Abstract
Abstract. Titin (also known as connectin) is a giant protein that spans half of the striated muscle sarcomere. In the I-band titin extends as the sarcomere is stretched, developing what is known as passive force. The I-band region of titin contains tandem Ig segments (consisting of serially linked immunoglobulin-like domains) with the unique PEVK segment in between (Labeit, S., and B. Kolmerer. 1995. Science. 270:293–296). Although the tandem Ig and PEVK segments have been proposed to behave as stiff and compliant springs, respectively, precise experimental testing of the hypothesis is still needed. Here, sequence-specific antibodies were used to mark the ends of the tandem Ig and PEVK segments. By following the extension of the segments as a function of sarcomere length (SL), their respective contributions to titin's elastic behavior were established. In slack sarcomeres (∼2.0 μm) the tandem Ig and PEVK segments were contracted. Upon stretching sarcomeres from ∼2.0 to 2.7 μm, the “contracted” tandem Ig segments straightened while their individual Ig domains remained folded. When sarcomeres were stretched beyond ∼2.7 μm, the tandem Ig segments did not further extend, instead PEVK extension was now dominant. Modeling tandem Ig and PEVK segments as entropic springs with different bending rigidities (Kellermayer, M., S. Smith, H. Granzier, and C. Bustamante. 1997. Science. 276:1112–1116) indicated that in the physiological SL range (a) the Ig-like domains of the tandem Ig segments remain folded and (b) the PEVK segment behaves as a permanently unfolded polypeptide. Our model provides a molecular basis for the sequential extension of titin's different segments. Initially, the tandem Ig segments extend at low forces due to their high bending rigidity. Subsequently, extension of the PEVK segment occurs only upon reaching sufficiently high external forces due to its low bending rigidity. The serial linking of tandem Ig and PEVK segments with different bending rigidities provides a unique passive force–SL relation that is not achievable with a single elastic segment.
Titin is a giant filamentous protein that, in addition to the thin and thick filaments, constitutes the third myofilament system of striated muscle. In the sarcomere, titin molecules span the entire 1–2-μm distance from the Z-line to the M-line. Previous studies have revealed that the A-band region of the molecule is rendered inextensible due to its tight association with the thick filament, whereas the I-band region behaves elastically as the sarcomere undergoes changes in length. The elastic properties of the I-band region of titin are primarily responsible for the passive force that is generated when unactivated (i.e., passive) muscle is stretched. Passive force is present in actively contracting muscle as well, where it helps maintain the structural integrity of the sarcomere and thereby ensures efficient muscle contraction. (For recent reviews and original citations see Fürst and Gautel, 1995; Trinick, 1996; Wang, 1996; Labeit et al., 1997; Maruyama, 1997.) The recently elucidated primary structure of human soleus titin (Labeit and Kolmerer, 1995) indicates that in the I-band titin is composed mainly of two types of segments: tandem Ig segments, consisting of serially linked Ig-like domains, and the PEVK segment that has a unique sequence (70% of its residues are P-proline, E-glutamate, V-valine, and K-lysine). The PEVK and tandem Ig segments have been suggested to act as low- and high-stiffness segments of the molecule, respectively (Labeit and Kolmerer, 1995).
In earlier studies of the elastic behavior of titin, Trombitás et al. (1995) and Granzier et al. (1996) found that in slack sarcomeres (where passive force is zero) the elastic I-band portion of titin is not straight, rather, it is in a contracted state. Passive force was proposed to be determined by the entropic force arising from the straightening of the I-band region of titin during modest stretch and by the unraveling (denaturation) of domains at high degrees of sarcomere extension. Subsequently, Gautel and Goulding (1996) and Linke et al. (1996) investigated whether extension of the elastic I-band segment occurs uniformly along the elastic segment. Using immunofluorescence, Linke et al. (1996) followed the location of an antibody (N2A) that labels the unique sequence NH2-terminal of the PEVK segment. The titin segment between the N2A epitope and the Z-line extended predominantly during small amplitude stretch. However, upon moderate to extreme stretch extension of the segment between the N2A epitope and the A-band (PEVK segment and ∼25 Ig domains) became predominant. Gautel and Goulding (1996) used an antibody (MG1) that labels titin some distance NH2-terminal of the PEVK segment and, by using immunoelectron microscopy, monitored the distances from the epitope to the Z-line and to the A-band as a function of sarcomere length (SL)1. The titin segment between the MG1 epitope and the Z-line (containing Ig domains) was found to continuously extend with sarcomere stretch, albeit with a decreased rate in sarcomeres longer than ∼2.8 μm. The segment between the MG1 epitope and the A-band (containing both the PEVK segment and ∼25 Ig-like domains) extended linearly as sarcomeres were stretched along a wide SL range. Gautel and Goulding (1996) and Linke et al. (1996) explained the observations with a model in which the tandem Ig and PEVK segments extend sequentially.
Recent experiments with single molecules have yielded new insights into the elastic properties of titin (Kellermayer et al., 1997; Rief et al., 1997; Tskhovrebova and Trinick, 1997; Tskhovrebova et al., 1997). The force versus extension curves of the titin molecule measured with laser tweezers revealed that at small to moderate extensions the wormlike chain (WLC) behavior of an unfolded region dominates the behavior of the entire molecule (Kellermayer et al., 1997). The unfolded region may contain the PEVK segment, because the preponderance of proline residues and charge clusters along the PEVK sequence may prevent the formation of stable tertiary structure folds (Labeit and Kolmerer, 1995), suggesting that the PEVK behaves as a denatured polypeptide. Upon stretching the single titin molecule with high enough forces and with appropriately slow rates, unfolding of globular domains occurred (Kellermayer et al., 1997). To test whether the PEVK segment behaves as unfolded protein in situ and whether unfolding of Ig domains takes place under physiological conditions, the behavior of tandem Ig and PEVK segments must be precisely followed during passive stretching of the sarcomere.
In this work sequence-specific antibodies were used to mark the boundaries of the PEVK segment, making it possible for the first time to follow its extension as sarcomeres were stretched. In addition, we also marked the tandem Ig segments and studied their extensible behavior. As the number of residues in the PEVK segment and the number of Ig-like domains of the tandem Ig segments varies in titins from different muscles we used human soleus muscle whose titin has been sequenced (Labeit and Kolmerer, 1995). This allowed us to calculate the contour length of the unfolded PEVK segment and also to convert the measured extensions of PEVK and tandem Ig segment to average extension per single amino acid and average extension per single Ig-like domain, respectively. It was found that extension of the tandem Ig segments dominated at SLs between slack (∼2.0 μm) and ∼2.7 μm, whereas PEVK extension dominated at SLs greater than ∼2.7 μm. It was possible to effectively simulate the extension of the tandem Ig and PEVK segments with the WLC model (Bustamante et al., 1994; Marko and Siggia, 1995) using the properties of the single titin molecule (Kellermayer et al., 1997). According to our analysis, throughout the physiological SL range the Ig-like domains maintain their folded structure, and the PEVK segment behaves as a permanently unfolded polypeptide. The serial linking of a permanently folded and a permanently unfolded segment in titin, due to the segments' significantly different bending rigidities, provides passive muscle with a unique mechanical stretch response.
Materials and Methods
Fiber Preparations and Mechanics
Single fibers were dissected from human soleus muscle (biopsies obtained in accordance with protocol “Role of titin in human muscle tissue 2,” Washington State University) and were both chemically and mechanically skinned. Fibers were kept continuously in relaxing solution that contained high levels of protease inhibitors (for example see Granzier and Irving, 1995), and were used within 48 h of harvest. SL was obtained by using laser diffraction. To measure the passive force–SL relation, relaxed fibers were slowly stretched (35 nm/s per sarcomere) from their slack SL to a predetermined amplitude, followed by a release back to the slack length. To measure maximal active force, fibers were activated (SL 2.5 mm) with 10−4 M free Ca2+. (For additional technical details see Granzier and Irving, 1995; Granzier et al., 1996.)
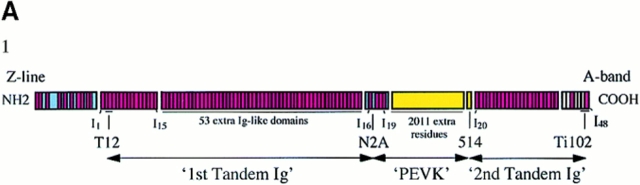
Immunoelectron Microscopy
Fibers were stretched (∼0.25 μm/sarcomere/s) and held for 10 min followed by fixation, immunolabeling, embedding, and sectioning (Trombitás et al., 1991; Granzier et al., 1996). The examined antibodies and their binding sites along the sequence of human soleus titin are listed in Fig. 1 A1. The T12 epitope location is based on Sebestyén et al. (1995). N2A is an affinity-purified polyclonal antibody (Linke et al., 1996) that was raised against the expressed titin sequence from the N2A splice pathway (base pairs 15607–15957 of the human soleus titin sequence). Antibody 514 is an affinity-purified polyclonal antibody that was raised against a synthetic peptide containing COOH-terminal PEVK amino acid residues 4596– 4606 of human cardiac titin. Ti102 is a monoclonal antibody that was raised against the genetically expressed cardiac titin fragment I47–I48 (Jin, 1995; Li et al., 1995). Z-line to epitope distances were measured from electron micrographs after high-resolution scanning (UMAX, UC-1260) and digital image processing using custom-written macros for the image analysis program NIH Image (v. 1.6, Wayne Rasband, National Institutes of Health). For spatial calibration, the electron microscope's (JEOL-1200 EX; JEOL Ltd., Tokyo, Japan) magnification was used.
Figure 1.
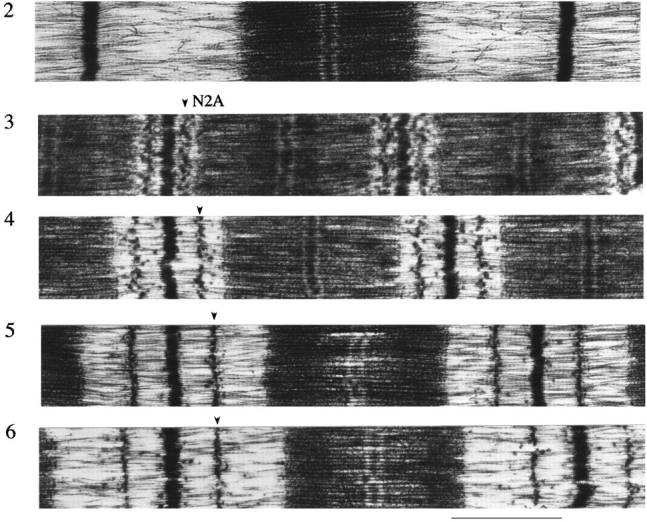
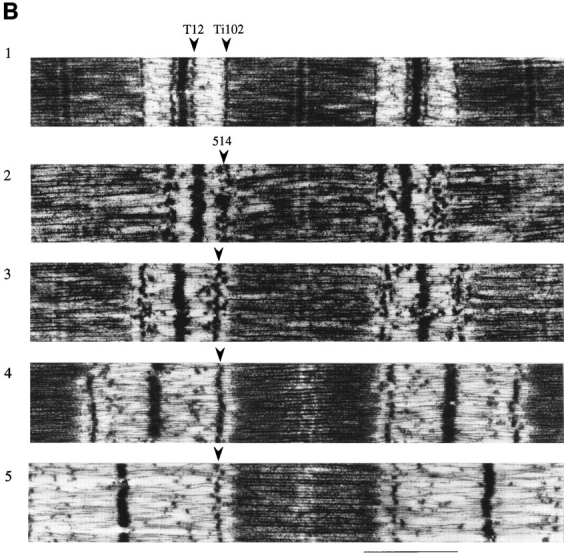
Electron micrographs of sarcomeres labeled with antititin antibodies T12, N2A, 514, and Ti102. (A1) sequence of I-band segment of human soleus muscle titin (Labeit and Kolmerer, 1995). (A2) Control. (A3–A6) Labeled with N2A. (B1) Labeled with T12 and Ti102. (B2–B5) Labeled with 514. (A1, red and white) Ig-like and fibronectin-like domains, respectively. (Blue) Unique sequence. (Yellow) PEVK segment. Domain numbering according to that of cardiac titin with extra domains and amino acid residues of human soleus titin indicated (Labeit and Kolmerer, 1995). Bar, 1.0 μm.
Calculations
A recent comparison of several entropic elasticity models (Kellermayer et al., 1997) revealed that the properties of the single titin molecule can be described best with the WLC model. In the present work we assumed that titin behaves as two WLCs in series: the tandem Ig segment and the PEVK segment. For a WLC, the external force (F) is related to the fractional extension (z/L) as:
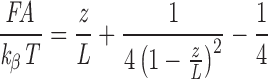 |
1 |
where A is the persistence length, k β is Boltzmann's constant, T is absolute temperature, z is the end-to-end length (extension), and L is the contour length (equal to the end-to-end length of the chain stretched with infinite force; Bustamante et al., 1994; Marko and Siggia, 1995). The contour length of the fully extended PEVK segment is ∼820 nm, calculated from its 2,174 residues (Labeit and Kolmerer, 1995) and a maximal residue spacing of ∼0.38 nm (Cantor and Schimmel, 1980). The contour length of the native tandem Ig segments was estimated by plotting the measured end-to-end length of the tandem Ig segments (Fig. 3) versus passive force−1/2 (passive force measured in single fibers) and extrapolating to infinite force (for example see Kellermayer et al., 1997). The contour lengths obtained by this method for the first and second tandem Ig segments (Fig. 1 A1) were 340 and 140 nm, respectively. Because tandem Ig and PEVK segments are in series, they bear equivalent forces, and the fractional extension (z/L) of one segment can be calculated as a function of the fractional extension of the other. From the fractional extensions of the PEVK and tandem Ig segments the corresponding SLs were calculated using 0.1 mm as the distance from the center of the Z-line to the T12 epitope and 1.6 mm as the A-band width. For additional details see Granzier et al. (1997).
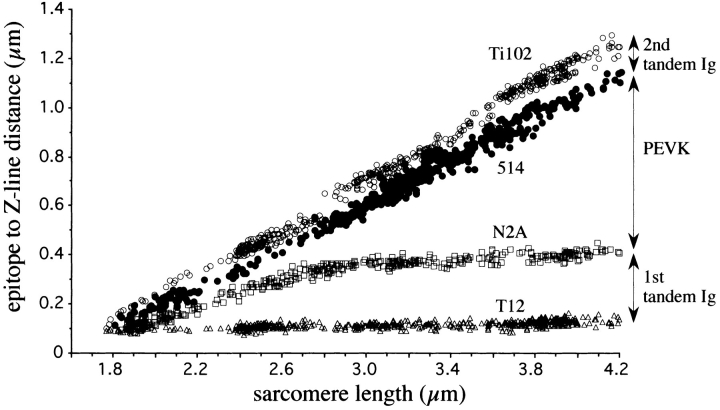
Figure 3.
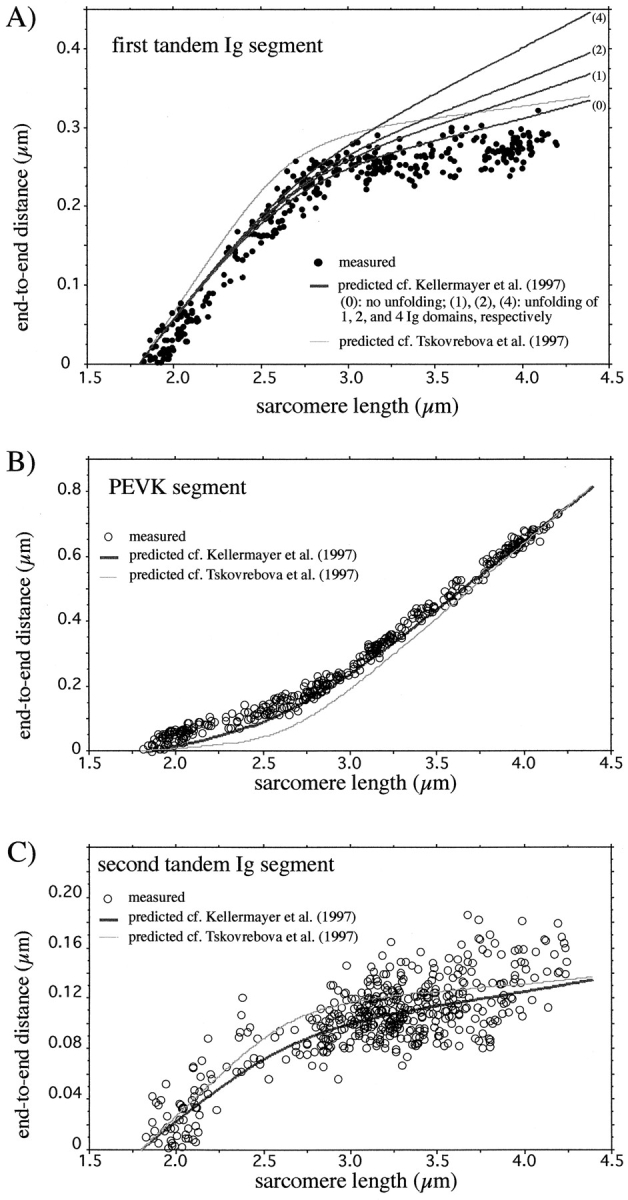
SL dependence of the end-to-end length of tandem Ig and PEVK segments. (Solid lines) Predicted extensions assuming that tandem Ig and PEVK segments behave as wormlike entropic elasticities in series. Predictions based on Kellermayer et al. (1997) assume persistence lengths of tandem Ig and PEVK segments of 15 and 2 nm, respectively. Lines indicated by 0, 1, 2, and 4 assume that 0, 1, 2, and 4 Ig domains of the first-tandem Ig segment were permanently unfolded (A, 2 nm; L, 38 nm). Predictions based on Tskhovrebova et al. (1997) assume persistence lengths of tandem Ig and PEVK segments of 4.8 and 0.15 nm, respectively.
Results
Human soleus fibers were stretched and held at constant length for 10 min and subsequently fixed, immunolabeled, and then processed for electron microscopy. Sequence-specific antititin antibodies (T12, N2A, 514, and Ti-102) were used to mark the boundaries of the tandem Ig and PEVK segments. Between the T12 and N2A epitopes lies the first tandem Ig segment, between N2A and 514 epitopes the PEVK segment, and between 514 and Ti102 epitopes the second tandem Ig segment (Fig. 1 A1). From the electron micrographs (examples are shown in Fig. 1), the epitope-to-Z-line distances and the end-to-end lengths of the tandem Ig and PEVK segments were determined as a function of SL.
In short sarcomeres, the four epitopes merged (Fig. 2), indicating that the elastic I-band segment extends from near the T12 epitope to near the Ti102 epitope. In slack sarcomeres (i.e., in sarcomeres where passive force is zero), the end-to-end length of the elastic segment was ∼130 nm (Table I). Because this length is much smaller than the ∼480-nm contour length of the native tandem Ig segments (see below), the segment between the T12 and Ti102 epitopes is not fully extended in slack sarcomeres; rather, it occupies a contracted state. As sarcomeres were stretched from their slack length of ∼2.0 μm to a length of ∼2.75 μm, titin extension was confined primarily to the tandem Ig segments. In contrast, upon further stretch, extension occurred primarily within the PEVK segment (Fig. 3). Thus, tandem Ig extension dominates between the slack SL and ∼2.75 μm, and PEVK extension dominates at longer lengths.
Figure 2.
Z-line to epitope distances as a function of SL. The T12 epitope maintains a fixed distance from the Z-line. Other epitopes are merged with T12 at a SL of ∼1.8 μm, but depart from T12 as sarcomeres are stretched.
Table I.
Structural Properties
| Slack sarcomeres | ||
| Length | 1.98 ± 0.15 μm (n = 22) | |
| T12-Ti102 distance | 130 nm* | |
| Sarcomere length range 4.1–4.2 μm | ||
| 1st Tandem Ig segment | ||
| End-to-end length | 284 ± 16 nm | |
| Interdomain spacing‡ | 4.3 ± 0.2 nm | |
| PEVK segment | ||
| End-to-end length | 730 ± 18 nm | |
| PEVK residue spacing‡ | 0.33 nm | |
| 2nd Tandem Ig segment | ||
| End-to-end length | 138 ± 16 nm | |
| Interdomain spacing‡ | 4.9 ± 0.6 nm |
Determined from linear regression analysis of data shown in Fig. 2.
Assumption: 1st tandem Ig segment contains 66 Ig-like domains; 2nd tandem Ig domain contains 28 Ig/Fn-like domains; PEVK contains 2174 residues (to account for three Ig domains 13 nm was subtracted from measured N2A-514 distance).
To determine the forces required to extend the tandem Ig and PEVK segments, we measured the passive force–SL relation of human soleus muscle fibers. The magnitude of passive force was expressed relative to that of active force established at a SL of ∼2.5 μm. Passive force at 2.75 μm SL was 4.1 ± 1.9% (mean ± SD; n = 4) of maximal active force, whereas at 3.75 μm it was 40 ± 10%. Thus, extending the tandem Ig and PEVK segments requires physiologically significant passive force levels.
The behavior of the tandem Ig and PEVK segments in response to sarcomere stretch was simulated by the WLC model of entropic elasticity (Bustamante et al., 1994; Marko and Siggia, 1995). Experiments on single molecules have indicated that the PEVK segment may behave as a permanently unfolded polypeptide, with properties strikingly different from those of the rest of the molecule. In the study by Kellermayer et al. (1997), the unfolded region was found to behave as a WLC with a persistence length (a measure of the chain's bending rigidity) of ∼2 nm. In contrast, the native structure has a persistence length of ∼15 nm (Higuchi et al., 1993). Titin was assumed to behave as two WLCs in series: the tandem Ig segment and the PEVK segment. For a WLC, the external force (F) can be calculated from its persistence length (A), and fractional extension (z/L), where (z) is the end-to-end length, and (L) is the contour length (Bustamante et al., 1994; Marko and Siggia, 1995). Because WLCs in series bear equivalent forces, the relative fractional extensions of PEVK and tandem Ig segments at a given force level can be calculated. From the fractional extensions and the contour lengths of the PEVK and tandem Ig segments, their end-to-end lengths were calculated as a function of SL (see Materials and Methods).
A comparison of the simulated and experimental results is shown in Fig. 3. The main difference was that at Sls < 2.8 μm the predicted end-to-end lengths of the PEVK segment were slightly less than measured. This may indicate that, at those Sls, the persistence length of the PEVK segment is slightly larger than the assumed 2 nm. This would result in an increase in the measured end-to-end length of the PEVK segment for a given degree of sarcomere stretch (Materials and Methods). A larger persistence length at Sls < 2.8 μm may be explained if either the entire PEVK segment or subsegments within the PEVK were to assume a favored conformation(s). An alternative explanation is that at Sls < 2.8 μm the PEVK segment interacts weakly with actin (Guttierez, G., A. van Heerden, J. Wright, and K. Wang. Biophys. J. 72:279a (Abstr.)). A possible effect of Ig domain unfolding on the predicted curves was estimated by assuming that a certain number of Ig domains (1, 2, and 4) were preunfolded, and that each behaved as a WLC with persistence length of 2 nm and a contour length of 38 nm [(residue spacing) × (number of residues): 0.38 nm × 100)]. The data were most accurately simulated by assuming that the Ig domains were fully folded (Fig. 3 A).
Tskhovrebova et al. (1997) have measured 4.8 and 0.15 nm for the persistence length of the folded regions of the titin molecule and the PEVK segment, respectively. These values are significantly smaller than those measured by Kellermayer et al. (1997) and used in the calculations above. To compare the different reported persistence lengths, we simulated the in situ titin behavior with the shorter persistence lengths measured by Tskhovrebova et al. (1997) as well. The predicted extensions were somewhat larger than measured for the tandem Ig segments and smaller than measured for the PEVK segment at SLs less than ∼3.5 μm (Fig. 3). Overall the simulation was less optimal than when using the values reported by Kellermayer et al. (1997). Considering the 0.6–220-nm persistence length range of amino-acid homopolymers (Cantor and Schimmel, 1980), the persistence length of the PEVK segment may have been underestimated by Tskhovrebova et al. (1997).
Discussion
The elastic region of human soleus titin extends in situ from near the T12 epitope to near the Ti102 epitope, and is composed mainly of tandem Ig segments and the PEVK segment (Figs. 1 and 2). When slack sarcomeres are stretched, the elastic titin segment does not extend uniformly. Instead, in sarcomeres stretched to a length of ∼2.75 mm, titin extension is confined primarily to the tandem Ig segments, whereas upon further stretch, extension occurs primarily within the PEVK segment. These findings strongly support the sequential extension model of titin elasticity (Labeit and Kolmerer, 1995; Gautel and Goulding, 1996; Linke et al., 1996). To understand how it is possible that titin's distinct segments extend differently, we simulated the segmental extension measurements obtained here with the mechanical properties of the single titin molecule (Kellermayer et al., 1997; Tskhovrebova et al., 1997). Modeling the tandem Ig and PEVK segments as serially linked WLC with persistence lengths of 15 and 2 nm, respectively, resulted in extensions strikingly close to our measurements (Fig. 3). The simulation provides an explanation for the sequential extension of the two types of segments: significant extension of the high-persistence length tandem Ig segments occurs at low force whereas extending the low-persistence length PEVK segment requires much higher forces (Eq. 1).
Modeling the tandem Ig and PEVK segments as serially linked WLCs results in the most effective simulation when Ig domain unfolding is assumed to be absent (Fig. 3 A), indicating that in situ, all of the Ig domains occupy the folded configuration. Assuming that Ig unfolding is indeed absent, the Ig interdomain spacing at the longest SL studied (4.2 mm) was calculated to be ∼4.5 nm (Table I). This distance can be accommodated by the native structure of the Ig domains along with their short polypeptide linking sequence (Improta et al., 1996). Since the in situ SL is unlikely to extend beyond 4.2 μm (Lieber et al., 1994; Ledvina and Segal, 1995), Ig unfolding may be absent along the entire physiological SL range. Stretching single molecules using scanning force microscopy or optical trapping results in the unfolding of Ig/Fn domains with a rate constant that increases with force (Kellermayer et al., 1997; Rief et al., 1997). Accordingly, a pronounced unfolding was expected to take place during the long time (∼10′) allowed by the preparative procedure. The unexpected lack of unfolding (Fig. 3 A) may result from an interaction between titin and other sarcomeric elements (Granzier et al., 1997), which could stabilize Ig domains, and allow muscle to avoid the energetically costly unfolding/refolding cycle. In conclusion, the effective simulation of tandem Ig extension that results from assuming permanently folded Ig domains, indicates that Ig unfolding is likely to be absent throughout the physiological SL range.
The average PEVK residue spacing in the longest sarcomeres studied (4.2 mm) was ∼0.33 nm (Table I). This distance can be accommodated by the PEVK segment if it is fully unfolded and extended to nearly its maximal length (Cantor and Schimmel, 1980). Because the simulation of PEVK extension (see above) assumed WLC behavior without unfolding/refolding transitions, the closeness of the measured and simulated data (Fig. 3 B) indicates that the PEVK segment behaves as an unfolded polypeptide at all SL. Avoiding unfolding/refolding transitions will minimize energy loss (hysteresis) during stretch–release cycles (Kellermayer et al. 1997). However, some hysteresis may still be present. For example, time delays in straightening of thermal fluctuations along the PEVK segment may give rise to hysteresis during stretch–release cycles with high speed (Seifert et al., 1996). In conclusion, the PEVK segment extends from a contracted state to a straightened state as sarcomeres are stretched. Like other recently discovered proteins with unfolded but biologically active domains (Daughdrill et al., 1997; Plaxco and Groß, 1997), titin may be a protein for which the unfolded state is functionally important.
The N2A antibody has recently been used to follow the NH2-terminal end of the PEVK segment (Linke et al., 1996). This antibody together with the novel antibody specific to the COOH-terminal PEVK region (antibody 514) made it possible for the first time to study how the entire PEVK segment extends as the sarcomere is stretched (Fig. 3 B). The Z-line to N2A epitope distances measured here (Fig. 2) are in general agreement with those obtained on rat soleus myofibrils using immunofluorescence (Linke et al., 1996), suggesting that soleus titins of different species are conserved. In contrast, different observations were made on rabbit psoas fibers by Gautel and Goulding (1996) using an antibody (MG1) that labels near the N2A epitope. The distance from the Z-line to the MG1 epitope increased by ∼180 nm between SLs 2.0 and 2.7 μm, and by an additional ∼100 nm between SLs 2.7 and 3.5 μm. In this study (Fig. 2), the Z-line to N2A distance remained almost constant at SLs greater than ∼2.7 μm. The conflicting results might be caused by differences in the properties of human soleus and rabbit psoas titins, or by differences in the experimental protocols used. Our work indicates an absence of Ig-unfolding in sarcomeres stretched and then held in that state for ∼10′. However, due to the kinetic nature of unfolding (Rief et al., 1997; Kellermayer et al., 1997), Ig domain unfolding may occur if sarcomeres are held in the stretched state long enough. Thus, in principle, a long preparation procedure may have caused the increase in Z-line-to-MG1 distances in sarcomeres longer than ∼2.75 μm (Gautel and Goulding, 1996).
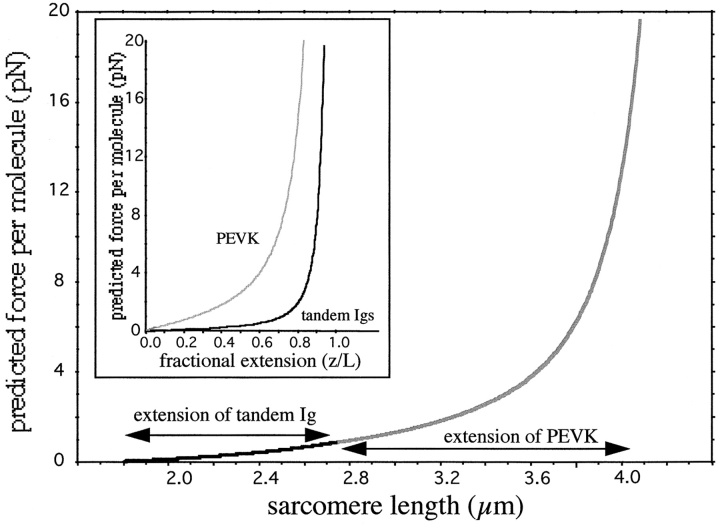
The mechanical functions of titin include passively restoring stretched or shortened sarcomeres to their slack length (Trombitás et al., 1993, 1997; Helmes et al., 1996), maintaining the central position of A-bands in contracting sarcomeres (Horowits et al., 1987) and limiting SL inhomogeneity amongst series-coupled sarcomeres (Granzier and Pollack, 1990; Granzier et al., 1991). To perform these important physiological functions, the relation between titin's force and SL can neither be too shallow (Horowits et al., 1987; Granzier and Pollack, 1990; Granzier et al., 1991), nor too steep, as this would prevent sarcomeres from operating over a wide range of lengths, which in some skeletal muscles ranges from ∼2.5 to 3.75 μm (Lieber et al., 1994). Titin meets these functional requirements by serially linking two types of segments with strikingly different elastic properties. Due to their high-persistence length, the tandem Ig segments extend at low forces (Fig. 4, inset) and are unable to provide a physiologically relevant passive force range by themselves. In combination with the PEVK segment, however, the tandem Ig segments may behave as length regulators that set the SL at which PEVK-based passive force generation begins (Fig. 4). Conversely, the low-persistence length PEVK segment requires high forces for moderate extension (Fig. 4, inset) and is unable to provide a wide SL range with physiologically relevant passive forces by itself. However, in series with the tandem Ig segments the PEVK segment may function as a passive force generator (Fig. 4) that develops intermediate forces (2–20 pN) at a wide range of SLs (2.7– 4.2 μm). In addition, the permanently unfolded state of the PEVK segment makes repeated stretch and release possible with minimal energy loss. Thus, the unfolded PEVK segment of low-persistence length in series with the folded tandem Ig segment of high-persistence length may provide an efficient way of developing physiologically useful levels of passive force over a wide SL range.
Figure 4.
Entropic force–SL relation of elastic region of titin modeled as two serially linked WLCs with different persistence length (tandem Ig and PEVK segments). Force increases modestly at lengths where tandem Ig extension predominates and steeply at lengths where PEVK extension predominates. Inset: relation between predicted entropic force and extension ratio (z/L) of PEVK segment and tandem Ig segment.
Footnotes
1. Abbreviations used in this paper: SL, sarcomere length; WLC, wormlike chain.
We are grateful to Dr. G. French for his help in obtaining muscle biopsies and to Dr. Gyöngyi Kellermayer and Mark McNabb for their excellent technical assistance.
This work was supported by grants from the National Institutes of Health (HL-47053 to M. Greaser and AR-42652 to H. Granzier) and Deutshce Forschungsgemeinschaft, La668/2-3 to S. Labeit. H. Granzier is an Established Investigator of the American Heart Association.
Address correspondence and reprint requests to Henk Granzier, Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, WA 99164-6520. Tel.: (509) 335-3390. FAX: (509) 335-4650. E-mail: granzier@wsunix.wsu.edu
Dr. Kellermayer's present address is Central Laboratory, University Medical School of Pécs, Pécs, H-7626, Hungary.
References
- Bustamante C, Marko J, Siggia E, Smith S. Entropic elasticity of λ-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- Cantor, C., and P. Schimmel. 1980. The Behavior of Biological Macromolecules. Freeman and Company, San Francisco. 849–1371.
- Daughdrill G, Chadsey M, Karlinsey J, Hughes K, Dahlquist F. The C-terminal half of anti-sigma factor, FlgM, becomes structured when bound to its target, σ. Nat Struct Biol. 1997;4:285–291. doi: 10.1038/nsb0497-285. [DOI] [PubMed] [Google Scholar]
- Fürst D, Gautel M. The anatomy of a molecular giant: how the sarcomeric cytoskeleton is assembled from molecules of the immunoglobulin superfamily. J Mol Cell Cardiol. 1995;27:951–959. doi: 10.1016/0022-2828(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Gautel M, Goulding D. A molecular map of titin/connectin elasticity reveals two different mechanisms acting in series. FEBS Lett. 1996;385:11–14. doi: 10.1016/0014-5793(96)00338-9. [DOI] [PubMed] [Google Scholar]
- Granzier H, Pollack G. The descending limb of the force-sarcomere length relation of the frog revisited. J Physiol. 1990;421:595–615. doi: 10.1113/jphysiol.1990.sp017964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Irving T. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Akster H, ter Keurs H. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol. 1991;260:C1060–C1070. doi: 10.1152/ajpcell.1991.260.5.C1060. [DOI] [PubMed] [Google Scholar]
- Granzier H, Helmes M, Trombitás K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophys J. 1996;70:430–442. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H, Kellermayer M, Helmes M, Trombitás K. Titin elasticity and mechanism of passive force development in rat cardiac myocytes probed by thin-filament extraction. Biophys J. 1997;73:2043–2053. doi: 10.1016/S0006-3495(97)78234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmes M, Trombitás K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res. 1996;79:603–611. doi: 10.1161/01.res.79.3.619. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Nakauchi Y, Maruyama K, Fujime F. Characterization of β-connectin (titin 2) from striated muscle by dynamic light scattering. Biophys J. 1993;65:1906–1915. doi: 10.1016/S0006-3495(93)81261-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R, Podolsky R. The positional stability of thick filaments in activated skeletal muscle: evidence for the role of titin filaments. J Cell Biol. 1987;105:2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improta S, Politou A, Pastore A. Immunoglobulin-like modules from titin I-band: extensible components of muscle elasticity. Structure. 1996;4:323–337. doi: 10.1016/s0969-2126(96)00036-6. [DOI] [PubMed] [Google Scholar]
- Jin J-P. Cloned rat cardiac titin class I and class II motifs: expression, purification, characterization, and interaction with F-actin. J Biol Chem. 1995;270:6908–6916. [PubMed] [Google Scholar]
- Kellermayer M, Smith S, Granzier H, Bustamante C. Folding- unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. Titins, giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B, Linke W. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res. 1997;80:290–294. doi: 10.1161/01.res.80.2.290. [DOI] [PubMed] [Google Scholar]
- Ledvina M, Segal S. Sarcomere length and capillary curvature of rat hindlimb muscles in vivo. J Appl Physiol. 1995;78:2047–2051. doi: 10.1152/jappl.1995.78.6.2047. [DOI] [PubMed] [Google Scholar]
- Li Q, Jin J-P, Granzier H. The effect of genetically expressed cardiac titin fragments on in vitro actin motility. Biophys J. 1995;69:1508–1518. doi: 10.1016/S0006-3495(95)80021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber R, Loren G, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol. 1994;71:874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- Linke W, Ivemeyer M, Olivieri M, Kolmerer B, Rüegg J, Labeit S. Towards a molecular understanding of the elasticity of titin. J Mol Biol. 1996;261:62–71. doi: 10.1006/jmbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- Marko J, Siggia E. Stretching DNA. Macromolecules. 1995;8:8759–8770. [Google Scholar]
- Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB J. 1997;11:341–345. doi: 10.1096/fasebj.11.5.9141500. [DOI] [PubMed] [Google Scholar]
- Plaxco K, Groß M. The importance of being unfolded. Nature. 1997;386:657–659. doi: 10.1038/386657a0. [DOI] [PubMed] [Google Scholar]
- Rief M, Gautel M, Oesterhelt F, Fernandez J, Gaub H. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- Sebestyén M, Wolff J, Greaser M. Characterization of a 5.4 kb cDNA fragment from the Z-line region of rabbit cardiac titin reveals phosphorylation sites for proline-directed kinases. J Cell Sci. 1995;108:3029–3037. doi: 10.1242/jcs.108.9.3029. [DOI] [PubMed] [Google Scholar]
- Seifert U, Wintz W, Nelson P. Straightening of thermal fluctuations in semiflexible polymers by applied tension. Phys Rev Lett. 1996;77:5389–5392. doi: 10.1103/PhysRevLett.77.5389. [DOI] [PubMed] [Google Scholar]
- Trinick J. Cytoskeleton: titin as scaffold and spring. Curr Biol. 1996;6:258–260. doi: 10.1016/s0960-9822(02)00472-4. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Baatsen P, Kellermayer M, Pollack G. Nature and origin of gap filaments in striated muscle. J Cell Sci. 1991;100:809–814. doi: 10.1242/jcs.100.4.809. [DOI] [PubMed] [Google Scholar]
- Trombitás G, Pollack, Wright J, Wang K. Elastic properties of titin filaments demonstrated using a “freeze-break” technique. Cell Motil Cytoskel. 1993;24:274–283. doi: 10.1002/cm.970240408. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Jin J-P, Granzier H. The mechanically active domain of titin in cardiac muscle. Circ Res. 1995;77:856–861. doi: 10.1161/01.res.77.4.856. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Greaser M, Pollack G. Interaction between titin and thin filaments in intact cardiac muscle. J Muscle Res Cell Motil. 1997;18:345–351. doi: 10.1023/a:1018626210300. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Direct visualization of extensibility in isolated titin molecules. J Mol Biol. 1997;265:100–106. doi: 10.1006/jmbi.1996.0717. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J, Sleep J, Simmons R. Elasticity and unfolding of single molecules of the giant muscle protein of titin. Nature. 1997;387:308–312. doi: 10.1038/387308a0. [DOI] [PubMed] [Google Scholar]
- Wang K. Titin/connectin and nebulin: giant protein rulers of muscle structure and function. Adv Biophys. 1996;33:123–132. [PubMed] [Google Scholar]