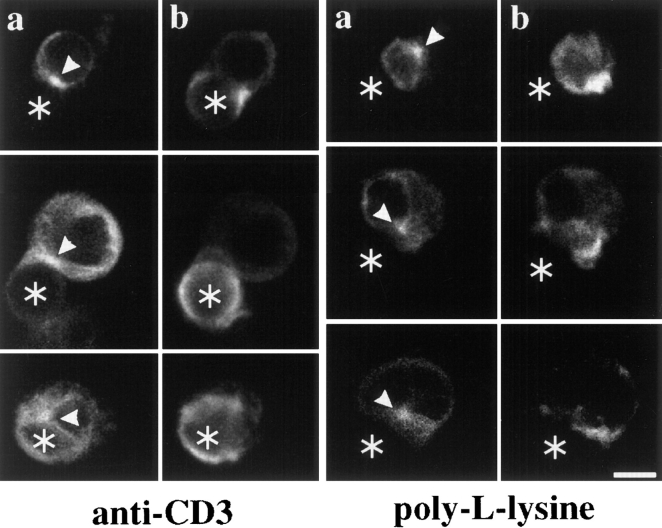
Previous T cell polarization studies performed in a B cell–T cell system demonstrated the dependence of cytoskeletal rearrangements during conjugate formation on the presence of antigen, implying the participation of the TCR in these events (Kupfer and Dennert, 1984). The involvement of accessory molecules including integrins and coreceptors such as CD4/CD8 made it difficult to evaluate to what extent TCR-linked pathways were involved and sufficient for T cell cytoskeletal polarization. To address this question, we set up an in vitro assay using Jurkat T cells as effectors, and antibody-coated, cell-sized latex microspheres as APCs. Previous studies have demonstrated that such artificial APCs, either coated with TCR-specific antibodies or antigen–major histocompatibility complexes (MHC), could induce early and late signaling events, such as tyrosine phosphorylation and exocytosis, in T cells (Anel et al., 1994; Mescher, 1992). In contrast to soluble or plate-bound TCR-specific antibodies, the use of latex beads allowed us to study signaling events in response to a focal stimulus. In a first set of experiments, we investigated whether triggering of the TCR alone was sufficient to induce reorientation of the microtubule cytoskeleton and polarized actin polymerization. Latex beads coated with the CD3ε-specific antibody Leu4 were mixed with Jurkat T cells at a 1:2 ratio and incubated for 30 min at 37°C. Formed conjugates were fixed and stained for tubulin and F-actin. The position of the MTOC within the T cells was analyzed microscopically. Only MTOCs directly underlying the bound latex bead were scored positive. In the majority of all conjugates (73 ± 2%), the T cell MTOC was facing the latex bead contact area (Fig. 1 a). Polymerized actin was concentrated in a dense collar at the bead attachment site. F-actin-rich pseudopods protruded from the T cell surface, and in many cases surrounded the bead (Fig. 1 b). Similar results were obtained at shorter incubation times (10–20 min) and with latex beads coated with the Jurkat Tiβ-specific antibody C305 (data not shown). In contrast, the position of the MTOC was random when conjugates between Jurkat T cells and poly-l-lysine-treated beads were analyzed (Fig. 1 a). Furthermore, no F-actin accumulation at the bead contact site could be observed (Fig. 1 b).
Figure 1.
Anti–TCR-coated latex beads induce MTOC reorientation and polarized actin polymerization in Jurkat T cells. Jurkat T cells were mixed at a 1:2 ratio with anti–CD3ε-coated latex beads (left) or poly-l-lysine-coated latex beads (right). After 30 min at 37°C, conjugates were stained with the antitubulin antibody YOL1/34 (a) and rhodamine-phalloidin to visualize F-actin (b). The position of cell-bound latex beads is indicated by an asterisk, the position of the MTOC by an arrowhead. Bar, 5 μm.
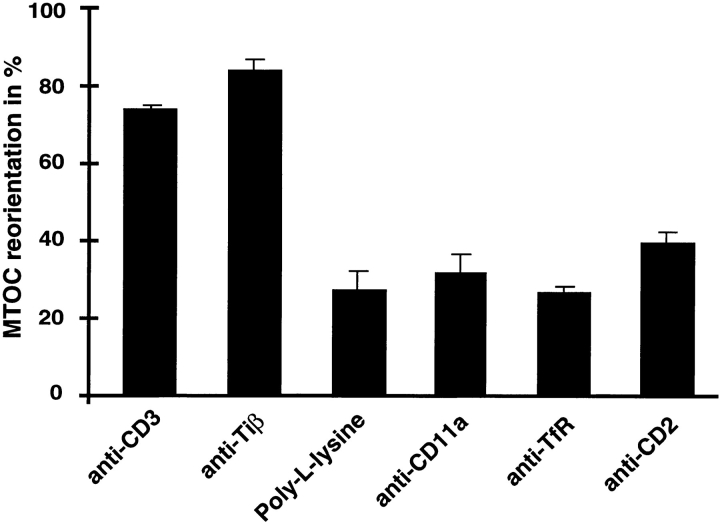
To determine whether cytoskeletal polarization was specifically associated with TCR cross-linking, antibodies directed against a variety of T cell surface molecules (CD11a/ LFA-1, transferrin receptor [TfR] and CD2) and poly-l-lysine were bound to latex beads and used in the conjugate formation assay. All surface molecules analyzed were expressed at comparable levels in Jurkat T cells and similar numbers of bead–cell conjugates were formed (data not shown). As summarized in Fig. 2, TCR/CD3-specific beads displayed MTOC polarization in ∼80% of all scored conjugates, whereas beads coated with either anti–CD11a, anti–TfR, anti–CD2, or poly-l-lysine were randomly positioned (31 ± 5, 26 ± 2, 39 ± 4, and 26 ± 6% of scored conjugates, respectively), demonstrating that cytoskeletal polarization is specifically associated with TCR/CD3 cross-linking.
Figure 2.
MTOC reorientation in Jurkat cells is specifically associated with TCR/CD3 cross-linking. Jurkat T cells were mixed at a 1:2 ratio with latex beads coated either with poly-l-lysine or antibodies against CD11a/LFA-1, the transferrin receptor, and CD2. Conjugates were stained with antitubulin antibody. Each column represents the average of at least four individual experiments in which >100 conjugates were scored for MTOC reorientation. MTOCs positioned between the bead contact site and the T cell nucleus, in close proximity to the T cell plasma membrane, were scored as positive for reorientation.
Clustering of Chimeras Containing the Cytoplasmic Domain of CD3ε or TCR-ζ Can Induce MTOC Reorientation and Actin Polymerization
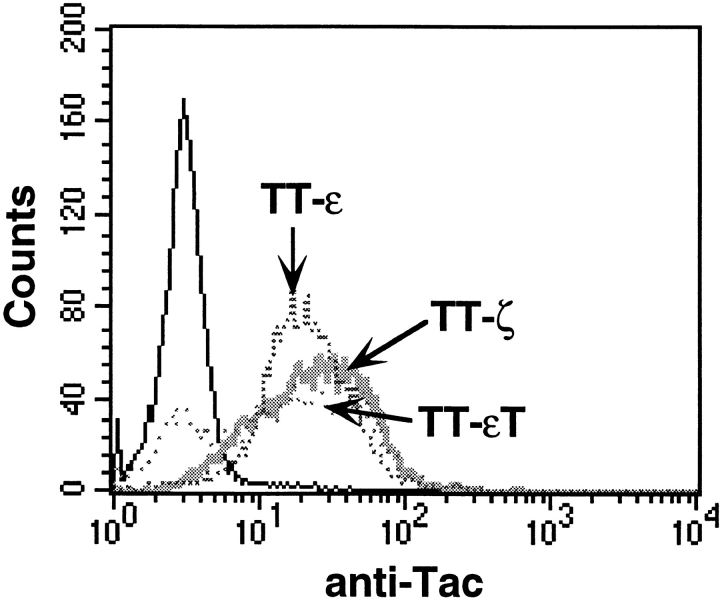
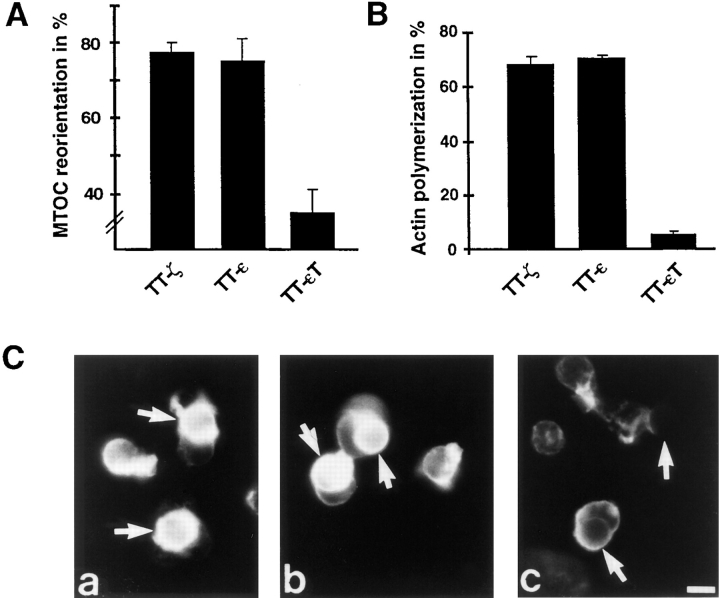
Several studies support the view that the presence of multiple ITAMs in the TCR might serve the purpose of signal amplification (Irving et al., 1993; Shinkai et al., 1995). However, single TCR subunits can associate with distinct SH2-containing signaling molecules and show qualitative differences in the induction of certain cellular responses (Combadiere et al., 1996; Osman et al., 1996). This suggests that distinct TCR subunits could couple the receptor to qualitatively different signaling pathways. To evaluate the ability of single TCR chains to induce cytoskeletal changes, we analyzed Jurkat T cells stably expressing a chimera between the extracellular and transmembrane domain of CD25 (Tac) with either the cytoplasmic tail of TCR-ζ (TT-ζ), CD3ε (TT-ε), or a truncated form of CD3ε (TT-εT) containing only the first three amino acids of the cytoplasmic tail. An ITAM motif is present once in the CD3ε chain and three times in the TCR-ζ chain. The ITAMs in both chains differ in their nonconsensus sequences and were shown to bind signaling molecules with different affinities (Osman et al., 1996). As demonstrated previously (Letourneur and Klausner, 1991; Shinkai et al., 1995), TT chimeras are expressed as monomeric molecules and do not associate with endogenous CD3 subunits. Clones with similar surface expression of the chimeric proteins (Fig. 3) were chosen and mixed with anti–Tac-coated latex beads for 10 min. The percentage of conjugate formation was comparable (data not shown). As shown in Fig. 4 A, clustering of the cytoplasmic tail of either TCR-ζ or CD3ε was sufficient to induce MTOC reorientation. The extent of MTOC reorientation triggered by either chimera was comparable and was similar to levels induced by cross-linking of the complete TCR-CD3 complex. Furthermore, both chimeras could induce actin polymerization (Fig. 4, B and C). In contrast, no cytoskeletal polarization was detected towards beads bound to the truncated TT-εT chimera. These results suggest that both the ζ and ε chains can trigger qualitatively similar signals, which are normally generated by the TCR and induce T cell polarization. Furthermore, the presence of one ITAM motif was sufficient to induce cytoskeletal rearrangements, and the number of ITAMS had no obvious quantitative effect on the induction of cytoskeletal polarization. However, in transient expression experiments where chimera surface expression was lower, the TT-ε chimera induced MTOC reorientation less efficiently than TT-ζ (see below and data not shown). This result supports the view that multiple ITAMs can act as signal amplifiers under suboptimal activation conditions.
Figure 3.
Chimera surface expression in stable Jurkat-derived clones expressing chimeras between Tac and the cytoplasmic tail of TCR-ζ (TT-ζ), CD3ε (TT-ε), or a truncated version of CD3ε (TT-εT). Stable clones were analyzed for chimera surface expression by FACS® analysis using an FITC-labeled Tac-specific antibody. The solid line represents untransfected Jurkat T cells.
Figure 4.
Clustering of chimeras containing the cytoplasmic domain of CD3ε or TCR-ζ can induce MTOC reorientation and actin polymerization. Stable Jurkat-derived clones expressing chimeras between Tac and the cytoplasmic tail of TCR-ζ (TT-ζ), CD3ε (TT-ε), or a truncated version of CD3ε (TT-εT) were analyzed. Clones were mixed at a 1:2 ratio with latex beads coated with anti–Tac. After incubation for 10 min at 37°C, conjugates were processed as in Fig. 1. (A) Conjugates were scored for MTOC reorientation. Averages of four different experiments are shown. To better visualize the differences in MTOC reorientation, the scale of the graph starts at 25%, an arbitrarily set background value obtained with poly-l-lysine-coated beads. (B) Only conjugates between single latex beads and single Jurkat cells were scored for actin polymerization. Three different experiments were averaged. (C) Conjugates between anti–Tax latex beads and Jurkat clones expressing either TT-ζ (a), TT-ε (b), or TT-εT (c) were stained for F-actin. Latex beads are indicated by an arrow. Bar, 5 μm.
MTOC Reorientation and Actin Polymerization Require the Presence of an Intact ITAM
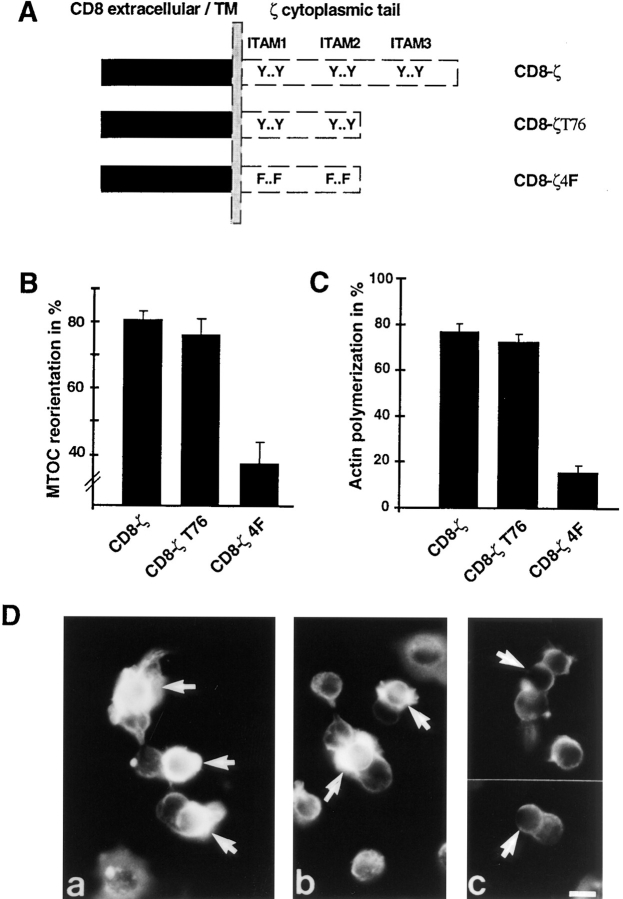
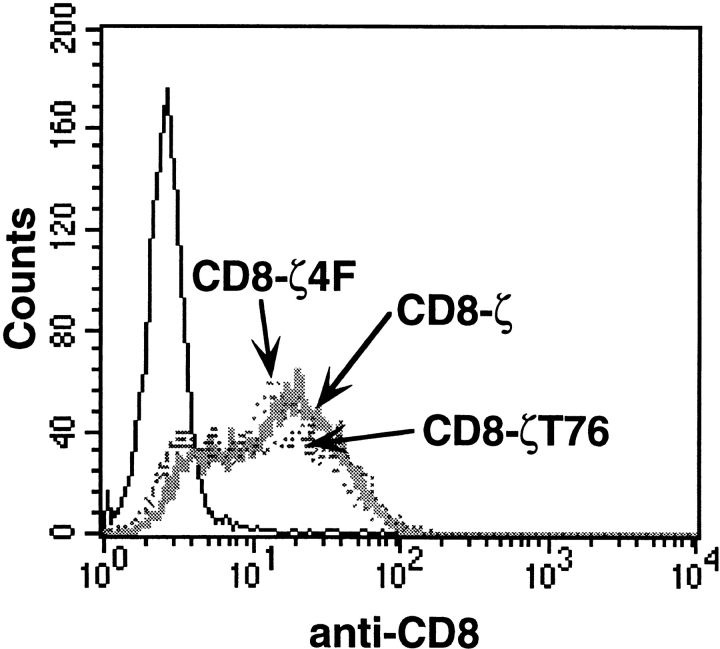
ITAM phosphorylation is essential for signaling through the TCR in many cellular responses. Phosphorylated ITAMs serve as docking sites for downstream signaling molecules such as ZAP-70 (Osman et al., 1996). Furthermore, phosphorylated ITAMs can mediate the activation-induced association of TCR components with the actin cytoskeleton. In particular, the third ITAM of the TCR-ζ chain has been reported to be crucial for F-actin interaction (Rozdzial et al., 1995). To investigate the role of ITAM phosphorylation and F-actin binding in coupling the TCR to T cell polarization, we analyzed stable clones expressing a chimera between the extracellular/transmembrane domain of human CD8 and either the entire cytoplasmic tail of TCR-ζ (CD8-ζ), the cytoplasmic tail of TCR-ζ truncated after the second ITAM (CD8-ζT76), or the CD8-ζT76 chimera in which all four ITAM tyrosines were mutated to phenylalanine (CD8-ζ4F) (Fig. 5 A). Clones with comparable surface expression were used (Fig. 6). As shown before for the TT-ζ expressing clone, cross-linking of the CD8-ζ chimera efficiently induced MTOC reorientation and actin polymerization at the bead attachment site (Fig. 5, B and C). No obvious qualitative difference was observed for the CD8-ζT76 chimera, which lacks the third ITAM, suggested to mediate F-actin binding. Focal actin polymerization was as frequently detected with CD8-ζT76 as in the CD8-ζ chimera (Fig. 5 C). As judged by immunofluorescence microscopy, no significant difference in the extent of F-actin accumulation was observed (Fig. 5 D). Our data suggest that the observed clustering of F-actin at the site of TCR engagement occurs in the absence of the third ζ chain ITAM. In contrast, analysis of CD8-ζ4F demonstrated the strict dependence of both MTOC reorientation and actin polymerization on the tyrosine phosphorylation of the ITAM motif. Mixing of clone CD8-ζ4F with anti–CD8-coated latex beads still yielded conjugates, but MTOC positioning was random (38 ± 7%) (Fig. 5 B). The frequency and extent of actin polymerization was also dramatically reduced (Fig. 5, C and D). These results indicate that ITAM phosphorylation is required for TCR-induced cytoskeletal polarization.
Figure 5.
MTOC reorientation and actin polymerization require ITAM phosphorylation. (A) Schematic representation of the expressed CD8 chimeras. (B) Conjugates between the CD8 chimera–expressing clones and anti–CD8-coated latex beads were analyzed for MTOC reorientation as in Fig. 2. Results of six different experiments are averaged. (C) Actin polymerization in single bead–T cell conjugates was scored as in Fig. 4. Averages of three different experiments are shown. (C) Actin polymerization in response to CD8-ζ (a), CD8-ζT76 (b), and CD8-ζ4F (c) cross-linking was analyzed as in Fig. 4. The position of latex beads is indicated by an arrow. Bar, 5 μm.
Figure 6.
CD8 chimera surface expression in stable Jurkat-derived clones. Stable clones were analyzed for chimera surface expression by FACS® analysis using an FITC-labeled CD8-specific antibody. The solid line represents untransfected Jurkat T cells.
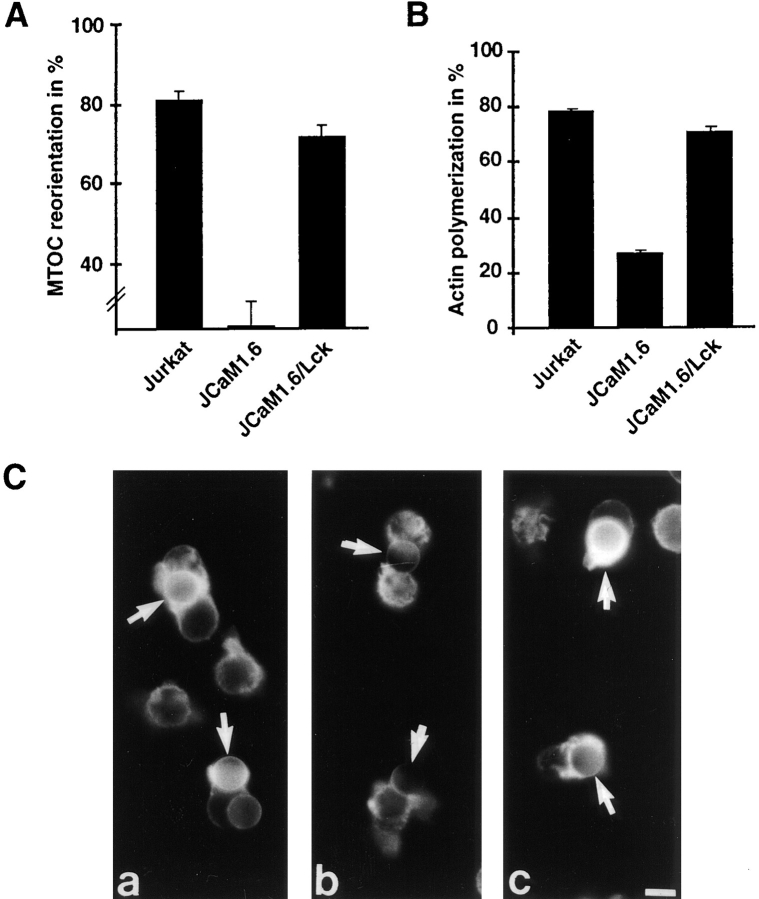
To corroborate this finding, we analyzed cytoskeletal rearrangements in the mutant Jurkat cell line JCaM1.6, which lacks functional Lck. Src family kinases and in particular Lck have been shown to be responsible for ITAM phosphorylation in T cells (Iwashima et al., 1994; van Oers et al., 1996). Consistent with the failure to phosphorylate ζ and CD3 chains, TCR-ligation of JCaM1.6 fails to induce tyrosine phosphorylation, calcium influx, or CD69 expression (Straus and Weiss, 1992). Analyzed in our conjugate formation assay, JCaM1.6 failed to polarize towards CD3-specific latex beads. MTOC positioning was random (30 ± 4% of scored conjugates) and no actin polymerization was detected (Fig. 7, A and B). Reconstitution of JCaM1.6 with wild-type murine Lck (JCaM1.6/Lck) restored TCR-induced cytoskeletal changes comparable with that observed in the parental Jurkat cell line (Fig. 7, A and B). Similar results were obtained with the mutant Jurkat cell line J45.01, which lacks functional CD45 (data not shown). CD45 is a transmembrane tyrosine phosphatase required for the activation of Src family kinases (Weiss and Littman, 1994). Taken together, these results support the view that ITAM phosphorylation and subsequent recruitment of downstream signaling molecules to the TCR are a prerequisite for cytoskeletal alterations.
Figure 7.
MTOC reorientation and actin polymerization are abolished in the Lck-deficient cell line JCaM1.6. Jurkat T cells and the signaling mutant JCaM1.6 were bound to anti–CD3ε beads and scored as described in Fig. 2 for (A) MTOC reorientation and (B and C) actin polymerization. C shows actin polymerization in Jurkat T cells (a), the Lck-deficient mutant JCaM1.6 (b), and JCaM1.6/Lck reconstituted with murine Lck (c). Arrows point to cell-bound beads. Averages of seven (A) and three (B) experiments are shown. Bar, 5 μm.
Overexpression of a Dominant-Negative ZAP-70 Mutant Impairs MTOC Reorientation
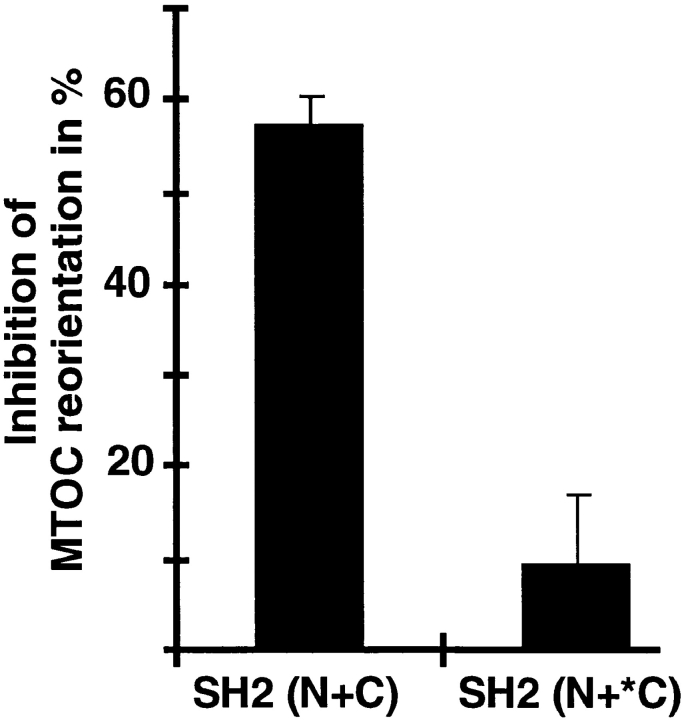
A crucial step in TCR-mediated signaling is the recruitment of ZAP-70 to phosphorylated ITAMs after TCR engagement. Recruitment leads to tyrosine phosphorylation and activation of ZAP-70, which, in turn, contributes to the activation of both early and late signaling cascades. Several studies suggest that ZAP-70 plays in important role in TCR-mediated signaling. For example, lack of functional ZAP-70 in humans and mice results in severe developmental and functional defects in the T cell compartment (Arpaia et al., 1994; Chan et al., 1994; Elder et al., 1994). Furthermore, introduction of a dominant-negative mutant form of ZAP-70 into T cells suppresses TCR-mediated tyrosine phosphorylation of several signaling molecules and activation of NF-AT, a nuclear factor essential for inducible interleukin 2 gene expression (Qian et al., 1996). To assess the role of the ZAP-70–ITAM interaction in TCR-mediated MTOC reorientation, we transiently cotransfected Jurkat cells with the activating chimera TTε and the dominant-negative ZAP-70 mutant SH2 (N+C) (Qian et al., 1996). SH2 (N+C) is thought to inhibit TCR signaling by blocking phosphorylated ITAMs (Qian et al., 1996). Transfected cells were mixed with anti–Tac latex beads 36 h later, and conjugates were formed for 10 min at 37°C. Similar to our studies in stable clones, engagement of TT-ε induced MTOC reorientation, although to a lesser extent (67 ± 1% of the conjugates). As expected, engagement of transiently expressed TT-εT failed to induce MTOC reorientation (20 ± 6%) and served as a negative control. Coexpression of dominant-negative ZAP-70 markedly reduced MTOC reorientation by ∼50–60%, whereas expression of a mutant SH2 (N+C) form (SH2 (N +*C), (Qian et al., 1996), unable to bind to ITAMs, had only minimal effects (Fig. 8). Expression of both mutants did not affect TT-ε surface expression (data not shown). Moreover, comparable results were obtained in Jurkat TAg cells (data not shown). These results demonstrate that engagement of transiently expressed activating chimeras can induce MTOC reorientation. Moreover, a dominant-negative mutant of ZAP-70 encompassing the two SH2 domains impairs both TCR-mediated MTOC reorientation (shown here) and NF-AT gene activation (Qian et al., 1996). This suggests that both responses are mediated by the same proximal signaling events requiring molecules that interact with phosphorylated ITAMs.
Figure 8.
Expression of dominant-negative ZAP-70 mutant impairs ITAM-mediated MTOC reorientation. Jurkat T cells were cotransfected with TT-εT or TT-ε (30 μg), together with 40 μg of empty vector or plasmids encoding different ZAP-70 mutants. 36 h later, cells were mixed with anti–Tac latex beads and incubated for 10 min at 37°C. Conjugates were fixed in paraformaldehyde and stained with antitubulin antibody. Percent MTOC inhibition equals X−N/M−N, where X is the percent MTOC reorientation triggered by TT-ε in the presence of dominant-negative ZAP-70, M is the percent MTOC reorientation triggered by TT-ε alone, and N is the percent MTOC reorientation triggered by TT-εT. Results are the average of three independent experiments. The averaged percent MTOC reorientation for each condition was 20 ± 6% for TT-εT plus vector, 67 ± 1% for TT-ε plus vector, 40 ± 6% for TT-ε plus SH2 (N+C), and 64 ± 5% for TT-ε plus SH2 (N+*C).
MTOC Reorientation Occurs in the Absence of Ras Activation
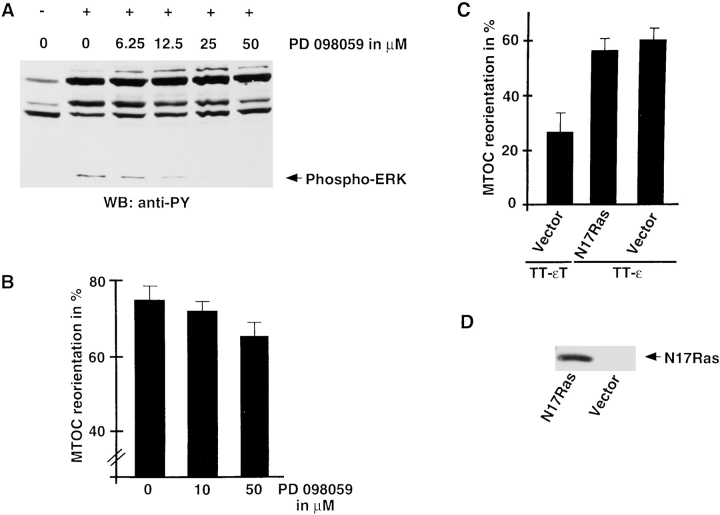
Two major signaling pathways, namely the Ras and the PLC-γ1 pathway, are activated after TCR engagement. Activation of the small GTPase Ras by the TCR induces the extracellular signal-regulated kinase (ERK 1,2) cascade involving several cytoplasmic serine/threonine kinases. ERKs phosphorylate transcription factors of the AP-1 family and are necessary for TCR-mediated NF-AT gene activation and T cell development (Alberola-Ila et al., 1995; Izquierdo-Pastor et al., 1995; Swan et al., 1995). In certain cell types, ERKs are associated with microtubules (Morishima-Kawashima and Kosik, 1996; Reszka et al., 1995) and phosphorylate microtubule-associated proteins (MAPs). MAPs stimulate the polymerization of new and preexisting microtubules. Interestingly, these microtubule-promoting properties are lost after in vitro phosphorylation by ERKs (Hoshi et al., 1992). Thus, TCR-induced ERK activation could influence microtubule dynamics. To address this question, we took advantage of the recently described compound PD 098059, a selective inhibitor of the ERK-activating enzyme MAPK/ERK kinase (Dudley et al., 1995). To characterize the effect of PD 098059 on TCR-induced ERK activation, Jurkat T cells were preincubated for 30 min with increasing concentrations of PD 098059 and were stimulated for 10 min by the addition of C305-coated latex beads. Cells were then lysed and analyzed for tyrosine phosphorylation by immunoblotting. As shown in Fig. 9 A, incubation with PD 098059 resulted in a dose-dependent inhibition of ERK phosphorylation in response to TCR engagement, whereas the phosphorylation status of various other proteins remained unchanged. The identity and quantity of ERK detected was confirmed by stripping and reblotting the same membrane with a phospho-ERK–specific antibody (data not shown). This experiment demonstrates that PD 098059 indeed specifically inhibits ERK phosphorylation. However, the inhibitor had no significant effect on MTOC reorientation (Fig. 9 B), even at concentrations that substantially reduced ERK phosphorylation. This suggests that ERKs are not involved in the regulation of cytoskeletal events associated with MTOC reorientation.
Figure 9.
MTOC reorientation is mediated by Ras-independent pathways. (A) Jurkat T cells were incubated with the indicated concentrations of PD 098059 in DMSO for 30 min, followed by a 10-min stimulation with C305-coated latex beads (+). Untreated control cells were mixed with poly-l-lysine-coated beads (−). Cells were lysed and whole cell lysates were analyzed by immunoblotting with an antiphosphotyrosine antibody. (B) Jurkat T cells were incubated with the indicated concentrations of PD 098059 for 30 min. Subsequently, C305-coated latex beads were added at a 1:2 ratio and conjugates were incubated for 10 min. Conjugates were fixed and stained with antitubulin antibody. The average of four independent experiments is shown. (C) TAg Jurkat cells were cotransfected with TT-εT or TT-ε (30 μg) together with 40 μg of empty vector or a plasmid encoding the dominant-negative Ras mutant N17Ras. 36 h later, cells were mixed with anti–Tac latex beads and incubated for 10 min at 37°C. Conjugates were fixed in paraformaldehyde and stained with antitubulin antibody. The averaged percent MTOC reorientations of two independent experiments are shown. (D) Lysates from 106 Tag Jurkat cells either cotransfected with TT-ε and empty vector or N17Ras were analyzed for the presence of mutant Ras with a Ha-Ras-specific mAb.
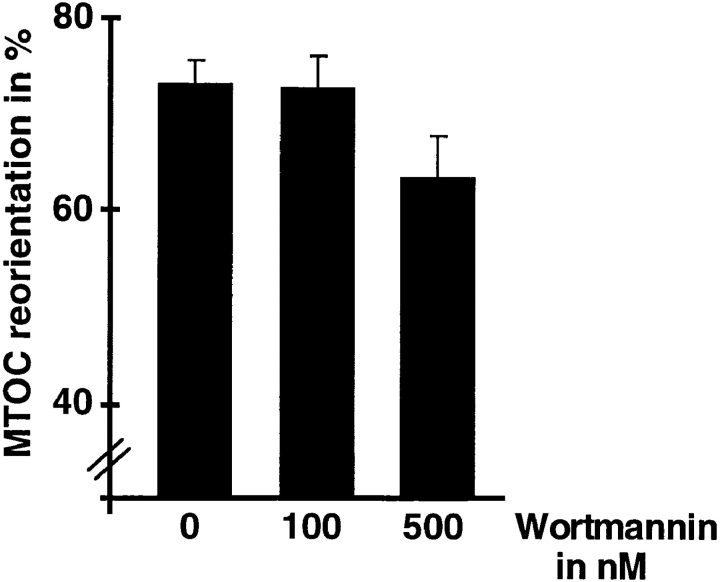
Besides ERKs, the small GTPase Rac-1 and phosphatidylinositol 3 kinase (PI-3 kinase) have been recently identified as Ras effector molecules (Genot et al., 1996; Rodriguez-Viciana et al., 1994). Interestingly, both PI-3 kinase and CDC42, a close family member of Rac-1, have been shown to be involved in B cell–induced MTOC reorientation of a murine T cell hybridoma (Stowers et al., 1995). Thus, TCR-mediated Ras activation might induce MTOC reorientation via ERK-independent pathways. To investigate this possibility, we transiently coexpressed the activating TT-ε chimera with the dominant-negative Ras mutant Ha-N17Ras in TAg Jurkat cells. Earlier studies have demonstrated that overexpression of this Ras mutant efficiently inhibits TCR-mediated events such as NF-AT/IL-2 gene activation or T cell development (Alberola-Ila et al., 1995; Rayter et al., 1992). However, when analyzed in the polarization assay, the mutant did not significantly affect MTOC reorientation (Fig. 9 C), although high expression levels could be detected by Western blotting (Fig. 9 D). Furthermore, MTOC reorientation in Jurkat T cells pretreated with the specific PI-3 kinase inhibitor Wortmannin was comparable to cells pretreated with the vehicle alone (Fig. 10). These results further substantiate our inhibitor experiment and suggest that Ras-mediated effector pathways are not involved in TCR-mediated microtubule reorganization. In contrast to earlier studies (Stowers et al., 1995), inhibition of PI-3 kinase did not affect MTOC reorientation in our assay system. This discrepancy might reflect differences in the assay systems used.
Figure 10.
PI-3 kinase is not required for TCR-mediated MTOC reorientation in Jurkat T cells. Jurkat T cells were incubated with the indicated concentrations of Wortmannin for 30 min. Subsequently, Leu4-coated latex beads were added at a 1:2 ratio and conjugates were incubated for 30 min. Conjugates were fixed and stained with antitubulin antibody. The average of four independent experiments is shown.
Discussion
Upon binding to an APC, T cells undergo rapid and dramatic cytoskeletal changes that ensure the polarized delivery of an effector response. To date, T cell polarization has been mainly studied in a B cell–T cell system, where complex receptor–ligand interactions complicate the identification of the participating signaling pathways. In this report, we describe a simple bead-based conjugate formation assay to study this response. Jurkat T cells were chosen as model effector cells. Although this leukemic cell line may differ in many aspects from primary T cells, it has been a powerful tool in dissecting signaling pathways involved in T cell activation. Jurkat T cells can be easily manipulated by transient transfection approaches, and several TCR-signaling mutants have been generated and characterized. APCs have been replaced by antibody-coated latex beads. Since antibody-induced TCR cross-linking may induce stronger signals than TCR engagement by peptide–MHC complexes, our assay system may underscore the need for accessory molecules such as adhesion receptors or coreceptors. Furthermore, T cell activation with immobilized antibodies may not exactly reflect the role of receptor membrane motility and recycling in the generation of an intracellular signal. Nevertheless, the assay system should be useful to characterize the minimal requirements for polarized cytoskeletal rearrangements and to identify key-regulators of this mechanism. Our analysis provides evidence that TCR engagement alone is sufficient to induce MTOC reorientation and focal F-actin accumulation, suggesting that TCR-dependent signaling pathways can regulate cytoskeletal dynamics.
The induction of actin polymerization through antigen receptor activation has been previously observed in a variety of cell types including T cells, B cells, and macrophages (Greenberg et al., 1991; Melamed et al., 1991; Parsey and Lewis, 1993), but so far the underlying signaling pathways have been elusive. In contrast to cell activation by soluble factors, antigen-induced T cell activation is triggered by spatially constrained ligands; thus, TCR-induced cytoskeletal changes could influence the cellular response in several ways. First, cytoskeleton-dependent changes in cell shape and motility could increase the frequency of APC contacts, eventually resulting in the formation of stable conjugates (Negulescu et al., 1996). Second, actin polymerization could be required for the rapid reorganization and concentration of accessory molecules and receptors at the T cell–APC contact site, which would further increase conjugate stability and result in the formation of signaling complexes (Monks et al., 1997; Shaw and Dustin, 1997). Focally clustered F-actin could also serve as a spatial cue, recruiting proteins involved in MTOC reorientation. Such a role for F-actin has been postulated in the mechanism of spindle rotation during the embryogenesis of Caenorhabditus elegans (Waddle et al., 1994). Third, reorientation of the MTOC and organellar reorganization could ensure the delivery of a polarized immune response by effector T cells to a specific target cell in crowded environments such as lymphoid organs.
Two complexes within the TCR, namely the TCR-ζ and CD3 chains, couple the receptor via ITAMs to the intracellular signaling pathways. Interestingly, individual phosphorylated ITAMs bind differentially to SH2-containing signaling molecules in vitro (Osman et al., 1995, 1996) and could therefore activate distinct signaling pathways (Combadiere et al., 1996; Letourneur and Klausner, 1992). In our experiments, TCR-ζ and CD3ε were equally able to trigger MTOC reorientation and actin polymerization in stable clones. However, when transiently expressed at lower levels, CD3ε induced MTOC reorientation less efficiently compared with ζ, supporting the concept that multiple ITAMs can serve as signal amplifiers. ITAM-mediated signal amplification may be crucial when MTOC reorientation is induced by a physiological stimulus; i.e., a peptide–MHC complex. Earlier studies have demonstrated that MTOC reorientation in antigen-specific T cell–B cell conjugates was highly antigen-dose dependent and occurred inefficiently at low antigen levels (Kupfer et al., 1987). Antibody-induced TCR cross-linking, as used in our study, may induce a more potent signal than TCR engagement by peptide–MHC complexes. Thus, the requirements for multiple ITAM-containing TCR/CD3-chains may differ between the in vitro system and the in vivo situation.
A signaling molecule that binds preferentially to ζ ITAMs, but not to the ε ITAM in vitro, is the p85 regulatory subunit of PI-3 kinase (Osman et al., 1996). Interestingly, inhibition of PI-3 kinase with the specific inhibitor Wortmannin completely abolished MTOC reorientation and actin polymerization in a B cell–T cell hybridoma conjugate assay (Stowers et al., 1995). This result suggests that PI-3 kinase is a regulator of T cell polarization. Tested in our system, a TT-ε chimera readily induces cytoskeletal polarization. This indicates that PI-3 kinase is either activated independently of its recruitment to the CD3ε ITAM or is not involved in the cytoskeletal responses in our assay. Since the PI-3 kinase inhibitor Wortmannin had no effect on T cell polarization in our hands, we favor the second interpretation. The discrepancy observed between the two systems could reflect either cell line–specific differences or the different modes used to induce polarization. To generate a strong enough signal, B cell–T cell conjugates may need the contribution of adhesion molecules that may signal via a PI-3 kinase-dependent mechanism. Furthermore, efficient signaling by peptide–MHC complexes requires the serial engagement of TCRs (Valitutti et al., 1995a ). PI-3 kinase could be involved in the recruitment and concentration of TCR molecules at the B cell–T cell contact area.
ITAM phosphorylation is a key event in T cell activation. Our analysis of ζ-chain chimeras and Jurkat mutants demonstrates that MTOC reorientation and actin polymerization require the presence of at least one intact ITAM and a functional ITAM-phosphorylating Src-family kinase. Similarly, Cox et al. (1996) showed recently that clustering of the ITAM-containing Fc receptor γ subunit can trigger submembranous actin assembly in the chicken B cell line DT40. Actin polymerization required ITAM phosphorylation and the presence of the PTK Syk. In T cells, the Syk/ZAP-70 family member ZAP-70 can bind to phosphorylated ITAMs of all TCR signaling subunits with very high affinity (Osman et al., 1996). Recruitment of ZAP-70 to the receptor results in ZAP-70 phosphorylation and activation. Overexpression of a dominant-negative mutant of ZAP-70, which blocks phosphorylated ITAMs, markedly reduced MTOC reorientation in our studies. This result suggests that ZAP-70 or another phospho-ITAM-binding molecule may be involved in the regulation of microtubule dynamics. In fact, both Zap-70 and Syk can associate and efficiently phosphorylate α-tubulin in vitro (Huby et al., 1995; Peters et al., 1996). Furthermore, two recent reports provide evidence that T cell activation leads to the rapid tyrosine phosphorylation of α-tubulin (Cardine et al., 1995; Ley et al., 1994). Importantly, phosphorylated α-tubulin was not incorporated into microtubules, but remained in the soluble, unpolymerized tubulin pool. Syk was found to phosphorylate a site in the COOH terminus of α-tubulin, which coincides with a region involved in MAP binding (Peters et al., 1996). Since MAPs are involved in regulating the polymerization status of tubulin, it is possible that TCR-induced activation of ZAP-70 leads to the phosphorylation of α-tubulin and the subsequent dissociation of MAPs. This in turn could destabilize existing microtubules and lower the pool of tubulin monomers available for polymerization. Such changes in microtubule dynamics would occur locally at the site of TCR engagement and might result in the repositioning of the MTOC. Although the model described is very attractive, the possibility remains that dominant-negative ZAP-70 impairs MTOC reorientation by inhibiting the binding of other SH2-containing effector molecules to ITAMs. Taking advantage of membrane-targeted ZAP-70/Syk chimeras, future experiments will assess whether independent activation of this PTK family is sufficient to induce T cell polarization.
Proteins involved in the regulation of microtubule dynamics are often good substrates for ERKs and protein kinase C (PKC) (Gelfand, 1991). Members of both kinase families are known to be activated upon T cell activation. Thus, apart from the aforementioned ZAP-70-mediated mechanism, changes in the microtubule cytoskeleton could be regulated by one of these pathways. Our experiments show that MTOC reorientation can occur in the absence of Ras activation. Neither overexpression of dominant-negative Ras, nor inhibition of MAPK/ERK significantly affected MTOC reorientation. These results suggest that Ras-independent pathways regulate microtubule rearrangements in response to TCR engagement. In earlier studies, MTOC reorientation was shown to be dependent on extracellular calcium (Kupfer et al., 1987). Incubation of B cell–T cell couples in the presence of EGTA reduced MTOC reorientation from >90% to 55 ± 5%. Chelation of extracellular calcium showed similar effects in our conjugate formation assay (data not shown). MTOC reorientation was significantly reduced, but not abolished (from 78% to 50 ± 8%). This suggests that MTOC reorientation may in part be regulated by a calcium-dependent pathway. Indeed, repositioning of the MTOC could be a complex event involving the shortening of microtubules and the subsequent anchoring of the MTOC at the site of TCR engagement. Thus, impairment of one of these events could result in partial MTOC reorientation. A more simple explanation relates to the fact that intracellular calcium stores are not depleted under our assay conditions. Thus, release of intracellular calcium may be sufficient to partially activate calcium-dependent effector molecules. Interestingly, Ca2+/calmodulin-dependent kinase family members phosphorylate MAPs (Gelfand, 1991). Thus, several kinase families might participate in the regulation of MTOC reorientation.
The assay system described here should prove useful to further characterize the effector molecules involved in T cell cytoskeleton dynamics. Once identified, it will be interesting to assess the physiological role of these effectors in maintaining a polarized intercellular immune response.
Footnotes
The authors thank members of the Weiss laboratory for helpful discussions, and in particular Drs. D. Yablonski and N.S.C. van Oers for critical reading of the manuscript. We gratefully acknowledge the technical assistance of T. Laroche and the laser scanning microscope.
This work was supported in part by grants from the Boehringer Ingelheim Fonds (B. Lowin-Kropf) and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation (DRG-1356 to V. Smith Shapiro).
Address all correspondence to Dr. A. Weiss, Howard Hughes Medical Institute, U 426, 3rd and Parnassus Avenues, UCSF, San Francisco, CA 94143-0724. Tel.: (415) 476-1291. Fax: (415) 502-5081.
B. Lowin-Kropf's present address is Ludwig Institute for Cancer Research, Lausanne Branch, 1066 Epalinges, Switzerland.
1. Abbreviations used in this paper: APC, antigen-presenting cell; ERK, extracellular signal-regulated kinase; ITAM, immunoreceptor tyrosine-based activation motif; MAP, microtubule-associated protein; MHC, major histocompatibility complex; MTOC, microtubule-organizing center; PI-3, phosphatidylinositol 3; PTK, protein tyrosine kinase; SH2, src-homology-domain 2; TAg, T antigen; TCR, T cell antigen receptor; ZAP-70, zeta-associated protein 70.
References
- Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- Anel A, Richieri GV, Kleinfeld AM. A tyrosine phosphorylation requirement for cytotoxic T lymphocyte degranulation. J Biol Chem. 1994;269:9506–9513. [PubMed] [Google Scholar]
- Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+thymic selection in humans lacking ZAP-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Cardine AM, Kirchgessner H, Eckerskorn C, Meuer SC, Schraven B. Human T lymphocyte activation induces tyrosine phosphorylation of α-tubulin and its association with the SH2 domain of the p59fyn protein tyrosine kinase. Eur J Immunol. 1995;25:3290–3297. doi: 10.1002/eji.1830251214. [DOI] [PubMed] [Google Scholar]
- Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo W-L, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Combadiere B, Freedman M, Chen L, Shores EW, Love P, Lenardo MJ. Qualitative and quantitative contributions of the T cell receptor ζ chain to mature T cell apoptosis. J Exp Med. 1996;183:2109–2117. doi: 10.1084/jem.183.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Chang P, Kuosaki T, Greenberg S. Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J Biol Chem. 1996;271:16597–16602. doi: 10.1074/jbc.271.28.16597. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow T. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand VI. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO (Eur Mol Biol Organ) J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MA, Dazin PE, Weiss A. At least two non-antigen-binding molecules are required for signal transduction by the T cell antigen receptor. Proc Natl Acad Sci USA. 1988;85:8613–8617. doi: 10.1073/pnas.85.22.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S, El J, Khoury, Di Virgilio F, Kaplan EM, Silverstein SC. Ca2+-independent F-actin assembley and disassembley during Fc receptor-mediated phagocytosis in mouse macrophages. J Cell Biol. 1991;113:757–767. doi: 10.1083/jcb.113.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskelton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Otha K, Gotoh Y, Mori A, Murofushi H, Sakai H, Nishida E. Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces alteration in their function. Eur J Biochem. 1992;203:43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- Huby RDJ, Carlile GW, Ley SC. Interaction between the protein-tyrosine kinase ZAP-70, the proto-oncoprotein Vav, and tubulin in Jurkat T cells . J Biol Chem. 1995;270:30241–30244. doi: 10.1074/jbc.270.51.30241. [DOI] [PubMed] [Google Scholar]
- Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Pastor M, Reif K, Cantrell D. The regulation and function of p21ras during T cell activation and growth. Immunol Today. 1995;16:159–164. doi: 10.1016/0167-5699(95)80134-0. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci USA. 1991;88:775–779. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells: IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Swain SL, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer H, Monks CRF, Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J Exp Med. 1994;179:1507–1515. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor ζ family proteins. Proc Natl Acad Sci USA. 1991;88:8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3ε. Science. 1992;255:79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Ley SC, Verbi W, Pappin DJC, Druker B, Davies AA, Crumpton MJ. Tyrosine phosphorylation of α tubulin in human T lymphocytes. Eur J Immunol. 1994;24:99–106. doi: 10.1002/eji.1830240116. [DOI] [PubMed] [Google Scholar]
- Melamed I, Downey GP, Aktories K, Roifman CM. Microfilament assembly is required for antigen-receptor-mediated activation of human B lymphocytes. J Immunol. 1991;147:1139–1146. [PubMed] [Google Scholar]
- Mescher MF. Surface contact requirements for activation of cytotoxic T lymphocytes. J Immunol. 1992;149:2402–2405. [PubMed] [Google Scholar]
- Monks CRF, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Kosik K. The pool of MAP kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996;7:893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- Osman N, Lucas SC, Turner H, Cantrell D. A comparison of the interaction of Shc and the tyrosine kinase ZAP-70 with the T cell antigen receptor ζ chain tyrosine-based activation motif. J Biol Chem. 1995;270:13981–13986. doi: 10.1074/jbc.270.23.13981. [DOI] [PubMed] [Google Scholar]
- Osman N, Turner H, Lucas S, Reif K, Cantrell DA. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor ζ subunits and the CD3 γ, δ, and ε chains. Eur J Immunol. 1996;26:1063–1068. doi: 10.1002/eji.1830260516. [DOI] [PubMed] [Google Scholar]
- Parsey MV, Lewis GK. Actin polymerization and pseudopod reorganization accompany anti-CD3-induced growth arrest in Jurkat T cells. J Immunol. 1993;151:1881–1893. [PubMed] [Google Scholar]
- Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates α-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- Poo W-J, Conrad L, Janeway CA., Jr Receptor-directed focusing of lymphokine release by helper T cells. Nature. 1988;332:378–380. doi: 10.1038/332378a0. [DOI] [PubMed] [Google Scholar]
- Qian D, Mollenauer MN, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayter SI, Woodrow M, Lucas SC, Cantrell DA, Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO (Eur Mol Biol Organ) J. 1992;11:4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanheaesbroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Rozdzial MM, Malissen B, Finkel TH. Tyrosine-phosphorylated T cell receptor ζ chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3:623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- Ryser J-E, Rungger-Brandle E, Chaponnier C, Gabbiani G, Vassalli P. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J Immunol. 1982;128:1159–1162. [PubMed] [Google Scholar]
- Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Ma A, Cheng H-L, Alt FW. CD3ε and CD3ζ cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995;2:401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Stowers L, Yelon D, Berg LJ, Chant S. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci USA. 1995;92:5027–5031. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D, Weiss A. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995a;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995b;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddle JA, Cooper JA, Waterston RH. Transient localized accumulation of actin in Caenorhabditis elegans blastomeres with oriented asymmetric divisions. Development (Camb) 1994;120:2317–2328. doi: 10.1242/dev.120.8.2317. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weiss A, Stobo JD. Requirements for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Chouaib S, Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 erythrocyte receptor determinants. J Immunol. 1986;137:1097–2003. [PubMed] [Google Scholar]