Abstract
Abstract. Vascular endothelial growth factor (VEGF) is an important regulator of vasculogenesis, angiogenesis, and vascular permeability. In contrast to its transient expression during the formation of new blood vessels, VEGF and its receptors are continuously and highly expressed in some adult tissues, such as the kidney glomerulus and choroid plexus. This suggests that VEGF produced by the epithelial cells of these tissues might be involved in the induction or maintenance of fenestrations in adjacent endothelial cells expressing the VEGF receptors. Here we describe a defined in vitro culture system where fenestrae formation was induced in adrenal cortex capillary endothelial cells by VEGF, but not by fibroblast growth factor. A strong induction of endothelial fenestrations was observed in cocultures of endothelial cells with choroid plexus epithelial cells, or mammary epithelial cells stably transfected with cDNAs for VEGF 120 or 164, but not with untransfected cells. These results demonstrate that, in these cocultures, VEGF is sufficient to induce fenestrations in vitro. Identical results were achieved when the epithelial cells were replaced by an epithelial-derived basal lamina-type extracellular matrix, but not with collagen alone. In this defined system, VEGF-mediated induction of fenestrae was always accompanied by an increase in the number of fused diaphragmed caveolae-like vesicles. Caveolae, but not fenestrae, were labeled with a caveolin-1–specific antibody both in vivo and in vitro. VEGF stimulation led to VEGF receptor tyrosine phosphorylation, but no change in the distribution, phosphorylation, or protein level of caveolin-1 was observed. We conclude that VEGF in the presence of a basal lamina-type extracellular matrix specifically induces fenestrations in endothelial cells. This defined in vitro system will allow further study of the signaling mechanisms involved in fenestrae formation, modification of caveolae, and vascular permeability.
Fenestrae are specialized plasma membrane microdomains in endothelial cells that are involved in vascular permeability. They appear as circular discontinuities of ∼60 nm in diameter and usually occur in clusters in the most attenuated regions of the endothelial cell (for review see Palade et al., 1979). Fenestrated endothelia are found in endocrine organs, the gastrointestinal tract, kidney glomeruli, and specific regions of the brain, such as choroid plexus, and circumventricular organs, as well as in many tumors (Rhodin, 1974; Coomber et al., 1987, 1988; Shibata, 1989; Roberts and Palade, 1997). In contrast, endothelial cells found within connective tissues, muscle, skin, lung, and brain generally do not possess fenestrations. The molecular basis for this phenotypic diversity of endothelial cells is not known (Risau, 1995).
Previous in vitro studies aimed to identify factors involved in fenestrae formation reported that extracellular signals (e.g., TGFβ, retinoic acid, serotonin), the tumor promotor phorbol myristate acetate (PMA)1, the actin-disrupting agent cytochalasin B, and calcium ions, as well as extracellular matrix components, have some effect on fenestrae formation (Milici et al., 1985; Lombardi et al., 1986, 1988; Steffan et al., 1987; Carley et al., 1988; McGuire et al., 1992; Gatmaitan et al., 1996). However, the relevance of these factors in fenestrae induction under physiological conditions in vivo is not known. In addition, no molecular markers are available to detect the fenestrated phenotype in rapid screening assays.
Vascular endothelial growth factor (VEGF), which exists in several alternative splice variants (Tischer et al., 1991; Breier et al., 1992; Poltorak et al., 1997), is not only an endothelial growth factor but was initially characterized as a vascular permeability factor secreted by tumor cells (Senger et al., 1983; Keck et al., 1989). We have previously shown that VEGF is continuously expressed in epithelial cells of adult organs with fenestrated endothelium, such as choroid plexus and kidney glomeruli (Breier et al., 1992). Also, the persistent expression of mRNA for the corresponding VEGF receptors-1 and -2 in endothelial cells of these organs has been described (Millauer et al., 1993; Breier et al., 1995; Simon et al., 1995). Since VEGF is highly expressed during embryonic vasculogenesis and angiogenesis, but is downregulated in most adult tissues when endothelial proliferation has ceased, we speculated that the continuous expression of VEGF might be involved in the induction or maintenance of endothelial fenestrations (Breier et al., 1992). Indeed, it has recently been shown that VEGF application in vivo directly and rapidly induced fenestrae in the continuous endothelium of skeletal muscle and skin (Roberts and Palade, 1995) and in the neovasculature of VEGF-secreting tumors (Roberts and Palade, 1997). Although these studies provide compelling evidence that VEGF is involved in fenestrae formation in vivo, in vitro model systems are required to characterize the molecular mechanisms by which VEGF induces fenestrae.
The exact mechanisms (e.g., transcellular versus paracellular) and structures (e.g., plasmalemmal vesicles versus cell–cell junctions) responsible for increased vascular permeability are still controversial (Jain, 1988; Dvorak et al., 1995; Roberts and Palade, 1997). Compelling evidence exists that caveolae are involved in transcytotic transport of solutes across the endothelium (Ghitescu et al., 1986; Predescu et al., 1994, Schnitzer et al., 1994). Caveolae (also termed plasmalemmal vesicles) are small invaginations at the cell surface that measure 50–100 nm in diameter and often surround fenestrae (Palade et al., 1979). It is known that fenestrae are highly permeable for water and small solutes (Levick and Smaje, 1987). Electron microscopic studies have shown that most fenestrae are spanned by a nonmembraneous diaphragm that is also found in caveolae and in fused clustered vesicles, also referred to as vesiculo-vacuolar organelles (VVOs; Dvorak et al., 1996). However, the exact structural and functional relationship between these vesicular structures remains unclear.
Caveolin is an integral membrane component of caveolae that is now generally accepted as a marker protein for this organelle. Caveolin (now termed caveolin-1) belongs to a multigene family of at least three different members: caveolin-1, -2, and -3 (Kurzchalia et al., 1992; Way and Parton, 1995; Scherer et al., 1996; Song et al., 1996; Tang et al., 1996). Two alternate start sites in the gene of caveolin-1 give rise to α and β isoforms (Scherer et al., 1995). The structurally homologous caveolin proteins show overlapping but distinct tissue distribution. Although caveolin-1 was found to be a critical component of the caveolar coat (Rothberg et al., 1992), its function is still unknown. Caveolae have been implicated in signal transduction (Anderson, 1993; Sargiacomo et al., 1993; Lisanti et al., 1994), transcytosis (Ghitescu et al., 1986; Predescu et al., 1994, Schnitzer et al., 1994), cholesterol transport (Murata et al., 1995; Smart et al., 1996), regulation of tissue factor (Mulder et al., 1996), and endothelial nitric oxide synthase function (Garcia-Cardena et al., 1996; Shaul et al., 1996); (for reviews see also Kurzchalia and Parton, 1996; Parton, 1996; Couet et al., 1997b ). Despite the similarity between caveolae, VVOs, and fenestrae, no information has been available on the distribution of caveolin-1 in the latter two structures.
Here we report the establishment of a novel in vitro system for studying the conditions under which endothelial cells develop fenestrations in response to VEGF. Progressively better defined conditions show that VEGF induces a robust and consistent increase in the number of fenestrations and fused diaphragmed vesicles. This increase was accompanied by a decrease in labeling of fused vesicles and fenestrae for caveolin-1, a marker protein of plasmalemmal vesicles or caveolae. VEGF stimulation initiated receptor tyrosine phosphorylation and signal transduction in endothelial cells. However, no change in phosphorylation or protein levels of caveolin-1 was observed. Our data demonstrate that, under defined in vitro conditions, such as the presence of a basal lamina-type matrix, VEGF is sufficient to induce fenestrations in vitro.
Materials and Methods
Materials
Laboratory reagents were purchased from Sigma Chemical Co. (Deisenhofen, Germany) unless otherwise stated. Anti–caveolin-1 antibodies were described previously (Dupree et al., 1993) or were purchased from Santa Cruz Biotechnology, Inc. (C-20; Heidelberg, Germany) or Transduction Laboratories, (C13630; Exeter, UK). For staining of endothelial cells, a rabbit polyclonal antibody against von Willebrand factor (Dako Corp., Hamburg, Germany) or monoclonal antibodies against platelet endothelial cell adhesion molecule-1 (Mec 13.3; Vecchi et al., 1994) and endoglin (MJ 7/18; Ge et al., 1994) were used. Antiphosphoserine and anticytokeratin antibodies were purchased from Sigma Chemical Co. A rabbit polyclonal serum and a rat monoclonal antibody against flk-1 were gifts from H. App (SUGEN, Inc., Redwood City, CA), and S.-I. Nishikawa and H. Kataoke (Kyoto University, Kyoto, Japan), respectively. Antibodies against phosphotyrosine were obtained from Transduction Laboratories and the anti-VEGF antibody was prepared as previously described (Breier et al., 1992). As a control for equal protein loading on gels, a monoclonal anti-d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody from Biogenesis (Poole, UK) was used. Peroxidase-conjugated secondary antibodies (reactive with either rabbit or mouse IgG) were from Pierce Chemical Co. (Rockford, IL) or Amersham Buchler GmbH (Braunschweig, Germany). For immunolabeling in light and electron microscopy, FITC, rhodamine, Cy-3, or gold-conjugated antibodies against rabbit, mouse, or rat IgG were obtained from The Jackson ImmunoResearch Laboratories (West Grove, PA), Amersham Buchler GmbH, or Aurion (BioTrend, Cologne, Germany). As negative controls for primary antibodies, unconjugated rabbit, rat, or mouse IgG from Sigma Chemical Co. and Vector Labs Inc. (Burlingame, CA) were included.
Cell Culture
Media and other cell culture reagents were obtained from Life Technologies, Inc. (Eggenstein, Germany). Cloned bovine adrenal cortex endothelial cells (ACE), a gift of J. Folkman (Harvard Medical School, Boston, MA); Folkman and Haudenschild, 1980) were used at passages 15–20. Stock cultures were grown on collagen-coated dishes (collagen G; Seromed, Berlin, Germany) in low glucose DME containing 10% donor calf serum (Sigma Chemical Co.). Choroid plexus epithelial cells were isolated from mice of embryonic day 12.5 (Stadler et al., 1994), cultured on laminin (Upstate Biotechnology Inc., Lake Placid, NY)-coated dishes, and then routinely checked for expression of cytokeratin by immunofluorescence microscopy. The mouse mammary epithelial cell lines overexpressing VEGF 120 or 164 were generated by calcium-phosphate transfection of EpH4 cells (Oft et al., 1996) with cytomegalovirus-based expression vectors (pRC/CMV; Invitrogen BV, Leek, The Netherlands) that contained the respective mouse VEGF cDNAs (Breier et al., 1992). Cell clones resistant to G418 (Life Technologies, Inc.) were selected and tested for secretion of the respective VEGF protein into the medium by immunoblot analysis as published (Breier et al., 1992). Preparation of extracellular matrix–coated dishes was performed essentially as described (Gospodarowicz, 1984). Cocultures were generated by simultaneously seeding epithelial cells and twice the number of endothelial cells on tissue culture plates that were either coated for 16 h at 4°C with laminin (cocultures with choroid plexus epithelial cells) or for 16 h at 37°C with collagen (cocultures with EpH4 cells). The cells were grown for ∼4–7 d in DME supplemented with 10% FCS (PAN Systems GmbH, Nuernberg, Germany) until the cultures had reached confluency. Human umbilical vein endothelial cells (HUVEC) were prepared by the method of Jaffe et al. (1973) as modified by Jarrell et al. (1984) and then cultured as described in detail elsewhere (Lampugnani et al., 1992). Human or murine recombinant VEGF was provided by H. Weich (Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany), and human basic FGF was obtained from Boehringer Mannheim (Mannheim, Germany).
Electron Microscopy
Conventional Microscopy.
Confluent cell monolayers were washed with Hanks modified salt solution (HMSS), pH 7.4, and then fixed for 4 h in 2% glutaraldehyde (Polysciences Inc., Warrington, PA) in HMSS, pH 7.4, 4°C, followed by two washes in 0.2 M cacodylate buffer for 10 min each and then postfixed for 1 h in 1% OsO4. After washing, the cells were scraped from the dishes, centrifuged in buffer for 5 min, and then dehydrated by a graded ethanol series. En bloc staining was performed in 6% uranyl acetate/70% ethanol for ⩾1 h or overnight in the dark. Samples were embedded in Araldite (Serva, Heidelberg, Germany), sectioned with an ultramicrotome (model Ultracut R; Leica, Inc., St. Gallen, Switzerland) for electron microscopy, stained with lead citrate, and then examined in an electron microscope (model EM10; Carl Zeiss, Inc., Thornwood, NY). For morphometric analysis, thin sections were cut for each specimen from three levels at a distance of 30 μm. In every section, all profiles of ACE cells, fenestrations, and fused diaphragmed vesicles were counted. Clusters of diaphragmed vesicles, clusters of fenestrae, and isolated fenestrae were counted as single entities.
Immunoelectron Microscopy.
Cell cultures and mouse tissues were washed in HMSS buffer and fixed in the same buffer supplemented with 2% paraformaldehyde, 0.05% glutaraldehyde, pH 7.4, for 2 h at 4°C. After washing in PBS, the pellets obtained by centrifugation were enclosed in 2% agar, embedded in Lowicryl K4M resin (Polysciences, Inc., Eppelheim, Germany), and then polymerized at −35°C under UV light. Ultrathin sections were cut and mounted on nickel or copper grids and then immunolabeled. The sections were blocked for 30 min at room temperature with TBS containing 5% BSA, 0.1% cold-water fish skin gelatin, 5% normal serum, and 0.1% Tween-20 (Serva), and then incubated with polyclonal anti–caveolin-1 antibody (C13630; Transduction Laboratories), diluted 1:100 or 1:50 in incubation buffer: 0.8% BSA, 0.1% cold-water fish skin gelatin, 20 mM NaN3, 0.1% Tween-20 in TBS. As negative controls, the primary antibody was omitted or nonimmune rabbit IgG at the same concentration as the primary antibody were used. After washing six times in incubation buffer, incubation with secondary antibody (goat anti–rabbit conjugated with 6- or 15-nm gold particles, diluted 1:40 in incubation buffer) was performed. The sections were subsequently washed and postfixed in 2% buffered glutaraldehyde for 5 min. Finally, the grids were washed in distilled water, air-dried, and then poststained with 6% aqueous uranyl acetate and lead citrate for 2 min.
Indirect Immunofluorescence
Cryosections of mouse embryos or adult mouse organs were fixed for 10 min in ice-cold methanol, air-dried, and then blocked for 15 min in normal goat serum. The sections were incubated for 1 h with the respective primary antibodies, washed three times with PBS, and then incubated for another hour with the appropriate fluorophore-conjugated secondary antibody. After a final wash, the sections were mounted in Mowiol 4-88 (Calbiochem, Bad Soden, Germany). Cells cultured on tissue culture dishes, glass coverslips, or chamberslides (Nunc, Naperville, IL) were fixed for 10 min with ice-cold methanol (for cytokeratin- and endothelial-specific staining) or for 20 min with 3% paraformaldehyde (for caveolin-1 staining), followed by incubation with 50 mM NH4Cl for 10 min and permeabilization with 0.2% Triton X-100 for 10 min. The cells were then rinsed, blocked with normal goat serum, and immunostained as described above. As negative controls, unconjugated rabbit, rat, or mouse immunoglobulins were used at the same concentration as the respective primary antibodies. Fluorescence was examined with a Zeiss Axiophot or Axiovert microscope (model 135; Carl Zeiss, Inc.).
Immunoprecipitation
ACE cells were stimulated with VEGF, pervanadate, or PMA at 37°C as indicated, pretreated for 1 h with 1 mM vanadate before lysis, and then washed in PBS containing 1 mM vanadate. The cells were solubilized on ice for 20 min either in Triton buffer (150 mM NaCl, 10 mM Tris, pH 7.5, 1% Triton X-100) or in SDS lysis buffer (Dupree et al., 1993) supplemented with a cocktail of phosphatase and proteinase inhibitors (1 mM vanadate, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF). For serine-phosphorylation studies, buffers additionally contained 400 nM okadaic acid (Calbiochem) and 50 mM NaF. The cells were scraped from the culture dishes and then the lysates were centrifuged at 13,000 rpm for 10 min at 4°C to pellet cellular debris. The supernatant volume corresponding to the same number of cells was either subjected to immunoprecipitation or aliquots were removed and boiled for 5 min after addition of 4× sample buffer (Laemmli, 1970). Immunoprecipitations were performed with antibodies either preabsorbed to protein A– or G–Sepharose beads (Pharmacia, Freiburg, Germany) or the Sepharose was added to preformed antibody–antigen complexes. The Sepharose-bound immune complexes were washed four times with lysis buffer (containing protease and phosphatase inhibitors), once with the same buffers without detergent, and then boiled in reducing sample buffer.
Immunoblotting
Conditioned media from control and transfected epithelial cells were concentrated using MICROSEP™ microconcentrators (Filtron, Karlstein, Germany) and then mixed with sample buffer. Proteins of media, total cellular extracts, and immunoprecipitates were separated by SDS-PAGE in 7, 12.5, or 15% gels, transferred onto 0.2-μm pore size nitrocellulose membranes (Schleicher & Schuell, Inc., Dassel, Germany), blocked with 3% BSA in PBST (0.1% Tween-20 in PBS), and then incubated with primary and peroxidase-conjugated secondary antibodies with six washes of PBST after each incubation. Protein–antibody complexes were visualized using the enhanced chemiluminescence Western blot detection system (Amersham Buchler GmbH).
RNA Extraction and Reverse Transcription (RT)-PCR Analysis
Choroid plexus was dissected from bovine brain and then frozen at −80°C. Glomeruli isolated from mouse kidneys (Kurtz et al., 1982) were stored at −80°C until used. Total cytoplasmic RNA was isolated from these tissues using the guanidinium thiocyanate method (Chomczynski and Sacchi, 1987) and RT was performed essentially as described (Wizigmann-Voos et al., 1995) using the following synthetic oligonucleotide primers for amplification: (5′-oligonucleotide) 5′-d(GTGCTCTCTTGGGTGCACTGG), and (3′-oligonucleotide) 5′-d(CACCGCCTTGGCTTGTCACAT). As a positive control, cDNAs encoding VEGF 120, 164, and 188 (Breier et al., 1992) were used as templates for amplification; the cDNA template was omitted from the reaction as negative control. The amplification products were separated on 1.5% agarose gels and visualized by ethidium bromide staining.
Results
Expression of VEGF Isoforms in Organs with Fenestrated Endothelium
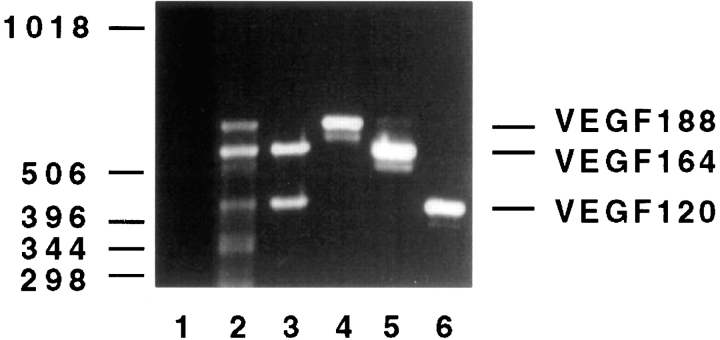
Since different isoforms of VEGF exist, it was conceivable that one particular isoform might possess the potential biological activity for induction of fenestrae. Therefore, we analyzed the expression pattern of VEGF isoforms in different adult organs using the RT-PCR technique. RNA from mouse heart, lung, kidney glomeruli, and bovine choroid plexus was extracted, reverse transcribed, and then amplified using different VEGF primer combinations. VEGF isoforms 164 and 120 were predominantly expressed in choroid plexus (Fig. 1, lane 3) and glomerulus (data not shown). In lung (Fig. 1, lane 2) and heart (data not shown), isoform 188 was also detectable. It appears, therefore, that the presence of VEGF in organs with fenestrated endothelium does not correlate with the predominant expression of a specific VEGF isoform, but may be related to a persistent VEGF expression in general.
Figure 1.
Expression of VEGF isoforms in bovine choroid plexus. RT-PCR analysis with VEGF-specific primers of RNA extracted from bovine or mouse tissues demonstrates predominant expression of mRNAs for VEGF isoforms 120 and 164 in choroid plexus (lane 3), whereas the 188 isoform was additionally expressed in lung (lane 2). The VEGF amplification products of 432-, 564-, and 636-bp correspond to VEGF 120, VEGF 164, and VEGF 188, respectively. As a positive control, full-length cDNAs of the three VEGF isoforms were amplified (lanes 4–6). The negative control without DNA template is shown in lane 1. The identity of the PCR products was confirmed by Southern blot analysis with a VEGF-specific probe (data not shown).
Continuous Expression of VEGF and VEGF Receptors in Organs with Fenestrated Endothelium
We have previously shown by in situ hybridization studies of embryonic and adult mouse tissues that the expression of VEGF and its receptors is downregulated in most adult tissues (Breier et al., 1992; Millauer et al., 1993). In contrast, VEGF expression was found to be maintained at a high level in adult organs with fenestrated endothelium (Breier et al., 1992; Brown et al., 1992; Jakeman et al., 1992; Monacci et al., 1993; Simon et al., 1995). For example, the mRNA for VEGF and its receptors is highly expressed in the endothelial cells of embryonic and adult choroid plexus as well as kidney glomeruli. To study the requirements for endothelial fenestrations in vitro, we have examined the choroid plexus in more detail by immunofluorescence microscopy. We found that VEGF receptor-2 (flk-1) was expressed in endothelial cells of embryonic and adult choroid plexus (data not shown) also at the protein level. In addition, the endothelial, but not epithelial, cells of choroid plexus were recognized by antibodies against caveolin-1 (data not shown). This finding is notable because high expression of caveolin-1 protein was reported for endothelial as well as epithelial cells (Kurzchalia et al., 1992; Lisanti et al., 1994). Taken together, these observations suggested a paracrine role for VEGF expressed by epithelial cells in the induction or maintenance of fenestrations in the adjacent endothelial cells in these tissues.
Cocultures of Endothelial Cells with Choroid Plexus Epithelial Cells
We used an in vitro coculture system in which freshly isolated murine choroid plexus epithelial cells (CPE) were plated together with bovine ACE. These primary endothelial cells, which express the VEGF receptors-1 and -2 (Plouet and Moukadiri, 1990; Vaisman et al., 1990), are derived from capillaries that are fenestrated in vivo, but lose fenestrae in culture (Folkman and Haudenschild, 1980; Furie et al., 1984). Cocultures formed confluent monolayers in which each cell type tended to grow in isolated clusters, and these were in direct contact with each other at their border areas (Fig. 2, a and b). Cultures of each cell type and cocultures were stained with antibodies against cytokeratin and von Willebrand factor to label epithelial and endothelial cells, respectively. Cultures of primary CPE cells consisted of ∼70% cytokeratin-positive epithelial cells. No endothelial cells could be detected with antibodies against the endothelial-specific antigens von Willebrand factor, platelet endothelial cell adhesion molecule-1, and endoglin (data not shown). Electron microscopic analysis of ACE cells revealed intact cell–cell junctions and basal–apical polarity (Fig. 2 c). The spindle-like appearance of these cells also allowed their identification in coculture. Intercellular contact zones were visible as areas where either extremely thin processes joined each other or extensions of larger cytoplasmic areas overlapped. In orthograde sections through the middle of the cell (indicated by the nucleus), relatively few caveolae and vesicle clusters were attached to the membrane and no difference in their distribution between the apical and basal surface was observed. However, in the processes distant from the perikaryon, the density of caveolae was increased (Fig. 2 d). Many caveolae were observed to be covered by a structure reminiscent of a diaphragm. However, fenestrations themselves were rarely observed in ACE cells cultured alone. The most striking difference between ACE cells cultured alone and in cocultures was the appearance of fenestrations and an increase in the number of fused vesicles with diaphragms in the endothelial cells (Fig. 2 e). The fenestrations, which were predominantly found in extremely attenuated areas of the endothelial cell, displayed the typical appearance of fenestrae in vivo. They had a diameter of ∼60 nm and were bridged by a thin diaphragm, which sometimes contained a central knob (see also Fig. 4). We conclude that CPE cells are able to induce endothelial fenestrations in vitro. Since CPE cells produce VEGF in vivo, we asked whether VEGF itself, in an epithelial context, induces fenestrae.
Figure 2.
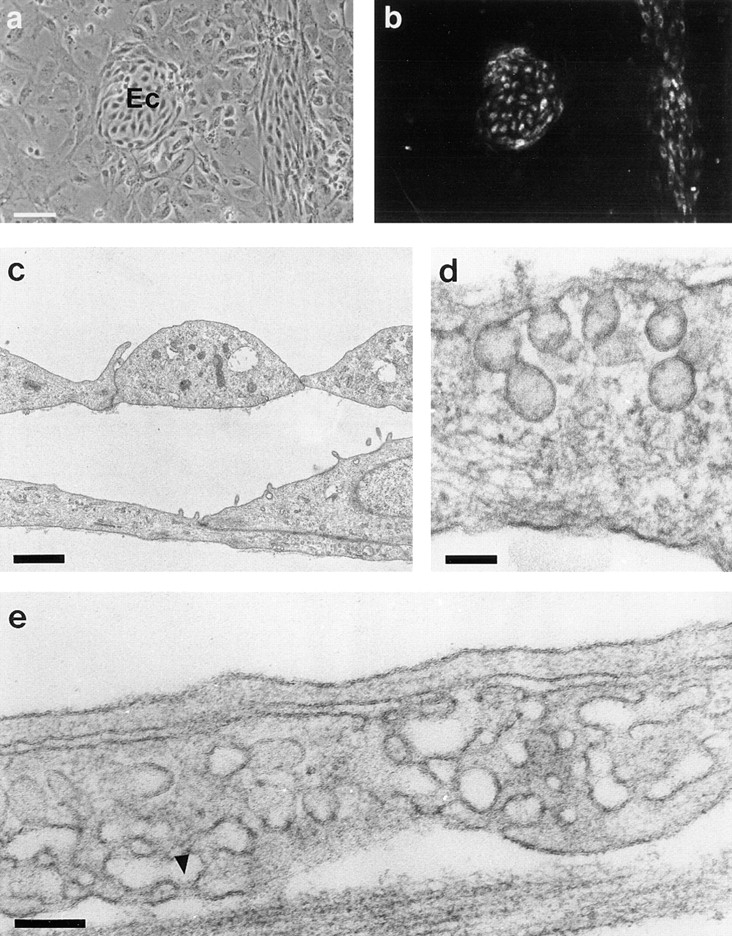
Coculture of ACE cells with isolated choroid plexus epithelial cells. (a) Light micrograph illustrating the difference in morphology between epithelial and endothelial cells (Ec). (b) Immunofluorescence staining with an antibody against von Willebrand factor of the same microscopic area shown in a. Islands of labeled endothelial cells within the unlabeled epithelial cell monolayer can be seen. (c and d) Transmission electron micrographs of ACE cells showing cell contacts and apical–basal morphology (c) and caveolar invaginations at the plasma membrane (d). (e) ACE cell in coculture with choroid plexus epithelial cells showing fused clustered vesicles with a diaphragm (arrowhead). Bars: (a) 100 μm; (c) 1 μm; (d and e) 0.1 μm.
Figure 4.

Electron micrograph of ACE cells cultured on basal-lamina type extracellular matrix after a 24-h treatment with VEGF. (a) ACE cells form long thin cellular processes that are interrupted by numerous fenestrations (arrowheads). (b) The endothelial cell is still connected to the underlying extracellular matrix (M). Fenestrations (arrowheads) are bridged by thin diaphragms, which sometimes contain a central knob (arrows). (c) ACE cell with a cluster of fused vesicles, which are separated only by thin diaphragms with a central knob (arrows). Bars: (a and b) 0.2 μm; (c) 0.1 μm.
Cocultures of Endothelial Cells with Stably Transfected Epithelial Cell Lines Overexpressing VEGF
To determine the direct effects of VEGF produced by epithelial cells on ACE cells, we used a mouse epithelial cell line (EpH4; Oft et al., 1996) that was stably transfected with either VEGF 164 or 120, as these were the two main isoforms found to be expressed in organs with fenestrated endothelium (refer to Fig. 1). Untransfected and VEGF-transfected epithelial cells displayed the typical cobblestone appearance of squamous epithelia with extensive tight junctions (data not shown). Immunoblot analysis of conditioned EpH4 media revealed that untransfected EpH4 cells exhibited only a very low basal expression of VEGF, whereas the transfected cell lines secreted high levels of the respective VEGF isoforms (Fig. 3 a). VEGF 120–transfected cells produced a higher amount of VEGF protein than VEGF 164–transfected cells (Fig. 3 a). The morphological appearance of the coculture was similar to the CPE/ACE cocultures, showing separate islands of tightly clustered cobblestone-shaped epithelial cells and spindle-shaped endothelial cells (Fig. 3, b and c). Growth of both epithelial and endothelial cells appeared to be accelerated in cocultures. Overexpression of VEGF by the transfected epithelial cells had a morphological effect on neighboring ACE cells, which was already visible at the light microscopic level. As illustrated in Fig. 3 c, ACE cells in cocultures with the transfected epithelial cells had a more elongated phenotype and developed small branches and sprouts, which were often concentrated in areas of endothelial cell islands close to epithelial cells. These were not observed in cocultures with untransfected cells. Electron microscopy of cocultures with transfected cells revealed an induction of fenestrae and a strong increase in the number of fused diaphragmed vesicles (Fig. 3, d–f). Frequently, fenestrae were found in thin processes of endothelial cells, thus resembling their appearance in vivo (Fig. 3 d). In addition, extremely thin cellular extensions enclosing an extracellular space or cellular process also exhibited numerous fenestrations (Fig. 3 f).
Figure 3.
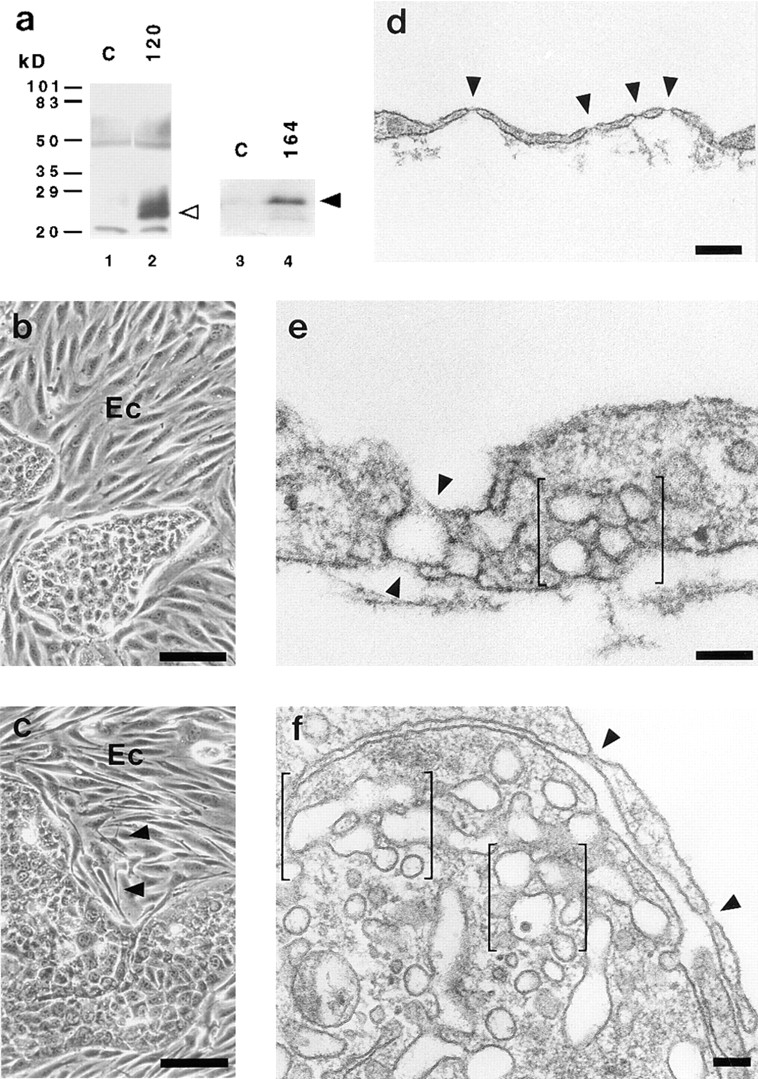
Cocultures of ACE cells with epithelial cells transfected with VEGF. (a) Transfected epithelial cells secrete VEGF into the medium. Conditioned media of normal and transfected epithelial cells were subjected to immunoblot analysis using anti-VEGF antibodies recognizing all known mouse VEGF isoforms. EpH4 cells stably transfected with cDNAs for VEGF 120 and 164 (lanes 2 and 4) secrete high levels of VEGF 120 (open arrowhead) and 164 (closed arrowhead) into the medium when compared to untransfected control cells (C; lanes 1 and 3). The broad band around 60 kD corresponds to the mol wt of BSA. (b and c) Light micrographs of cocultures of ACE cells with EpH4 cells, which were either untransfected (b) or transfected with the cDNA for VEGF 120 (c). The endothelial cells (Ec) appear to be more elongated and have developed small branches and sprouts (arrowheads) in cocultures with transfected EpH4 cells. (d–f) Electron micrographs showing sections through ACE cells cocultivated with VEGF 120–transfected epithelial cells. (d) Fenestrae (arrowheads) are arranged similarly to the in vivo situation in extremely attenuated areas of the endothelial cell. (e) Vertical section through a cellular process. Note fused clustered vesicles (bracket) combined with a dilated transendothelial channel (arrowheads) surrounding a larger vacuole. (f) Clusters of diaphragmed vesicles (brackets) and fenestrae (arrowheads) in ACE cells are shown. Bars: (b and c) 100 μm; (d) 0.25 μm; (e and f) 0.1 μm.
To quantify the effect of VEGF on ACE cells, a morphometric analysis was performed by counting the number of fenestrations and fused clustered vesicles with diaphragms and then expressing them in relation to the number of cellular profiles. Table I summarizes the quantitative effect of VEGF in cocultures of epithelial and endothelial cells. VEGF120-overexpressing EpH4 cells induced a seven- or eightfold increase in the number of fused clustered vesicles and fenestrae in ACE cells. The induction was less pronounced in cocultures with the VEGF 164–transfected cells, which may be attributed to the lower amount of VEGF secreted by this cell line. There was only a minor effect on fenestrae induction in cocultures with untransfected EpH4 cells compared to monocultures of ACE cells, which may be explained by the low basal expression of VEGF in control EpH4 cells. Taken together, these data demonstrate that VEGF induces an increase in the number of endothelial fenestrations and fused diaphragmed vesicles in vitro. In experiments where cocultures of transfected EpH4 cells with HUVEC instead of ACE cells were used, no effect was observed (data not shown).
Table I.
Fenestrae Induction in Cocultures
| Culture | [Fenestrae + clustered vesicles]/cell (mean) | SD | ||
|---|---|---|---|---|
| ACE | 0.0189 | 0.0033 | ||
| ACE + EPH4 | 0.0229 | 0.0042 | ||
| ACE + EPH4/VEGF 164 | 0.1231 | 0.0500 | ||
| ACE + EPH4/VEGF 120 | 0.1444 | 0.0632 |
Endothelial fenestrations and clustered diaphragmed vesicles in ACE cells in coculture with mouse mammary epithelial cells. Diaphragmed fenestrations and clustered vesicles were counted in ∼330 (ACE), 400 (ACE + EpH4), 390 (ACE + EpH4/ VEGF164), and 540 cells (ACE + EpH4/VEGF120) using sections from two independent experiments. Results were obtained from three section levels cut at a distance of 30 μm as described in Materials and Methods. The ratios of diaphragmed clustered vesicles and fenestrae number per cell profile were then determined. Student's t test was applied for pairwise comparison of the data and showed P < 0.001 for ACE versus ACE + EpH4/VEGF164; P < 0.005 for ACE versus ACE + EpH4/VEGF120; and P < 0.05 for ACE + EpH4 versus ACE + EpH4/VEGF120.
Effect of Purified VEGF on Endothelial Cells Grown on Complex Extracellular Matrix
To test the possibility that additional factors produced by the epithelial cells synergize with VEGF in fenestrae induction, we further reduced the complexity of our in vitro system and tested the effect of purified VEGF on endothelial cells. Since the importance of a specific extracellular environment for fenestrae induction had been demonstrated (Milici et al., 1985; Carley et al., 1988; McGuire et al., 1992), we tested the contribution of different extracellular matrices to the effect of VEGF. We cultivated the endothelial cells either on rat tail collagen, which is a mixture of type I and III collagen, or on a basal lamina-type extracellular matrix produced by corneal endothelial cells. We then incubated the cells for various time periods with recombinant VEGF 165 using concentrations (50–100 ng/ml) known to maximally induce autophosphorylation of VEGF receptors (Waltenberger et al., 1994). Similar to the cocultures, purified VEGF induced an elongated endothelial phenotype and the appearance of cell sprouts, which were first visible after 6 h of incubation and were much more pronounced after a 24-h treatment (data not shown). Electron microscopical analysis of ACE cells cultured on basal lamina-type matrix and stimulated for 24 h with VEGF revealed an increase in the number of fused diaphragmed vesicles and fenestrations to a similar extent as in the cocultures (Fig. 4; Table II). ACE cells formed long thin cellular processes, which were interrupted by numerous fenestrations (Fig. 4, a and b). In addition, large clusters of fused vesicles were found, frequently separated only by thin diaphragms that often contained a central knob (Fig. 4 c). Incubation periods shorter than 24 h did not show an effect on fenestrae formation. Consistent with the coculture studies, VEGF induced fenestrae formation only two- or threefold in HUVEC cells cultivated on the matrix (data not shown). As a control for the extracellular matrix effect, parallel cultures of ACE cells were plated on rat tail collagen. VEGF treatment of cells under these conditions did not induce fused vesicles and fenestrations, whereas ACE cells cultured on the basal lamina-type matrix showed a 10-fold increase (Table II). Since basic FGF has also been reported to induce fenestrae in vivo (Roberts and Palade, 1997), we tested if it could replace VEGF in our in vitro system. However, incubation of ACE cells cultured on basal lamina-type matrix with basic FGF had only a minor effect on the number of fenestrations and fused diaphragmed vesicles (Table II). In conclusion, our data show that the effect of VEGF on fenestrae induction in vitro is specific. In addition, in the presence of a basal lamina-type matrix, VEGF is also sufficient for the induction of fenestrations in ACE cells in vitro.
Table II.
Effects of Extracellular Matrix and Growth Factors
| Culture | [Fenestrae + clustered vesicles]/cell (mean) | SD | ||
|---|---|---|---|---|
| ACE/ECM | 0.0157 | 0.0064 | ||
| ACE/ECM + VEGF 165 | 0.1636 | 0.0121 | ||
| ACE/ECM + bFGF | 0.0372 | 0.0094 | ||
| ACE/collagen | 0.0077 | 0.0022 | ||
| ACE/collagen + VEGF 165 | 0.0033 | 0.0057 |
ACE cells were grown to confluency and then VEGF 165 (100 ng/ml) or bFGF (20 ng/ml) were added to the medium. After 24 h the cells were fixed and processed for electron microscopy. Diaphragmed fenestrations and clustered vesicles were then counted in 370 (ACE/ECM), 630 (ACE/ECM + VEGF165), 540 (ACE/ECM + bFGF), 415 (ACE/collagen), and 500 cells (ACE/collagen + VEGF165). The number of clustered vesicles and fenestrae in ACE cells grown on the complex matrix and stimulated with VEGF is comparable to that of cocultures of ACE cells with transfected epithelial cells (compare Tables I and II) and highly significant (P < 0.001). In contrast, bFGF has only a minor effect (P < 0.05) on fenestrae and clustered vesicles, whereas ACE cells grown on collagen were not inducible.
Caveolae, but Not Fenestrae or Fused Diaphragmed Vesicles, Can Be Labeled with Antibodies against Caveolin-1
Since the induction of endothelial fenestrations by VEGF was always accompanied by an increase in the number of fused clustered caveolae-like vesicles with diaphragms, we wondered if caveolar proteins might be common molecular components of these structures. Ultrathin Lowicryl sections of paraformaldehyde-fixed material were labeled with an antibody against caveolin-1, a marker protein of caveolae. We first confirmed the specificity of our approach by in situ labeling of mouse muscle capillaries, which have continuous endothelia extremely rich in caveolae. As shown in Fig. 5 a, a considerable fraction of the caveolae were immunolabeled with the antibody. The labeling efficiency was increased in cryosections compared to resin sections (data not shown). No labeling was seen if the primary antibody was omitted or replaced by nonimmune rabbit immunoglobulins (Fig. 5 b). Caveolin-1–positive vesicles could also be identified in endothelial cells of the choroid plexus dissected from adult mouse brain (Fig. 5 c). In contrast, the fenestrae of these endothelial cells were always devoid of anti–caveolin-1 immunoreactivity (Fig. 5 c).
Figure 5.
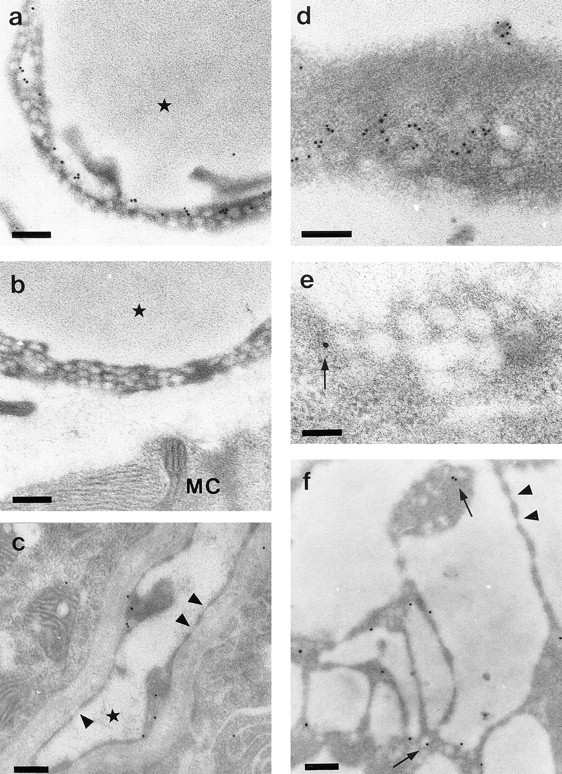
Immunoelectron microscopy using anti–caveolin-1 antibody. (a) Many caveolae are labeled with gold particles in mouse skeletal muscle capillaries. (b) Controls show no labeling for caveolin-1. (c) In capillaries of the choroid plexus, caveolin-1 labeling is found in caveolae but not in fenestrations (arrowheads). (d–f) Electron micrographs of ACE cells cocultured with VEGF 120–transfected epithelial cells. (d) Tangential section of an endothelial cell showing labeling of caveolae. (e) Tangential section through a grape-like cluster of diaphragmed vesicles. Only one vesicle is labeled (arrow). (f) Endothelial cell processes with multiple unlabeled fenestrae (arrowheads) and several gold-labeled caveolae (arrows). Capillary lumina are indicated by an asterisk. MC indicates muscle cell. Bars: (a–d and f) 0.2 μm; (e) 0.1 μm.
In vitro, we analyzed the cocultures of VEGF-transfected epithelial cells that had shown a strong induction of fenestrae and fused clustered vesicles. Fig. 5 d shows specific labeling of endothelial caveolae with anti–caveolin-1 antibodies in ACE cells. Specific labeling was only found in uncoated vesicles of apical or basal plasma membranes and was absent in control experiments when the first antibody was omitted. EpH4 cells revealed some immunopositive caveolae in their lateral membranes (data not shown). We never detected caveolin-1 in diaphragmed fenestrations (Fig. 5 f). Except for one gold particle, which may represent a recently fused caveola (Fig. 5 e), clustered diaphragmed vesicles were found to be immunonegative for caveolin-1.
Effects of VEGF on Caveolin-1 Expression and Phosphorylation in ACE Cells
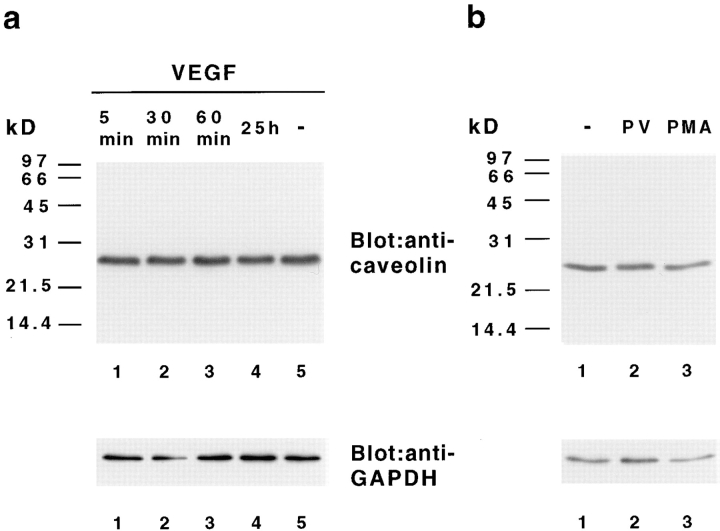
Since caveolin-1 was detected in endothelial caveolae, but not in fused clustered vesicles or fenestrae, we asked whether caveolin-1 is modified as a possible downstream target of VEGF signal transduction. We incubated ACE cells cultured on the basal lamina-type matrix for various time points with VEGF and performed immunoprecipitations with an antibody against VEGF receptor-2 and the anti–caveolin-1 antibody VIP-21N, which only recognizes the α isoform. VEGF rapidly induced the tyrosine phosphorylation of a 205-kD band, which was identified as the bovine form of the VEGF receptor-2 (Fig. 6 a), confirming the initiation of VEGF-induced signal transduction pathways in ACE cells. However, phosphorylation of caveolin-1 on tyrosine (Fig. 6 b) or serine residues (data not shown) was not observed at any time after VEGF treatment. As a control, treatment of ACE cells with the tyrosine phosphatase inhibitor pervanadate led to tyrosine phosphorylation and association of caveolin-1 with other phosphoproteins with molecular masses of ∼70, 30, 26, and 24-kD (Fig. 6, c and d; Vepa et al., 1997). In contrast to immunofluorescent labeling of caveolin-1 after pervanadate treatment, ACE cells stimulated with VEGF did not differ in caveolin-1 distribution compared to control cells (data not shown). Since we had observed that the number of fused caveolae-like vesicles increased in response to a longer treatment with VEGF, we tested if the amount of caveolin-1 protein was altered in these experiments. However, no change was observed in the expression level of caveolin-1 protein after treatment with VEGF, pervanadate, or PMA (Fig. 7). Taken together, our biochemical and immunofluorescence analyses demonstrate that induction of fenestrae in ACE cells in response to VEGF is not accompanied by a change in caveolin-1 phosphorylation, expression, or distribution.
Figure 6.
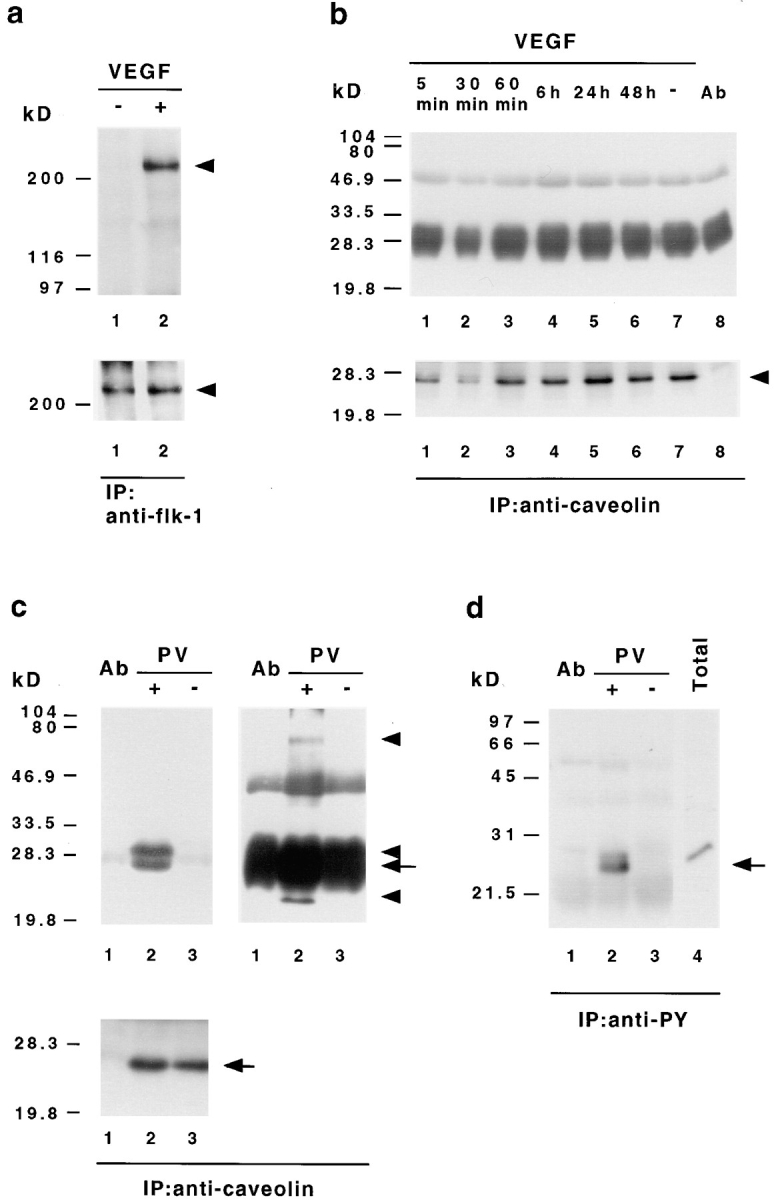
VEGF-induced signal transduction does not stimulate tyrosine phosphorylation of caveolin-1. (a) Tyrosine phosphorylation of bovine VEGF receptor-2 (flk-1) in ACE cells after VEGF stimulation. Endothelial cells were stimulated with (+) or without (−)100 ng/ml VEGF 165 for 5 min, extracted with Triton buffer, and then immunoprecipitated with anti–flk-1 antibodies. The precipitates were analyzed by Western immunoblotting with an antibody against phosphotyrosine (top). The same membrane was afterwards reprobed with an antibody recognizing flk-1 (bottom). In contrast to unstimulated cells (lane 1) bovine flk-1 (arrowhead) is found to be specifically phosphorylated on tyrosine after VEGF treatment (lane 2). (b) VEGF does not induce caveolin-1 tyrosine phosphorylation. ACE cells grown on basal lamina-type extracellular matrix were stimulated with VEGF 165 for the indicated time, extracted with SDS lysis buffer, immunoprecipitated with VIP-21N antibody, and then subjected to immunoblot analysis with antiphosphotyrosine antibody (top). Subsequent reprobing of the same membrane with anti–caveolin-1 antibody (bottom) indicated that equal amounts of caveolin-1 (arrowhead) have been precipitated. Protein bands at 50 and 30–25 kD corresponding to the IgG heavy and light chains eluted from the Sepharose, together with the bound proteins, are also present in immunoprecipitations without cell lysates (top, lane 8). (c and d) Pervanadate-induced tyrosine phosphorylation of caveolin-1 and associated proteins. ACE cells were treated with (+) or without (−) pervanadate (PV), extracted with SDS lysis buffer, and then subjected to immunoprecipitation with antibodies against caveolin-1 (c) or phosphotyrosine (d), followed by Western immunoblotting with antiphosphotyrosine (c, top) or anticaveolin-1 (d) antibodies. Blots of caveolin-1 immunoprecipitates were reprobed with anti–caveolin-1 antibody (c, bottom). The bands at 50 and 30–25 kD correspond to the IgG heavy and light chains. To reveal all precipitated phosphoproteins, two different exposures for the antiphosphotyrosine blots are shown in c. Note that the anticaveolin-1 antibody detects only one band in antiphosphotyrosine- and anti–caveolin-1 precipitates (c and d, arrows), whereas the antiphosphotyrosine antibody recognizes several proteins in the anticaveolin-1 precipitates (c, top arrowheads). As controls, immunoprecipitations without cell lysates (lanes 1 in c and d) and total cellular extracts (d, lane 4) are shown.
Figure 7.
VEGF does not alter the level of caveolin-1 protein. ACE cells were grown on basal-lamina matrix and treated with (a) VEGF 165 for the indicated time or (b) with pervanadate (PV, 20 min) or PMA (200 ng/ml, 1 h). Total cellular extracts were subjected to SDS-PAGE and immunoblot analysis with anti–caveolin-1 antibodies (top). The amount of caveolin-1 did not change after stimulation with VEGF, pervanadate, or PMA. Subsequent probing of the same membrane with anti-GAPDH antibody indicates equal amounts of protein in each lane (bottom).
Discussion
VEGF was first identified as a vascular permeability factor. After cloning murine VEGF and analyzing its distribution by in situ hybridization, we observed a strong correlation of VEGF expression with angiogenesis during embryonic development, e.g., in the brain (Breier et al., 1992). However, we found a continuous expression in epithelial cells of the choroid plexus and kidney glomerulus (podocytes) throughout development into the adult. Since the closely associated endothelial cells of these structures were fenestrated and also constitutively expressed VEGF receptors (Millauer et al., 1993; Breier et al., 1995), we speculated that VEGF might be involved in the induction or maintenance of endothelial fenestrations. This has been recently demonstrated in vivo by Roberts and Palade (1995, 1997) who showed that topical administration of VEGF 165 induced fenestrations in continuous microvascular endothelium of muscle and skin, and that tumor neovasculature induced by VEGF is fenestrated. However, in these in vivo systems, the questions regarding the responsible VEGF isoforms, the roles of contributing factors, supporting cells, and extracellular matrix are difficult to address. Biochemical studies on signal transduction and changes in molecular composition are almost impossible to perform in vivo due to tissue complexity and lack of sufficient material. Therefore, an in vitro system to study the effects of VEGF on fenestrae induction would be valuable.
To develop an in vitro system, we first used CPE in coculture with ACE. Wilting and Christ (1989) have demonstrated that grafting of CPE from quail into the coelomic cavity of chicken embryos induced the differentiation of fenestrated capillaries in vivo. In our in vitro cocultures of ACE cells with CPE, we also observed a strong induction of fenestrae. This prompted us to develop a system in which the direct effects of VEGF could be analyzed. We used a mammary epithelial cell line that by itself secreted very little endogenous VEGF under normal culture conditions. Epithelial cells stably transfected with VEGF cDNAs secreted a high level of VEGF and induced a seven- or eightfold increase in fenestrae and fused clustered vesicles in cocultured endothelial cells, thus providing direct evidence for a role of VEGF in fenestrae induction.
Using a complex basal lamina-type extracellular matrix, we showed that the addition of purified recombinant VEGF, but not bFGF, was sufficient to induce a ten-fold increase in fenestrations in endothelial cells. These progressively better-defined in vitro systems demonstrate for the first time that VEGF is a specific inducer of fenestrations in vitro. The fact that FGF has no major effect on fenestrae induction is interesting, because previous in vivo experiments have noted some effect of FGF (Roberts and Palade, 1997). In agreement with the hypothesis of these authors, we would suggest that FGF acts indirectly by upregulating VEGF or its receptors in the complex in vivo situation, but is unable to directly induce fenestrations in the defined in vitro system.
VEGF 120 and 164 induced fenestrations. Both isoforms are expressed in organs with fenestrated blood vessels (Bacic et al., 1995; Simon et al., 1995; this report) and bind to the high-affinity VEGF receptors-1 and -2 (Gitay-Goren et al., 1996; Keyt et al., 1996) that are expressed in endothelial cells of choroid plexus, kidney glomeruli, and cultured ACE cells (Plouet and Moukadiri, 1990; Vaisman et al., 1990; Millauer et al., 1993; Breier et al., 1995; Simon et al., 1995; this report). So far, different VEGF isoforms have not been observed to exhibit different biological effects (Birkenhager et al., 1996; Wilting et al., 1996; Schmidt and Flamme, 1997), although it was reported that VEGF 165 has an increased affinity for VEGF receptor-1 and a higher mitogenic potency compared to VEGF 121 (Keyt et al., 1996). In any case, our results imply a role for the VEGF isoforms 120 and 164 in fenestrae induction.
In addition to fenestrae formation, a striking induction of fused clustered caveolae-like vesicles with diaphragms was observed after VEGF treatment. Dilated plasmalemmal vesicles that aggregate to form clusters in which the vesicles are fused to one another and are separated by diaphragms provided with central knobs (Roberts and Palade, 1995) have also been referred to as VVOs (Kohn et al., 1992). VVOs have been observed in a small portion of normal venules in vivo (Feng et al., 1996), but seem to occur consistently in tumor vessels (Kohn et al., 1992; Qu et al., 1995). The function of VVOs was shown to be activated after treatment with VEGF in vivo (Feng et al., 1996). VEGF has also been localized to VVOs in tumor-associated microvascular endothelial cells (Qu et al., 1995). Considering the morphological relationship among VVOs, plasmalemmal vesicles, and endothelial fenestrations (e.g., size, presence of diaphragms, and cholesterol dependence) and the ocurrence of endothelial fenestrations as clusters in vivo, we decided to count clustered fenestrae and clustered diaphragmed vesicles together. By analyzing more than 4,500 cellular profiles in transmission electron microscopy, these structures appeared to be interrelated, but the exact temporal and spatial determinants of their formation is presently unclear. However, these observations raise the important question of whether caveolae can fuse and give rise to VVOs, and if subsequent VVOs can give rise to the so-called sieve plates (clusters of fenestrae found in the most attenuated periphery of endothelial cells).
Very little is known about the structural composition and modulation of caveolae, VVOs, and fenestrae. Caveolin-1 is a commonly accepted marker protein for caveolae (Rothberg et al., 1992; Parton et al., 1994; Smart et al., 1994, Stan et al., 1997). We attempted to correlate the distribution and biochemical modification of caveolin-1 with the distribution and induction of caveolae, fenestrae, and VVOs in vivo and in vitro. A considerable fraction of caveolae in endothelial cells of tissues and cocultures could be labeled with anti–caveolin-1 antibodies in immunoelectron microscopy. Clustered diaphragmed vesicles were only very rarely labeled and all fenestrae were negative for caveolin-1, suggesting that caveolin-1 may be specifically excluded from fused clustered vesicles and fenestrae. Therefore, it was interesting to investigate if modification of caveolin-1 after VEGF treatment correlated with the induction of fused clustered vesicles and fenestrae. VEGF did not induce a change in caveolin-1 distribution, tyrosine or serine phosphorylation, or alteration in the protein level of caveolin-1. A change in the distribution of caveolin-1 was observed only after pervanadate stimulation that caused increased tyrosine phosphorylation of caveolin-1 and associated proteins. Our results on tyrosine and serine phosphorylation of caveolin-1 are consistent with previous studies that demonstrated the activation of signaling in caveolae after stimulation with EGF, PDGF, or insulin in the absence of a consistent change in caveolin-1 phosphorylation (Scherer et al., 1994; Mastick et al., 1995; Liu et al., 1996). Although the induction of tyrosine phosphorylation of caveolin-1 after pervanadate treatment has been shown before (Vepa et al., 1997), the nature of the coprecipitated phosphoproteins and their possible relationship to previously identified caveolin-1–associated proteins (Scherer et al., 1994; Mastick et al., 1995; Liu et al., 1996) remains to be established. Whether VEGF receptors are concentrated in caveolae, as has been shown for PDGF receptor (Liu et al., 1996, 1997), EGF receptor (Smart et al., 1995), as well as other tyrosine kinase receptors (Wu et al., 1997), is not yet known, but is strongly suggested by the presence of a consensus binding site (WSF/YGVLLWEIF) for the caveolin-scaffolding domain (Couet et al., 1997a ) in their cytoplasmic tails. It is controversial whether caveolin-1 is regulated at the protein level (Glenney, 1989; Koleske et al., 1995; Mineo et al., 1996). Since we did not observe major changes in the amount of caveolin-1 protein after VEGF stimulation, the subcellular localization of caveolin-1 may be altered without concomitant protein degradation or synthesis. Several possibilities such as different caveolin-1 conformations or modifications not recognized by the available antibodies remain to be tested. Alternatively, palmitoylation or the oligomerization properties of caveolin-1 might be altered after VEGF treatment (Monier et al., 1995, 1996). However, the most likely explanation is that fusion of clustered caveolae-like vesicles leading to VVOs and fenestrae is accompanied by a loss of caveolin-1 protein. These observations suggest that although caveolin-1–coated vesicles might be involved in the formation or recruitment of fused diaphragmed vesicles and fenestrae, they do not comprise a structural entity of fenestrae themselves.
Previous in vivo experiments have shown that application of VEGF induced fenestrae in capillaries of skeletal muscle and skin (Roberts and Palade, 1995). Muscle and skin endothelial cells contain the highest number of caveolae and are sometimes found to be fenestrated (Korneliussen, 1975; Wolff, 1977). Therefore, it is possible that VEGF induces fenestrations only in an in vivo or in vitro environment that is permissive for fenestrae formation. One prerequisite for fenestrae formation seems to be a certain threshold number of caveolae. Brain microvessels have the lowest number of caveolae (Coomber and Stewart, 1985), and may be the least inducible endothelial cells. However, endothelial cells in the early embryonic perineural vascular plexus are fenestrated (Yoshida et al., 1988) and the neovasculature of some VEGF-secreting brain tumors has been found to possess endothelial fenestrations (Roberts and Palade, 1997). Fenestrated bovine ACE lose fenestrae when cultured (Folkman and Haudenschild, 1980; Furie et al., 1984), probably due to the withdrawal of VEGF-producing adrenal cortex epithelial cells. These previously fenestrated cells are the most responsive cells in terms of fenestrae inducibility analyzed in vitro so far. We have observed some, though weak, induction of fenestrations in HUVEC cells in vitro. Therefore, VEGF is able to induce fenestrae in previously nonfenestrated endothelial cells, but the magnitude of the response seems to be dependent on the origin of the endothelium. It is conceivable that, in contrast to embryonic endothelium that seems to be more plastic, the responsiveness of adult endothelium is restricted by the counteractive effects of the surrounding microenvironment, e.g., within the brain, to maintain blood–brain barrier and low permeability properties (Risau, 1995).
The other prerequisite for fenestrae formation seems to be an appropriate three-dimensional architecture of the endothelial cell. We demonstrate here that, in our in vitro system, the induction of fenestrae is completely dependent on a basal lamina-type extracellular matrix, because ACE cells cultured on collagen in the presence of VEGF were virtually devoid of fenestrations. These data are supported by our in vivo experiments of retroviral overexpression of VEGF in which we have rarely seen endothelial fenestrae in hypervascularized embryonic tissue (Flamme et al., 1995), and are consistent with the observation that an intact basal lamina forms rather late in vascular development (Risau and Lemmon, 1988). Therefore, both the correct extracellular matrix as well as activation of the relevant receptor seem to be necessary for the formation of fenestrae in endothelial cells. In vivo as well as in vitro, fenestrae are located in extremely attenuated regions of endothelial cells and are often organized as sieve plates. The first morphological effect observed in VEGF-treated endothelial cell cultures was the appearance of branch-like cell sprouts already visible after 6 h of stimulation. These thin cellular extensions also contained an extremely high density of caveolin-1 protein (data not shown). VEGF binding to and activation of its receptors may induce both the flattening of cellular processes and insertion of fenestrations. However, formation of cellular processes was also seen with bFGF (data not shown). Hence, it is unclear if this morphological change is associated with fenestrae induction or rather represents a general effect of VEGF and other growth factors on ACE cells.
Although the morphological characteristics and presence of fenestrae in specific vascular beds has been carefully analyzed, the molecular composition, induction, and maintenance of fenestrations in endothelial cells is still not well understood. Physiological studies have shown that fenestrae provide highly permeable pores for water and small solutes. More recently, based on the high density of cationic ferritin labeling of fenestrae, the anionic glycocalix present in fenestral pores has been investigated in more detail and shown to consist of filamentous plugs (Rostgaard and Qvortrup, 1997). However, this anionic coat may be quite variable, and even absent, in certain capillaries, e.g., in tumors (Roberts and Palade, 1997). These results raise the possibility that the permeability of endothelial fenestrations is not uniform but differs depending on the presence or absence of diaphragms, filamentous plugs, or the composition of the anionic glycocalix. Further insights into the functional heterogeneity of vascular territories in terms of permeability clearly require a molecular definition of fenestrae, diaphragms, caveolae, fused clustered caveolae-like vesicles, and VVOs. The availability of our in vitro system for fenestrae induction allows several important issues to be addressed, e.g., which signal transduction systems in endothelial cells are involved in the formation of cellular processes and fenestrae, and how the signals from VEGF receptor–tyrosine kinases are integrated with the putative integrin signals after their binding to the basal lamina-type extracellular matrix. Furthermore, the activity of VEGF as an endothelial growth versus permeability/fenestrae-inducing factor must be differentially regulated. It is conceivable that the VEGF-secreting epithelial cells induce a similar cascade of signals in the closely associated endothelial cells as required for growth and migration. However, the complex basal lamina and components therein, e.g., inhibitors, may modulate the signal so that no new capillaries form. This is consistent with the notion that epithelia are resistant to vascular invasion. The establishment of our in vitro system of endothelial fenestrations promises to be a useful model to further clarify the molecular mechanisms that are involved in the formation and function of endothelial fenestrations.
Footnotes
We thank B. Engelhardt, H. Drexler, S. Bamforth (all from Max Planck Institut für physiologische und klinische Forschung), U. Kniesel, and H. Gerhardt (Universität Tübingen) for their helpful comments on the manuscript.
This work was supported by the Max Planck Society and SUGEN, Inc.
Address all correspondence to Werner Risau, Max Planck Institut für Physiologische und Klinische Forschung, W.G. Kerckhoff Institut, Abteilung Molekulare Zellbiologie, Parkstr. 1, 61231 Bad Nauheim, Germany. Tel.: (49) 603-272-073. Fax: (49) 603-272-259. E-mail: wrisau@kerckhoff.mpg.de
1. Abbreviations used in this paper: ACE, adrenal cortex endothelial cells; CPE, choroid plexus epithelial cells; HMSS, Hanks modified salt solution; HUVEC, human umbilical vein endothelial cells; PMA, phorbol myristate acetate; RT, reverse transcription; VEGF, vascular endothelial growth factor; VVO, vesiculo-vacuolar organelle.
References
- Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic M, Edwards NA, Merrill MJ. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- Birkenhager R, Schneppe B, Rockl W, Wilting J, Weich HA, McCarthy JE. Synthesis and physiological activity of heterodimers comprising different splice forms of vascular endothelial growth factor and placenta growth factor. Biochem J. 1996;316:703–707. doi: 10.1042/bj3160703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development (Camb) 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Brown LF, Berse B, Tognazzi K, Manseau EJ, Van de Water L, Senger DR, Dvorak HF, Rosen S. Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int. 1992;42:1457–1461. doi: 10.1038/ki.1992.441. [DOI] [PubMed] [Google Scholar]
- Carley WW, Milici AJ, Madri JA. Extracellular matrix specificity for the differentiation of capillary endothelial cells. Exp Cell Res. 1988;178:426–434. doi: 10.1016/0014-4827(88)90411-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coomber BL, Stewart PA. Morphometric analysis of CNS microvascular endothelium. Microvasc Res. 1985;30:99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- Coomber BL, Stewart PA, Hayakawa K, Farrell CL, Del Maestro RF. Quantitative morphology of human glioblastoma multiforme microvessels: structural basis of blood-brain barrier defect. J Neuro-Oncol. 1987;5:299–307. doi: 10.1007/BF00148386. [DOI] [PubMed] [Google Scholar]
- Coomber BL, Stewart PA, Hayakawa EM, Farrell CL, Del Maestro RF. A quantitative assessment of microvessel ultrastructure in C6 astrocytoma spheroids transplanted to brain and to muscle. J Neuropathol Exp Neurol. 1988;47:29–40. doi: 10.1097/00005072-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997a;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Couet J, Li SW, Okamoto T, Scherer PE, Lisanti MP. Molecular and cellular biology of caveolae—paradoxes and plasticities. Trends Cardiovasc Med. 1997b;7:103–110. doi: 10.1016/S1050-1738(97)00001-7. [DOI] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO (Eur Mol Biol Organ) J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukocyte Biol. 1996;59:100–115. [PubMed] [Google Scholar]
- Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamme I, von Reutern M, Drexler HC, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. doi: 10.1006/dbio.1995.1291. [DOI] [PubMed] [Google Scholar]
- Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc UK. 1980;100:346–353. [PubMed] [Google Scholar]
- Furie MB, Cramer EB, Naprstek BL, Silverstein SC. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984;98:1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatmaitan Z, Varticovski L, Ling L, Mikkelsen R, Steffan AM, Arias IM. Studies on fenestral contraction in rat liver endothelial cells in culture. Am J Pathol. 1996;148:2027–2041. [PMC free article] [PubMed] [Google Scholar]
- Ge AZ, Butcher EC. Cloning and expression of a cDNA encoding mouse endoglin, an endothelial cell TGF-β ligand. Gene. 1994;138:201–206. doi: 10.1016/0378-1119(94)90808-7. [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102:1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitay-Goren H, Cohen T, Tessler S, Soker S, Gengrinovitch S, Rockwell P, Klagsbrun M, Levi B, Neufeld G. Selective binding of VEGF121 to one of the three vascular endothelial growth factor receptors of vascular endothelial cells. J Biol Chem. 1996;271:5519–5523. doi: 10.1074/jbc.271.10.5519. [DOI] [PubMed] [Google Scholar]
- Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- Gospodarowicz, D. 1984. Preparation of Extracellular Matrices Produced by Cultured Bovine Corneal Endothelial Cells: Their Use in Cell Culture. In Methods for preparation of media, supplements and substrata for serum-free animal cell culture. Alan R. Liss, Inc., New York. 275–293.
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992;89:244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell B, Levine E, Shapiro S, Williams S, Carabasi RA, Mueller S, Thornton S. Human adult endothelial cell growth in culture. J Vasc Surg. 1984;1:757–764. doi: 10.1067/mva.1984.avs0010757. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- Kohn S, Nagy JA, Dvorak HF, Dvorak AM. Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab Invest. 1992;67:596–607. [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H. Fenestrated blood capillaries and lymphatic capillaries in rat skeletal muscle. Cell Tissue Res. 1975;163:169–174. doi: 10.1007/BF00221724. [DOI] [PubMed] [Google Scholar]
- Kurtz A, Jelkmann W, Bauer C. Mesangial cells derived from rat glomeruli produce an erythropoiesis stimulating factor in cell culture. FEBS (Fed Eur Biochem Soc) Lett. 1982;137:129–132. doi: 10.1016/0014-5793(82)80330-x. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. And still they are moving. . . . dynamic properties of caveolae. FEBS (Fed Eur Biochem Soc) Lett. 1996;389:52–54. doi: 10.1016/0014-5793(96)00585-6. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi network–derived transport vesicles. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell–cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR, Smaje LH. An analysis of the permeability of a fenestra. Microvasc Res. 1987;33:233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Lombardi T, Montesano R, Furie MB, Silverstein SC, Orci L. Endothelial diaphragmed fenestrae: in vitro modulation by phorbol myristate acetate. J Cell Biol. 1986;102:1965–1970. doi: 10.1083/jcb.102.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi T, Montesano R, Furie MB, Silverstein SC, Orci L. In vitro modulation of endothelial fenestrae: opposing effects of retinoic acid and transforming growth factor β. J Cell Sci. 1988;91:313–318. doi: 10.1242/jcs.91.2.313. [DOI] [PubMed] [Google Scholar]
- Mastick CC, Brady MJ, Saltiel AR. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995;129:1523–1531. doi: 10.1083/jcb.129.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989–997. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- Milici AJ, Furie MB, Carley WW. The formation of fenestrations and channels by capillary endothelium in vitro. Proc Natl Acad Sci USA. 1985;82:6181–6185. doi: 10.1073/pnas.82.18.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993;264:C995–1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier S, Dietzen DJ, Hastings WR, Lublin DM, Kurzchalia TV. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS (Fed Eur Biochem Soc) Lett. 1996;388:143–149. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Smit JW, Bom VJ, Blom NR, Ruiters MH, Halie MR, van der Meer J. Association of smooth muscle cell tissue factor with caveolae. Blood. 1996;88:1306–1313. [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- Palade GE, Simionescu M, Simionescu N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl. 1979;463:11–32. [PubMed] [Google Scholar]
- Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouet J, Moukadiri H. Characterization of the receptor to vasculotropin on bovine adrenal cortex-derived capillary endothelial cells. J Biol Chem. 1990;265:22071–22074. [PubMed] [Google Scholar]
- Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Predescu D, Horvat D, Predescu S, Palade GE. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci USA. 1994;91:3014–3018. doi: 10.1073/pnas.91.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Nagy JA, Senger DR, Dvorak HF, Dvorak AM. Ultrastructural localization of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) to the abluminal plasma membrane and vesiculovacuolar organelles of tumor microvascular endothelium. J Histochem Cytochem. 1995;43:381–389. doi: 10.1177/43.4.7534783. [DOI] [PubMed] [Google Scholar]
- Rhodin, J.A.G. 1974. Histology. A Text and Atlas. Oxford University Press, New York. 803 pp.
- Risau W. Differentiation of endothelium. FASEB (Fed Am Soc Exp Biol) J. 1995;9:926–933. [PubMed] [Google Scholar]
- Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125:441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol–linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Mastick CC, Lodish HF. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, M., and I. Flamme. 1997. The in vivo activity of vascular endothelial growth factor isoforms in the avian embryo. Growth Factors. In press. [DOI] [PubMed]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson L, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdotheil nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989;78:561–571. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- Simon M, Grone HJ, Johren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- Stadler, E., T. Thomas, and M. Dziadek. 1994. In vitro culture of mouse fetal choroid plexus epithelial cells. In Cell Biology. A Laboratory Handbook. J.E. Celis, editor. Academic Press, Inc., NY. 109–115.
- Stan R, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae) Mol Biol Cell. 1997;8:595–605. doi: 10.1091/mbc.8.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan AM, Gendrault JL, Kirn A. Increase in the number of fenestrae in mouse endothelial liver cells by altering the cytoskeleton with cytochalasin B. Hepatology. 1987;7:1230–1238. doi: 10.1002/hep.1840070610. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265:19461–19466. [PubMed] [Google Scholar]
- Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnuerch H, Risau W, Ruco L, Mantovani A, Dejana E. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- Vepa S, Scribner WM, Natarajan V. Activation of protein phosphorylation by oxidants in vascular endothelial cells: identification of tyrosine phosphorylation of caveolin. Free Radical Biol Med. 1997;22:25–35. doi: 10.1016/s0891-5849(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS (Fed Eur Biochem Soc) Lett. 1995;376:108–112. doi: 10.1016/0014-5793(95)01256-7. [DOI] [PubMed] [Google Scholar]
- Wilting J, Christ B. An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 1989;255:487–494. doi: 10.1007/BF00218783. [DOI] [PubMed] [Google Scholar]
- Wilting J, Birkenhager R, Eichmann A, Kurz H, Martiny-Baron G, Marme D, McCarthy JE, Christ B, Weich HA. VEGF121 induces proliferation of vascular endothelial cells and expression of flk-1 without affecting lymphatic vessels of chorioallantoic membrane. Dev Biol. 1996;176:76–85. doi: 10.1006/dbio.1996.9993. [DOI] [PubMed] [Google Scholar]
- Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–1364. [PubMed] [Google Scholar]
- Wolff, J.R. 1977. Ultrastructure of the Terminal Vascular Bed as Related to Function. In Microcirculation. G. Kaley and B.M. Altura, editors. University Park Press, Baltimore, MD, and London, UK. 95–130.
- Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamada M, Wakabayashi K, Ikuta F. Endothelial fenestrae in the rat fetal cerebrum. Dev Brain Res. 1988;44:211–219. doi: 10.1016/0165-3806(88)90219-2. [DOI] [PubMed] [Google Scholar]