Abstract
We have analyzed the role of the protein kinase Chk1 in checkpoint control by using cell-free extracts from Xenopus eggs. Recombinant Xenopus Chk1 (Xchk1) phosphorylates the mitotic inducer Cdc25 in vitro on multiple sites including Ser-287. The Xchk1-catalyzed phosphorylation of Cdc25 on Ser-287 is sufficient to confer the binding of 14-3-3 proteins. Egg extracts from which Xchk1 has been removed by immunodepletion are strongly but not totally compromised in their ability to undergo a cell cycle delay in response to the presence of unreplicated DNA. Cdc25 in Xchk1-depleted extracts remains bound to 14-3-3 due to the action of a distinct Ser-287-specific kinase in addition to Xchk1. Xchk1 is highly phosphorylated in the presence of unreplicated or damaged DNA, and this phosphorylation is abolished by caffeine, an agent which attenuates checkpoint control. The checkpoint response to unreplicated DNA in this system involves both caffeine-sensitive and caffeine-insensitive steps. Our results indicate that caffeine disrupts the checkpoint pathway containing Xchk1.
Keywords: Cdc2, Chk1, 14-3-3 proteins, Cdc25, caffeine
The entry into mitosis is controlled by regulatory proteins that ensure the proper segregation of replicated chromosomes to daughter cells. The integrity of chromosomal DNA is under constant surveillance during the cell cycle. In eukaryotic cells, when chromosomes become damaged or cannot be replicated completely, mitosis is prevented by checkpoint mechanisms until two intact copies of the genome can be produced (Hartwell and Weinert, 1989; Murray, 1995; Elledge, 1996). A key target of these checkpoint pathways is the Cdc2–cyclin B complex, also known as maturation or M phase–promoting factor (MPF).1 The Cdc2–cyclin B complex, once activated at the G2/M transition, phosphorylates a myriad of proteins that carry out the various processes of mitosis such as nuclear disassembly and chromosome segregation (King et al., 1994; Morgan, 1997).
In the presence of damaged or unreplicated DNA, Cdc2–cyclin B is kept inactive due to inhibitory phosphorylation of the Tyr-15 and Thr-14 residues of Cdc2 (Nurse, 1997). These phosphorylations are carried out collectively by the kinases Wee1 and Myt1 (Featherstone and Russell, 1991; Igarashi et al., 1991; Parker et al., 1992; Mueller et al., 1995a ,b; Watanabe et al., 1995). At the onset of mitosis, the phosphatase Cdc25C removes these inhibitory phosphate groups and thereby activates Cdc2–cyclin B (Dunphy and Kumagai, 1991; Gautier et al., 1991; Strausfeld et al., 1991). The activity of Cdc25C is strictly regulated, being low during interphase and high at mitosis (Izumi et al., 1992; Kumagai and Dunphy, 1992). In Xenopus egg extracts, the activation of Cdc25C (hereafter referred to simply as Cdc25) at mitosis involves the stimulatory phosphorylation of its NH2-terminal regulatory domain by at least two kinases, including Cdc2–cyclin B itself and a Xenopus homologue of the Polo-like kinase called Plx1 (Hoffmann et al., 1993; Izumi and Maller, 1995; Kumagai and Dunphy, 1996).
In recent studies in humans and Xenopus, it has been shown that Cdc25 is negatively regulated during interphase by the binding of 14-3-3 proteins (Peng et al., 1997; Kumagai et al., 1998). The inactive form of Cdc25 found before mitosis is phosphorylated on Ser-216 and Ser-287 in humans and Xenopus, respectively. This phosphorylation, which occurs in a consensus 14-3-3 binding site, mediates the association between Cdc25 and 14-3-3 proteins. This phosphorylation-dependent interaction appears to suppress the activation and/or action of Cdc25. Mutants of Cdc25 that cannot be phosphorylated at this residue and thus cannot bind 14-3-3 proteins override the unreplicated/damaged DNA checkpoint(s), suggesting that Cdc25 is a target of checkpoint regulation (Peng et al., 1997; Kumagai et al., 1998).
In fission yeast, the protein kinase Chk1 is required for a cell cycle arrest in response to damaged DNA and also for the DNA replication checkpoint under certain circumstances (Walworth et al., 1993; al-Khodairy et al., 1994; Francesconi et al., 1997; Furnari et al., 1997). Chk1 acts downstream of several Rad gene products and is modified by phosphorylation upon DNA damage (Carr et al., 1995; Walworth and Bernards, 1996). Likewise, the kinase Rad53 from budding yeast is phosphorylated upon DNA damage in a manner that depends on several checkpoint control genes (Sanchez et al., 1996; Sun et al., 1996). In Drosophila, the grapes gene encodes a Chk1 homologue that appears to modulate cell cycle timing (Fogarty et al., 1997; Sibon et al., 1997). It has recently been shown that fission yeast Chk1 and its human homologue phosphorylate human Cdc25C on Ser-216, suggesting that Chk1 acts at least in part by regulating the binding of 14-3-3 to Cdc25C (Peng et al., 1997; Sanchez et al., 1997).
Xenopus egg extracts have provided a valuable in vitro system for studying the mechanisms of cell cycle control (Murray, 1991). Although early Xenopus embryos do not normally manifest the replication and damage checkpoints, egg extracts to which DNA has been added exogenously at a concentration typically found in somatic cells undergo a pronounced cell cycle arrest in response to replication inhibitors such as aphidicolin or DNA damaging agents (Dasso and Newport, 1990; Kumagai et al., 1998). Moreover, caffeine, an agent which disrupts the cell cycle arrest in either aphidicolin-treated or irradiated mammalian tissue culture cells by an unknown mechanism (Schlegel and Pardee, 1986), also overrides the unreplicated/ damaged DNA checkpoint(s) in Xenopus egg extracts (Dasso and Newport, 1990; Kumagai and Dunphy, 1995). These observations suggest that the protein components of such checkpoint pathways are stored in a latent form in unfertilized eggs.
In this report, we have cloned a Xenopus Chk1 homologue (Xchk1) and analyzed its role in checkpoint control in egg extracts. In immunodepletion studies, we have found that Xchk1 plays a role in the unreplicated/damaged DNA checkpoint(s) in this system. The phosphorylation of Cdc25 on Ser-287 by recombinant Xchk1 in vitro is sufficient to confer the binding of 14-3-3 proteins, but there appears to be an additional kinase with this substrate specificity in egg extracts. We find that caffeine acts by disrupting the function of Xchk1. However, a distinct, caffeine-insensitive pathway also plays a role in the checkpoint response. Taken together, these results indicate that a major facet of the checkpoint-mediated delay of the cell cycle in this system involves a caffeine-sensitive pathway containing Xchk1, Cdc25, and 14-3-3 proteins.
Materials and Methods
Cloning of a Xenopus Chk1 Homologue (Xchk1)
Two degenerate oligonucleotides corresponding to sequences conserved between Schizosaccharomyces pombe Chk1 and Drosophila Grapes were designed: TTT(G/C)(A/G)TAAIATIGAICCIGATGTIGG and A(G/A) IATIATICCICAI(G/C)(A/T)CCAITI(G/A)TCI(A/G)(C/T). A polymerase chain reaction (PCR) using Xenopus oocyte cDNA as the template yielded a major 300-bp fragment in addition to a few minor fragments. DNA sequencing revealed that the 300-bp fragment encodes a segment of protein with significant homology to Chk1. The 300-bp fragment was radiolabeled with 32P and used to screen a Xenopus oocyte cDNA library in the pAX-NMT vector to obtain a full-length cDNA clone (Mueller et al., 1995a ). A 2.2-kb ApaI-XhoI fragment encoding full-length Xchk1 was cloned into pBluescript SK- (pBS-Xchk1). A series of deletion clones was constructed by using the Erase-a-base kit (Promega Corp., Madison, WI). DNA sequencing of both strands was performed with an ABI model 373 automated sequencer (Perkin-Elmer Corp., Foster City, CA).
Production of Recombinant His6–Xchk1 and His6–Xchk1-N135A Proteins
An NcoI site at the start codon of Xchk1 was created by PCR using Pfu DNA polymerase with the primers CATGCCATGGCAGTTCCGTTTGTTGAAG and GGCCCTGGATTTAATCACTTC. The resulting PCR fragment was digested with NcoI and EcoRV to produce a 400-bp fragment encoding the NH2-terminal region of Xchk1. This fragment and a 1.7-kb EcoRV-XhoI fragment encoding the remaining COOH-terminal portion of Xchk1 were ligated to pFastBacHTa that had been digested with NcoI and XhoI. The kinase-negative mutant of Xchk1 (N135A) was created by PCR with the primers GAGATATCAAGCCTGAGGCCTTGCTTTTAG and AAACAGCTATGACCATGATTACGCC. This PCR fragment was digested with EcoRV and XhoI and cloned into pFastBacHTa together with 400-bp NH2-terminal NcoI-EcoRV fragment as described above. Baculoviruses encoding the recombinant His6–Xchk1 and His6–Xchk1-N135A proteins were produced using the Bac-to-Bac system (GIBCO BRL, Gaithersburg, MD). Recombinant proteins were isolated using nickel agarose beads as described (Kumagai and Dunphy, 1995).
Production of GST–Cdc25, GST–Cdc25-S287A, GST–Cdc25(254–316)-WT, and GST–Cdc25(254–316)-S287A Proteins in Bacteria
The BamHI-HindIII fragment of pFastBacHTa encoding His6–Cdc25-S287A (Kumagai et al., 1998) was cloned into pGEX–Cdc25 (Kumagai and Dunphy, 1992) that had been digested with BamHI and HindIII to yield pGEX–Cdc25-S287A. Glutathione-S-transferase (GST)–Cdc25 and GST–Cdc25-S287A were purified as described from Escherichia coli CAG629 transformed with pGEX–Cdc25 and pGEX–Cdc25-S287A, respectively (Kumagai and Dunphy, 1992). GST fusion protein constructs containing amino acids 254–316 of either wild-type Xenopus Cdc25 (GST– cdc25[254–316]-WT) or its S287A mutant (GST–Cdc25[254-316]-S287A) were produced by PCR with Pfu DNA polymerase (Stratagene, La Jolla, CA) using the primers CTAGAGGGATCCATGGCAATTCTTCTGTCGGGACCC and GGCCGTCGAATTCACGTCTCCTTTTCACTCTGAC and the appropriate DNA template. The PCR fragments were digested with BamHI and EcoRI and ligated into pGEX-2T that had been digested with BamHI and EcoRI. These GST fusion proteins were expressed in the E. coli strain BL21(DE3)pLysS and purified using glutathione agarose (Smith and Johnson, 1988).
Egg Extracts and Isolation of the Nuclear Fraction
Xenopus egg extracts were prepared as described (Murray, 1991). Egg extracts arrested in interphase due to the presence of unreplicated DNA routinely contained 1,000–3,000 demembranated Xenopus sperm nuclei/μl of extract and 100 μg/ml aphidicolin. Extracts arrested with damaged DNA contained 3,000 sperm nuclei/μl that had been treated with ultraviolet light (UV) as described previously (Kumagai et al., 1998). In some cases, caffeine was added to a final concentration of 5 mM from a 100-mM solution freshly dissolved in 10 mM Pipes-KOH, pH 7.5. To isolate the nuclear fraction, egg extracts were loaded onto buffer S containing 1.3 M sucrose, 100 mM KCl, 2.5 mM MgCl2, and 10 mM Hepes-KOH, pH 7.5, and then centrifuged at 5,000 g for 2 min in a swinging bucket rotor in an Eppendorf centrifuge (model 5417C; Eppendorf Scientific, Inc., Hamburg, Germany). The pellets were resuspended gently in 1 ml of buffer S and recentrifuged at 5,000 g for 2 min. For extracts that had been treated with caffeine, 5 mM of caffeine was included in buffer S.
Kinase Assays
Xchk1 was incubated with full-length His6–Cdc25 or GST–Cdc25(254– 316)-WT in 40 μl of kinase buffer (10 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 mM DTT) containing 2 μCi [γ-32P]ATP and 10 μM ATP.
Binding of 14-3-3ε Protein to Cdc25
Glutathione agarose beads (5 μl) containing either GST–Cdc25-WT, GST–Cdc25-S287A, or no protein were incubated with 0.3 μg His6– Xchk1, 0.3 μg His6–Xchk1-N135A, or neither protein in 50 μl of kinase buffer containing 1 mM cold ATP at 23°C for 30 min under agitation. Next, 1 μg Xenopus His6-14-3-3ε protein (Kumagai et al., 1998), 0.5% NP-40, and 1 mg/ml ovalbumin were added to the mixture, and the incubation was continued for another 30 min at 4°C. Beads were washed four times with 10 mM Hepes-KOH, pH 7.5, containing 500 mM NaCl and 0.5% NP-40 and then washed two times with Hepes-buffered saline (HBS; 10 mM Hepes-KOH, pH 7.5, and 150 mM NaCl). Proteins were eluted from the beads with 5 mM glutathione in HBS.
Production of 35S-labeled Xchk1 Protein
35S-labeled Xchk1 and Xchk1–N135A proteins were synthesized using pBS–Xchk1 and pBS–Xchk1-N135A as templates in the TNT in vitro transcription/translation system (Promega Corp.) in the presence of [35S]Translabel (ICN Biomedicals, Inc., Irvine, CA). pBS–Xchk1-N135A was created by cloning the BspMI-XhoI fragment from pFastBacHTa encoding His6–Xchk1-N135A into pBS–Xchk1 that had been digested with BspMI and XhoI. Reticulocyte lysates containing 35S-labeled proteins were added to egg extracts supplemented with 100 μg/ml of cycloheximide.
Production of Antibodies
Polyclonal rabbit antibodies against the purified His6–Xchk1 protein from insect cells were produced at a commercial facility (Berkeley Antibody Co., Richmond, CA). Antibodies were affinity-purified with His6–Xchk1 that had been coupled to CNBr-activated Sepharose 4B (Pharmacia Biotech, Inc., Piscataway, NJ). Antibodies against Xenopus Cdc25C and 14-3-3ε were described previously (Kumagai et al., 1998).
Depletion of Xchk1 from Egg Extracts
M phase extract (170 μl) was incubated with 10 μg of either affinity-purified Xchk1 antibodies or control rabbit IgG (Zymed Laboratories Inc., South San Francisco, CA) bound to 5 μl of Affiprep protein A beads (Bio-Rad Laboratories, Hercules, CA) at 4°C for 45 min. At the end of the incubation, the beads were removed by centrifugation. The supernatants were treated again with another batch of antibodies under the same conditions to yield double-depleted extracts.
Treatment of Xchk1 with Protein Phosphatase 2A
35S-Labeled Xchk1 was added to egg extract containing 1,000 sperm nuclei/μl, 100 μg/ml aphidicolin, and 100 μg/ml cycloheximide. The nuclear fraction was isolated as described above, dissolved in buffer N (10 mM Hepes-KOH, pH 7.5, 150 mM NaCl, 1% NP-40, 20 mM β-glycerolphosphate, 10 mM MgCl2, 5 mM EGTA, 1 mM orthovanadate, 10 μM phosphoserine, 10 μM phosphothreonine, and 10 μM phosphotyrosine) and centrifuged at 11,700 g for 5 min to remove insoluble proteins. Xchk1 was then immunoprecipitated with anti-Xchk1 antibodies bound to Affiprep protein A beads. Immunoprecipitates were washed once in buffer N containing 1 μM of microcystin, three times in buffer N, and three times in HBS. Immunoprecipitates were treated with 0.2 U protein phosphatase 2A (Upstate Biotechnology, Inc., Lake Placid, NY) for 30 min at 23°C in HBS containing 1 mM DTT and 100 μg/ml of bovine serum albumin.
Assay of Ser-287–specific Kinase Activity in Egg Extracts
Egg extracts were diluted 40-fold in EB buffer (80 mM β-glycerolphosphate, pH 7.3, 15 mM MgCl2, 20 mM EGTA, and 1 mM DTT). 10 μl of diluted extract was incubated with 2 μg of either GST–Cdc25(254–316)-WT or GST–Cdc25(254–316)-S287A in 20 μl of kinase buffer for 10 min at 23°C. Reactions were terminated with gel sample buffer and subjected to SDS-PAGE.
Results
Cloning of a Xenopus Chk1 Homologue (Xchk1)
To clone a Xenopus Chk1 homologue, we used degenerate PCR primers to amplify a segment of its complementary DNA (cDNA). The oligonucleotide primers were designed against homologous regions in the S. pombe Chk1 and Drosophila Grapes protein kinases. PCR amplification yielded an ∼300-bp fragment that was used to isolate a full-length Xenopus oocyte cDNA that encodes a protein with strong homology to the Chk1 family of protein kinases (EMBL/GenBank/DDBJ accession number AF053120). The open reading frame, which is preceded by two in-frame termination codons, encodes a 474-amino acid polypeptide (Xchk1) with a predicted M r of 54 kD (Fig. 1). Xchk1 shares significant sequence identity with S. pombe Chk1 (28% identical residues) (Walworth et al., 1993; al-Khodairy et al., 1994), Drosophila Grapes (45% identical) (Fogarty et al., 1997; Sibon et al., 1997), and a human Chk1 homologue (78% identical) (Flaggs et al., 1997; Sanchez et al., 1997). The kinase domain of Xchk1, which resides in the NH2-terminal half of the molecule, is the most similar among species, but there are also several blocks of highly conserved sequences in its COOH-terminal region.
Figure 1.

Sequence alignment of Xenopus Chk1 (Xchk1), human Chk1, Drosophila Grapes, and S. pombe Chk1. Sequences were aligned using the Prettyplot program. Identical residues are boxed. Asterisk, conserved asparagine (N135) residue that is required for kinase activity; underline, sequences that were used to design degenerate PCR primers. These sequence data are available from GenBank/EMBL/DDBJ under accession number AF053120.
Xchk1 Phosphorylates Cdc25 on Ser-287 and Mediates 14-3-3 Binding
To study the biochemistry of Xchk1, we expressed a histidine-tagged version (His6–Xchk1) in baculovirus-infected Sf9 insect cells. We also produced a kinase-negative form of Xchk1 with a mutation in a catalytically essential residue (His6–Xchk1-N135A). His6–Xchk1 and His6–Xchk1-N135A could be purified to near homogeneity by chromatography on nickel agarose (Fig. 2 A). Recombinant His6–Xchk1 phosphorylates Xenopus Cdc25 efficiently in vitro (Fig. 2 B, lane 1). Thus, it appears that His6–Xchk1 isolated from baculovirus-infected Sf9 insect cells is catalytically active, even though these cells were not treated with replication inhibitors or DNA-damaging agents. Under these conditions, His6–Xchk1 also phosphorylates the S287A mutant of Cdc25 in which the critical serine in its 14-3-3 binding site has been changed to alanine (Fig. 2 B, lane 3). In contrast, the kinase-negative His6–Xchk1-N135A protein could not use either the wild-type or S287A form of Cdc25 as a substrate. To characterize the Xchk1-catalyzed phosphorylation of Cdc25 in greater detail, we isolated the 32P-labeled wild-type and S287A forms of Cdc25 and subjected them to tryptic phosphopeptide analysis (Fig. 2 C). These mapping studies indicated that His6–Xchk1 phosphorylates wild-type Cdc25 on multiple sites in vitro. One of these sites corresponds to Ser-287, because a single spot is missing from the tryptic phosphopeptide map of the 32P-labeled Cdc25-S287A mutant. In other experiments, we showed that His6–Xchk1 phosphorylates a GST fusion protein containing a small fragment of Cdc25 that includes the 14-3-3 binding site (GST–Cdc25[254-316]-WT) (Fig. 2 D, lane 1). In contrast, His6–Xchk1 did not phosphorylate a mutant form of this peptide containing alanine instead of serine at position 287 (GST–Cdc25[254-316]-S287A) (Fig. 2 D, lane 2).
Figure 2.
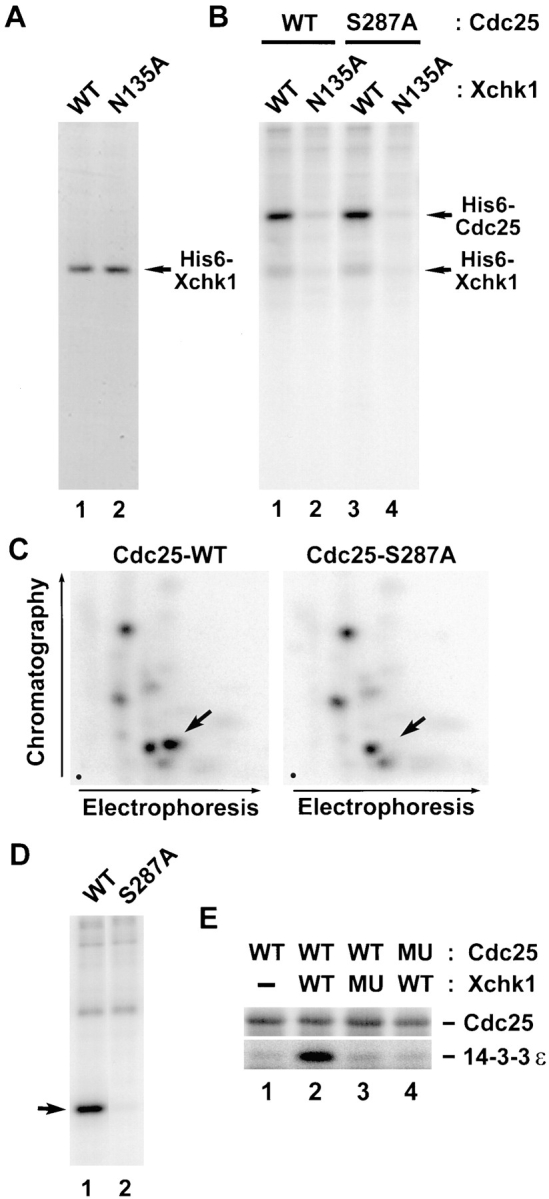
Xchk1 phosphorylates Cdc25 on Ser-287 and mediates binding of 14-3-3. (A) Recombinant His6–Xchk1 (lane 1) and His6–Xchk1-N135A (lane 2) were purified from baculovirus-infected insect cells, subjected to SDS-PAGE, and then stained with Coomassie blue. (B) Xchk1 phosphorylates Cdc25 efficiently in vitro. His6–Xchk1 (lanes 1 and 3) and His6–Xchk1-N135A (lanes 2 and 4) were incubated with either His6–Cdc25-WT (lanes 1 and 2) or His6–Cdc25-S287A (lanes 3 and 4) in kinase buffer containing 10 μM [γ-32P]ATP. Reactions were terminated with gel sample buffer and subjected to SDS-PAGE and autoradiography. (C) Xchk1 phosphorylates Cdc25 at Ser-287 and other sites. His6–Cdc25-WT and His6–Cdc25-S287A that had been incubated with His6–Xchk1 in the presence of 10 μM [γ-32P]ATP were processed for tryptic phosphopeptide mapping. Arrows, position of the peptide containing Ser-287; dots, origins in the lower left corner of each map. (D) Specific phosphorylation by His6– Xchk1 of a fragment of Cdc25 containing the 14-3-3 binding site. GST–Cdc25(254–316)-WT (lane 1) or GST–Cdc25(254–316)- S287A (lane 2) were incubated with His6–Xchk1 in the presence of [γ-32P]ATP and subjected to SDS-PAGE and autoradiography. Arrow, position of the wild-type and mutant GST fusion proteins. (E) Phosphorylation of Cdc25 by Xchk1 creates a 14-3-3 binding site. Bacterially-expressed GST–Cdc25-WT (lanes 1–3) and GST–Cdc25-S287A (lane 4) bound to glutathione agarose were incubated with His6–Xchk1 (lanes 2 and 4), His6–Xchk1-N135A (lane 3), or neither protein (lane 1) in kinase buffer containing 1 mM ATP at 23°C for 30 min. His6–14-3-3ε was then added to the mixture, and the incubation was continued at 4°C for an additional 30 min. At the end of the incubation, proteins specifically bound to the beads were eluted with glutathione and subjected to SDS-PAGE and immunoblotting with either anti-Cdc25 antibodies (top) or anti–14-3-3ε antibodies (bottom).
Previous studies have indicated that the phosphorylation of Cdc25C on Ser-216 and Ser-287 from humans and Xenopus, respectively, is necessary for the binding of 14-3-3 proteins (Peng et al., 1997; Kumagai et al., 1998). To ask whether the Xchk1-catalyzed phosphorylation of Xenopus Cdc25 can mediate 14-3-3 binding, we treated bacterially-expressed GST–Cdc25 and GST–Cdc25-S287A (both bound to glutathione agarose beads) with His6–Xchk1 and then added recombinant His6–14-3-3ε protein to the reaction. After reisolating the beads and eluting bound proteins with glutathione, we found that His6–14-3-3ε could associate specifically with GST–Cdc25 only if GST–Cdc25 had been phosphorylated previously by His6–Xchk1 (Fig. 2 E, lanes 1–3). In contrast, His6–14-3-3ε did not bind specifically to the GST–Cdc25-S287A mutant that had been treated with His6–Xchk1 (Fig. 2 E, lane 4), even though this incubation led to substantial phosphorylation of GST– Cdc25-S287A on sites distinct from Ser-287 (Fig. 2, B and C). Treatment of GST–Cdc25 with the kinase-negative His6–Xchk1-N135A mutant did not lead to binding of His6–14-3-3ε (Fig. 2 E, lane 3). In conclusion, the phosphorylation of Xenopus Cdc25 on Ser-287 by Xchk1 is sufficient for the binding of 14-3-3 proteins.
Immunodepletion of Xchk1 from Egg Extracts Reveals Xchk1-dependent and Xchk1-independent Checkpoint Control Pathways
To investigate the role of Xchk1 in checkpoint control, we set out to remove this protein from Xenopus egg extracts by immunodepletion. For this purpose, we used recombinant His6–Xchk1 to generate polyclonal antibodies that recognize a 54-kD polypeptide in immunoblots of Xenopus egg extracts (Fig. 3 A, lane 1). By using recombinant His6–Xchk1 as a standard, we estimate that endogenous Xchk1 is present at a concentration of 2 ng/μl (40 nM) in egg extracts. By comparison, the concentration of Cdc25C in these extracts is 10 ng/μl (140 nM) (Kumagai and Dunphy, 1992). By subjecting egg extracts to two rounds of immunodepletion with anti-Xchk1 antibodies bound to protein A beads, we were able to remove this protein quantitatively from egg extracts, as determined by immunoblotting with anti-Xchk1 antibodies (Fig. 3 A, lane 3). For comparison, no Xchk1 was removed by a mock immunodepletion with control antibodies (Fig. 3 A, lanes 1 and 2). Xchk1 could be restored to Xchk1-depleted extracts at close to the physiological level by the addition of recombinant His6–Xchk1 (Fig. 3 A, lane 4).
Figure 3.
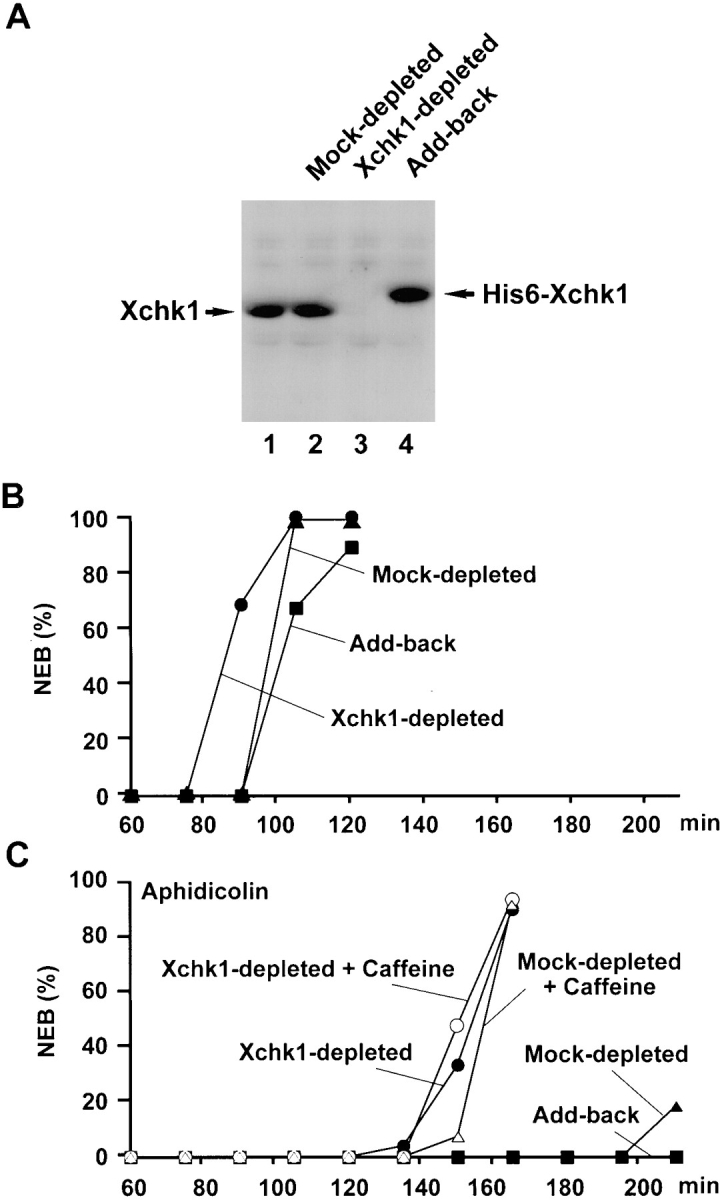
Immunodepletion of Xchk1 compromises checkpoint control in Xenopus egg extracts. (A) Immunodepletion of Xchk1 from Xenopus egg extracts. An M phase extract (lane 1) was treated with anti-Xchk1 antibodies (lanes 3 and 4) or control antibodies (lane 2) bound to protein A beads as described in Materials and Methods. In lane 4, His6–Xchk1 protein was added back to the Xchk1-depleted extract to a final concentration of 2 ng/μl. Samples (lanes 1–4 ) were processed for immunoblotting with anti-Xchk1 antibodies. (B) Effect of immunodepletion of Xchk1 on egg extracts lacking unreplicated DNA. M phase extracts were treated with anti-Xchk1 antibodies (•, ▪) or control antibodies (▴). In one set of samples (▪), His6–Xchk1 was added back to a concentration of 2 ng/μl. The extracts were activated with calcium, and the timing of NEB (nuclear envelope breakdown) was monitored by microscopy. (C) Effect of immunodepletion of Xchk1 on egg extracts containing unreplicated DNA. Extracts that had been treated with either anti-Xchk1 antibodies (•, ○, ▪) or control antibodies (▴, ▵) were incubated with 1,000 sperm nuclei/μl and 100 μg/ml aphidicolin in the presence (○, ▵) or absence (•, ▴, ▪) of 5 mM caffeine. In one case (▪), 2 ng/μl His6– Xchk1 was added back to the Xchk1-depleted extract.
Next, we compared cell cycle progression and checkpoint function in Xchk1-depleted extracts and mock-depleted control extracts. Typically, egg extracts in which a checkpoint has not been triggered enter mitosis between 75 and 105 min after the activation of the extract with calcium (Fig. 3 B). However, extracts containing both the replication inhibitor aphidicolin and a sufficient concentration of Xenopus sperm chromatin (>1,000 demembranated sperm nuclei/μl of egg extract) cannot enter mitosis for more than 3 h (Fig. 3 C). We observed that Xchk1-depleted extracts treated with aphidicolin entered mitosis substantially earlier (half-maximal nuclear envelope breakdown at ∼150 min) than mock-depleted extracts containing aphidicolin. Nonetheless, Xchk1-depleted extracts treated with aphidicolin were significantly delayed in mitotic entry in comparison with Xchk1-depleted extracts that had not been treated with aphidicolin (Fig. 3, B and C). Thus, it appears that the presence of unreplicated DNA can impede the entry into mitosis to some extent in Xchk1-depleted extracts but not nearly as efficiently as in mock-depleted extracts that contain Xchk1. Significantly, when His6–Xchk1 was added back to the Xchk1-depleted extracts, treatment with aphidicolin delayed mitotic entry as effectively as in mock-depleted extracts (Fig. 3 C). This observation indicates that the compromised checkpoint control in Xchk1-depleted extracts is due to the absence of Xchk1 and not to some non-specific effect of the immunodepletion procedure. Finally, Xchk1-depleted extracts lacking aphidicolin entered mitosis ∼15 min earlier than mock-depleted extracts or Xchk1-depleted extracts containing exogenously added His6–Xchk1 (Fig. 3 B). Interestingly, Xchk1 immunoprecipitated with anti-Xchk1 antibodies from interphase egg extracts lacking aphidicolin phosphorylates His6–Cdc25 or GST–Cdc25(254–316) as efficiently as baculovirus-expressed His6–Xchk1 from Sf9 insect cells (data not shown). Thus, Xchk1 may contribute to mitotic timing to some extent in the apparent absence of a checkpoint response.
Caffeine Disrupts the Checkpoint Control Pathway That Is Dependent upon Xchk1
Caffeine has been reported to override the replication and damage checkpoints in various experimental systems, but its mechanism of action and molecular target(s) have been unknown (Schlegel and Pardee, 1986; Dasso and Newport, 1990; Kumagai and Dunphy, 1995; Poon et al., 1997). In Xenopus egg extracts containing unreplicated or damaged DNA, the inclusion of caffeine overrides the checkpoint-mediated delay of mitosis. As shown in Fig. 3 C, caffeine accelerates mitotic entry substantially in mock-depleted extracts containing aphidicolin. Nonetheless, these caffeine-treated extracts enter mitosis at a slower rate than extracts that lack unreplicated or damaged DNA (Fig. 3 C; see also Fig. 2 in Kumagai and Dunphy, 1995). However, the presence of caffeine did not affect mitotic timing in Xchk1-depleted extracts containing aphidicolin (Fig. 3 C). In particular, Xchk1-depleted extracts containing aphidicolin underwent nuclear envelope breakdown at the same time in the presence and absence of caffeine. Taken together, these results indicate that the checkpoint-mediated delay of mitotic entry involves collaborating Xchk1-dependent and Xchk1-independent pathways. The Xchk1-dependent pathway is sensitive to caffeine, whereas the Xchk1-independent pathway is unaffected by this agent.
14-3-3 Remains Bound to Cdc25 in Xchk1-depleted Extracts
Since in vitro phosphorylation of Cdc25 on Ser-287 by Xchk1 is sufficient for the binding of 14-3-3 proteins, we asked whether 14-3-3 could bind to Cdc25 in extracts lacking Xchk1 (Fig. 4 A). For this purpose, we subjected Xchk1-depleted and mock-depleted egg extracts to immunoprecipitation with antibodies against Xenopus 14-3-3ε and then immunoblotted the resulting immunoprecipitates with either anti-Cdc25 antibodies or anti–14-3-3ε antibodies (to monitor the recovery of 14-3-3ε). In particular, M phase extracts were treated with anti-Xchk1 or control antibodies, driven into interphase by activation with calcium, and then subjected to immunoprecipitation with anti–14-3-3ε antibodies. For this experiment, we chose to immunodeplete Xchk1 from M phase egg extracts because 14-3-3 proteins are not bound to Cdc25 at M phase (Peng et al., 1997; Kumagai et al., 1998). We observed that the amount of Cdc25 bound to 14-3-3ε in Xchk1-depleted extracts that had been triggered to enter interphase was similar to that bound in either mock-depleted extracts or Xchk1-depleted extracts to which His6–Xchk1 had been added back (Fig. 4 A, lanes 1–3). With the same batch of egg extract, we confirmed that 14-3-3ε binds to Cdc25 during interphase but not M phase and that the binding during interphase is similar in the presence and absence of unreplicated or damaged DNA (Fig. 4 A, lanes 4–7; Kumagai et al., 1998).
Figure 4.
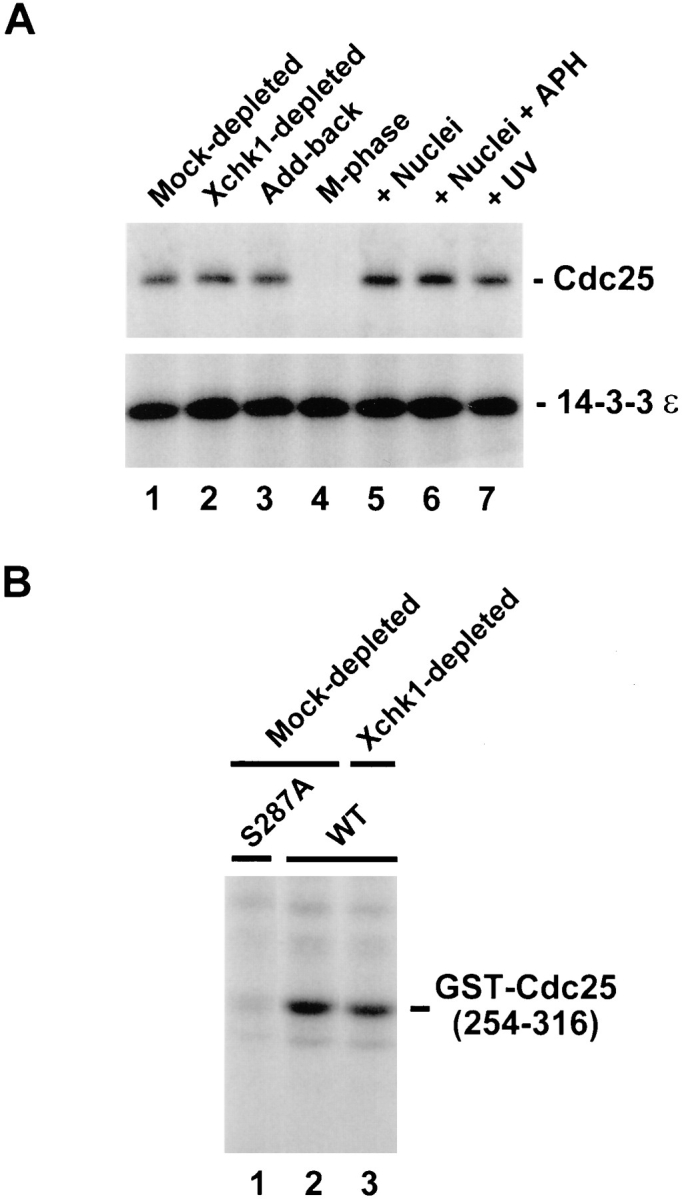
Cdc25 undergoes phosphorylation of Ser-287 and binds to 14-3-3ε in Xchk1-depleted extracts. (A) The 14-3-3ε protein binds to Cdc25 in Xchk1-depleted extracts. The 14-3-3ε protein was immunoprecipitated from a mock-depleted extract (lane 1), an Xchk1-depleted extract (lane 2), or an Xchk1-depleted extract supplemented with 2 ng/μl His6–Xchk1 protein (lane 3). For lanes 4–7, an M phase extract (lane 4) or interphase extracts (lanes 5–7) containing 1,000 sperm nuclei/μl (lane 5), 3,000 sperm nuclei/μl and 100 μg/ml aphidicolin (lane 6), or 3,000 UV-damaged sperm nuclei per μl (lane 7) were immunoprecipitated with anti–14-3-3ε antibodies. All extracts contained 100 μg/ml cycloheximide. In all cases, the anti–14-3-3ε immunoprecipitates were subjected to SDS-PAGE and immunoblotting with anti-Cdc25 (top) or anti–14-3-3ε (bottom) antibodies. APH, aphidicolin. (B) Mock-depleted extracts (lanes 1 and 2) and Xchk1-depleted extracts (lane 3) containing 1,000 sperm nuclei/μl were assayed for Ser-287–specific kinase activity using either GST–Cdc25(254– 316)-WT (lanes 2 and 3) or GST–Cdc25(254–316)-S287A (lane 1) as the substrate.
These findings suggest that a kinase distinct from Xchk1 can phosphorylate Ser-287 on Cdc25 in Xchk1-depleted extracts and thereby create a 14-3-3 binding site within Cdc25. To address this possibility more directly, we used the GST–Cdc25(254–316)-WT substrate to measure total Ser-287–specific kinase activity in both mock-depleted and Xchk1-depleted interphase extracts (Fig. 4 B). We observed that GST–Cdc25(254–316)-WT became phosphorylated quite efficiently in the Xchk1-depleted extract (Fig. 4 B, lane 3). The level of phosphorylation was ∼80% of that observed in a mock-depleted extract, as quantitated with a phosphorimager (Fig. 4 B, lane 2). This assay appears to detect phosphorylation of Ser-287 specifically because the GST–Cdc25(254–316)-S287A mutant was not radiolabeled under these conditions (Fig. 4 B, lane 1).
Exogenous Addition of Active Xchk1 Delays Mitosis in Egg Extracts in a Caffeine-resistant Manner
As another approach to assess the role of Xchk1 in mitotic timing, we added extra Xchk1 protein exogenously to cycling Xenopus egg extracts (Fig. 5). For this experiment, we added active His6–Xchk1 or the catalytically-inactive His6–Xchk1-N135A mutant to egg extracts at a final concentration of 10 ng/μl. Since the endogenous concentration of Xchk1 in these extracts is 2 ng/μl, this procedure leads to a sixfold increase in the level of Xchk1. We observed that the wild-type His6–Xchk1 protein elicited a substantial 60-min delay of mitosis (Fig. 5). In contrast, the kinase-negative His6–Xchk1-N135A protein had no effect on the timing of nuclear envelope breakdown (Fig. 5). Parenthetically, kinase-negative His6–Xchk1-N135A also had no effect on the cell cycle delay in aphidicolin-treated extracts, indicating that this mutant protein does not act as a dominant-negative competitor of checkpoint regulatory factors under these conditions (data not shown).
Figure 5.
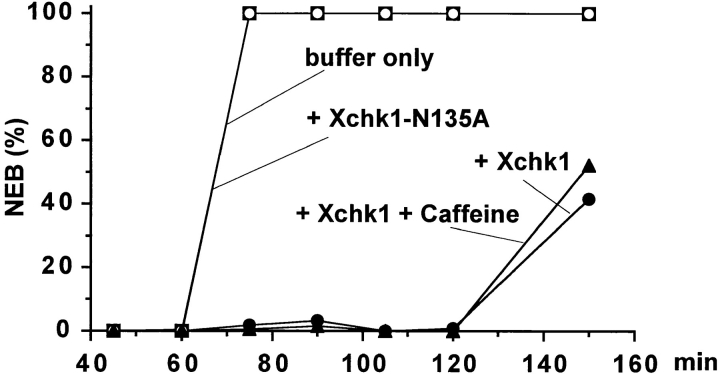
Exogenously added His6–Xchk1 delays mitosis in egg extracts in a caffeine-resistant manner. (A) His6–Xchk1 (•, ▴), His6–Xchk1-N135A (▪), or buffer only (○) was added to egg extracts containing 1,000 sperm nuclei/μl in the presence (▴) or absence (•, ▪, ○) of 5 mM caffeine. The timing of NEB was monitored by microscopy. Recombinant Xchk1 proteins were added to a final concentration of 10 ng/μl. Typically, caffeine did not affect mitotic timing significantly in the extracts containing buffer only or His6–Xchk1-N135A.
As described above, Xchk1-mediated checkpoint control is sensitive to caffeine. Therefore, we asked whether the delay of mitosis elicited by exogenously added His6– Xchk1 would be affected by this agent. As shown in Fig. 5, the presence of caffeine did not reverse the inhibitory effect of exogenously added His6–Xchk1 on the timing of mitosis. In similar experiments, treatment with caffeine did accelerate the entry into mitosis in aphidicolin-treated extracts containing only endogenous Xchk1 (refer to Fig. 3 C). These results suggest that caffeine does not directly inhibit the kinase activity of Xchk1. To address this issue more directly, we assayed the kinase activity of His6– Xchk1 toward recombinant Cdc25 in the presence and absence of 5 mM of caffeine. These assays were performed in the presence of 1 mM ATP, which is the endogenous concentration of ATP in egg extracts. We observed that caffeine had no effect on either the phosphorylation of Cdc25 by Xchk1 or the in vitro autophosphorylation of Xchk1 (data not shown). Collectively, our results suggest that caffeine does not directly inhibit Xchk1 or its downstream effects but instead compromises some process upstream of Xchk1 that is required for proper checkpoint regulation.
Caffeine Blocks the Checkpoint-dependent Phosphorylation of Xchk1
To further explore the functional properties of Xchk1, we examined the modification of this protein in the presence and absence of unreplicated or damaged DNA. For these experiments, we first prepared 35S-labeled Xchk1 protein and added it to egg extracts containing either aphidicolin or UV-damaged sperm chromatin. We observed that a portion of the labeled Xchk1 protein underwent an obvious modification in extracts containing unreplicated or damaged DNA, as evidenced by a decrease of electrophoretic mobility in SDS gels (Fig. 6 A, top, lanes 2 and 4). Similar results were obtained by immunoblotting the endogenous Xchk1 in egg extracts with anti-Xchk1 antibodies (data not shown). This modification appears to be phosphorylation, since it is reversed by treatment with protein phosphatase 2A (Fig. 6 B). Typically, we observed two closely spaced phosphorylated bands with reduced electrophoretic mobility in comparison with the major 54-kD Xchk1 band that was found in extracts lacking damaged or unreplicated DNA (Fig. 6 A, top, lane 1). When the nuclear fraction was isolated from these extracts, a higher proportion of the nuclear Xchk1 protein appeared to be modified in comparison with Xchk1 in the whole egg extract (Fig. 6 A, bottom, lanes 2 and 4). Significantly, caffeine completely abolished the checkpoint-associated phosphorylation of Xchk1 in the presence of either unreplicated or damaged DNA (Fig 6 A, top and bottom, lanes 3 and 5). This observation also argues that caffeine modulates the regulatory pathway containing Xchk1. Since only a portion of Xchk1 is hyperphosphorylated in response to unreplicated or damaged DNA and this hyperphosphorylated form proved to be difficult to isolate by immunoprecipitation, it is unclear at this time whether this phosphorylation directly alters the kinase activity of Xchk1.
Figure 6.
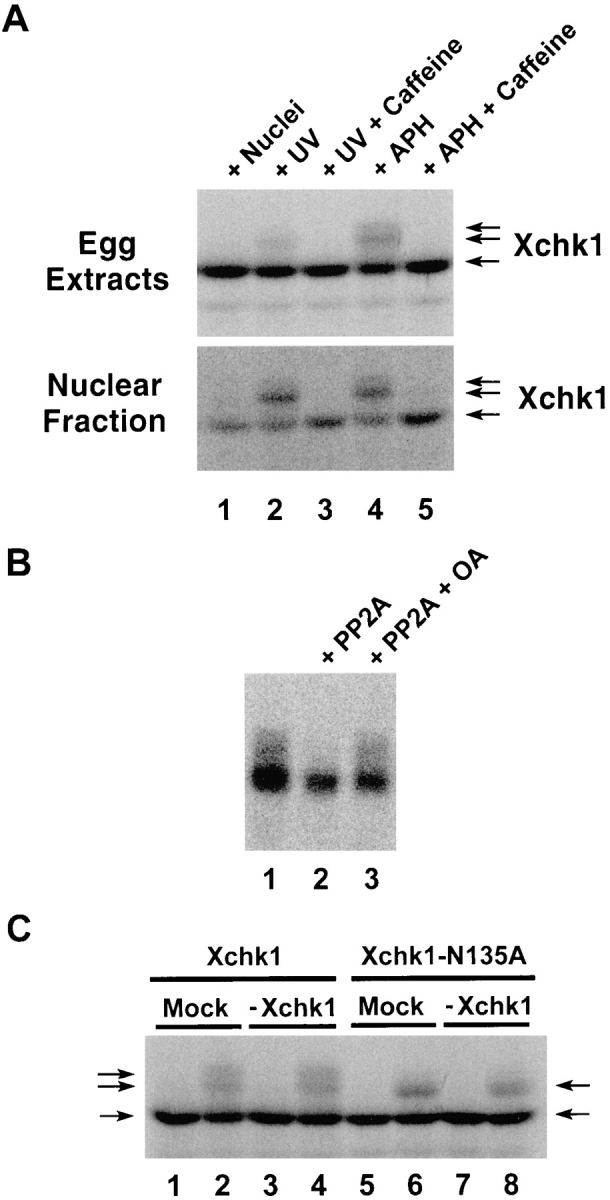
Xchk1 is modified when the DNA replication and DNA damage checkpoints are activated. (A) 35S-labeled Xchk1 was incubated in interphase egg extracts containing 3,000 sperm nuclei/μl (lane 1), 3,000 UV-damaged sperm nuclei/μl (lanes 2 and 3), or 3,000 sperm nuclei/μl and 100 μg/ml aphidicolin (lanes 4 and 5) in the presence (lanes 3 and 5) or absence (lanes 1, 2, and 4) of 5 mM caffeine. After 100 min of incubation at 23°C, 2 μl of each extract was taken for SDS-PAGE and autoradiography (top). Alternatively, 50 μl of each extract was centrifuged through a sucrose solution to isolate the nuclear fraction (bottom), which was also subjected to SDS-PAGE and autoradiography. APH, aphidicolin. (B) 35S-labeled Xchk1 was immunoprecipitated from interphase extracts containing 3,000 sperm nuclei/ μl and 100 μg/ml aphidicolin. The immunoprecipitates were incubated in phosphatase buffer containing no addition (lane 1), protein phosphatase 2A (lane 2), or protein phosphatase 2A and 3 μM okadaic acid (lane 3). PP2A, protein phosphatase 2A; OA, okadaic acid. (C) 35S-labeled Xchk1 (lanes 1–4) and Xchk1– N135A (lanes 5–8) were incubated for 100 min in either control-depleted (lanes 1, 2, 5, and 6) or Xchk1-depleted (lanes 3, 4, 7, and 8) extracts in the presence of either 3,000 sperm nuclei/μl (lanes 1, 3, 5, and 7) or 3,000 UV-damaged sperm nuclei/μl (lanes 2, 4, 6, and 8). Nuclear fractions were isolated as described above and subjected to SDS-PAGE and autoradiography.
To characterize further the phosphorylation of Xchk1, we asked whether it might be due to autophosphorylation or to the action of a distinct kinase. For these studies, we added 35S-labeled Xchk1 or the kinase-negative Xchk1– N135A mutant to either Xchk1-depleted or mock-depleted egg extracts that contained either UV-damaged or undamaged sperm chromatin (Fig. 6 C). As expected, the labeled Xchk1 protein underwent phosphorylation (upshift in SDS gels) in the extract containing damaged DNA but not in one containing undamaged DNA (Fig. 6 C). Similar results were obtained in extracts from which the endogenous Xchk1 protein had been removed by immunodepletion with anti-Xchk1 antibodies (Fig. 6 C, lanes 3 and 4). In extracts containing the 35S-labeled kinase-negative Xchk1–N135A protein, we observed somewhat different results. Although Xchk1–N135A was clearly modified in the presence of damaged DNA, the extent of the modification was reduced. In particular, the most highly phosphorylated form of Xchk1 was absent (Fig. 6 C, lanes 6 and 8). Since this partially phosphorylated form of Xchk1– N135A was also found in Xchk1-depleted extracts, this phosphorylation is presumably due to the action of a distinct kinase rather than autophosphorylation. However, since production of the most highly phosphorylated form of Xchk1 does depend upon the presence of catalytically active Xchk1, a portion of the checkpoint-induced modification of Xchk1 may represent autophosphorylation.
Discussion
In this report, we have analyzed the biochemical properties of the Xenopus Chk1 protein kinase (Xchk1) and its role in checkpoint control in Xenopus egg extracts. Recombinant Xchk1 phosphorylates the mitotic regulatory phosphatase Cdc25 on the critical serine (residue 287) in its 14-3-3 binding motif as well as some additional undefined sites. The Xchk1-catalyzed phosphorylation of Cdc25 on Ser-287 confers upon Cdc25 the ability to bind 14-3-3 proteins. In particular, Cdc25 that has been phosphorylated by Xchk1 in vitro binds efficiently to a recombinant form of 14-3-3ε, which is the major physiological partner of Cdc25 during interphase in Xenopus egg extracts (Kumagai et al., 1998). As described previously, the binding of 14-3-3 proteins correlates with negative regulation of the mitosis-inducing function of Cdc25C (Peng et al., 1997; Kumagai et al., 1998).
Various observations suggest that Xchk1 plays a significant role in the control of mitotic timing in Xenopus egg extracts. First, the addition of recombinant Xchk1 to egg extracts results in a strong inhibition of mitotic entry, whereas a kinase-negative form of Xchk1 has no effect. Secondly, immunodepletion of Xchk1 strongly compromises the ability of egg extracts to undergo a cell cycle arrest in response to the presence of unreplicated DNA. These two effects are consistent with a physiological role for Xchk1 in negatively regulating Cdc25. Finally, Xchk1 undergoes a marked phosphorylation in the presence of unreplicated or damaged DNA. This phosphorylation is abolished by caffeine under conditions in which this agent overrides checkpoint controls.
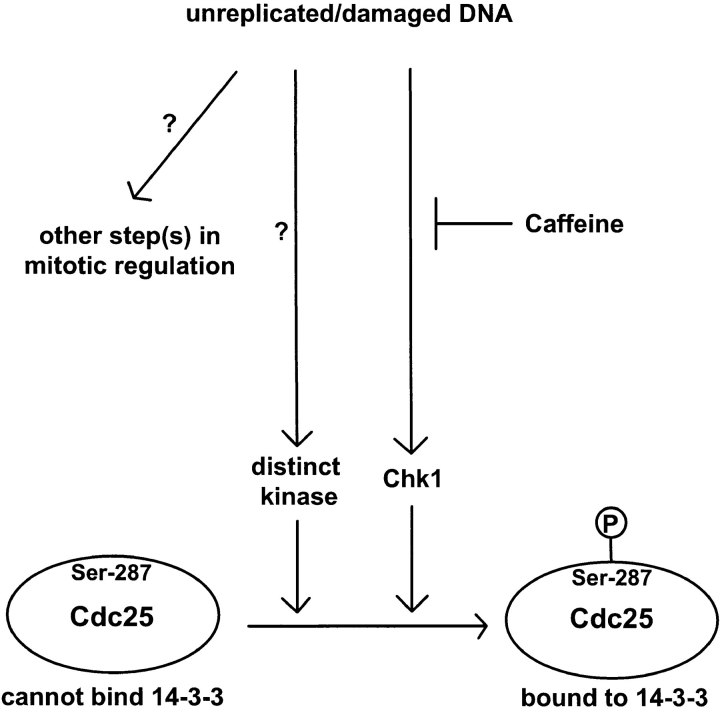
Overall, our results indicate that Xenopus egg extracts possess checkpoint regulatory molecules similar to those present in yeast and mammals (Carr, 1996; Nurse, 1997; Rhind et al., 1997; Saka et al., 1997; Sanchez et al., 1997; Weinert, 1997; Westphal, 1997). By analyzing Xchk1 and Cdc25 in egg extracts, we have learned about the complexity of the pathway that controls Cdc25 (Fig. 7). For example, Cdc25 can still associate with 14-3-3 proteins in extracts from which Xchk1 has been removed by immunodepletion, indicating that a kinase distinct from Xchk1 can phosphorylate Ser-287 of Cdc25. The identity of this kinase is presently unknown. A human kinase called C-TAK1 (Cdc twenty-five C–associated protein kinase 1) can phosphorylate Cdc25C on its 14-3-3 binding site in vitro, but the role of this enzyme in Cdc25C regulation in vivo has not been established (Peng et al., 1998). Similarly, it has been shown that the fission yeast kinase Cds1 overlaps functionally with Chk1 (Boddy et al., 1998). We have also found the pathway containing Xchk1, although it plays a major role in the checkpoint arrest in response to unreplicated/damaged DNA, does not appear to be fully responsible. In particular, Xchk1-depleted extracts containing unreplicated DNA, although strongly compromised in their checkpoint response, nonetheless enter mitosis at a significantly delayed rate relative to control extracts. This observation suggests that the checkpoint arrest involves both Xchk1-dependent and Xchk1-independent pathways, which appear to collaborate in the control of mitotic timing.
Figure 7.
Model for checkpoint regulation in Xenopus egg extracts. Refer to text for details.
Caffeine has been a useful tool in discerning the existence of these collaborating pathways (Schlegel and Pardee, 1986; Dasso and Newport, 1990; Poon et al., 1997). Furthermore, our studies have provided the first molecular insight into how this simple compound disrupts checkpoint regulation. Caffeine overrides the unreplicated/damaged DNA checkpoint in Xenopus extracts, but nonetheless caffeine-treated extracts enter mitosis more slowly than control extracts in which a checkpoint has not been triggered. This observation could mean that caffeine is not fully effective at inhibiting its target(s) or that the checkpoint response involves multiple interacting pathways, not all of which are sensitive to caffeine. Our results favor the latter possibility because the attenuated cell cycle delay observed in Xchk1-depleted extracts containing unreplicated or damaged DNA is completely insensitive to caffeine. In contrast, extracts containing the normal amount of endogenous Xchk1 and unreplicated DNA enter mitosis at a considerably accelerated pace in the presence of caffeine. This observation, coupled with the fact that caffeine completely abolishes the checkpoint-dependent phosphorylation of Xchk1, argues that the Xchk1-dependent pathway in Xenopus egg extracts is caffeine sensitive. Caffeine appears not to directly inhibit Xchk1, since it does not block the phosphorylation of Cdc25 by recombinant Xchk1 in vitro at a physiological concentration of ATP. Also, caffeine does not override the delay of mitosis in egg extracts in response to the exogenous addition of active recombinant His6–Xchk1. Our results suggest that caffeine acts at some point upstream of Xchk1. This view is supported by the observation that the checkpoint-associated phosphorylation of Xchk1 is blocked entirely by caffeine.
Much remains to be learned about the biochemical details of how Xchk1 inhibits mitosis when unreplicated DNA is present. Earlier studies have indicated that mutation of the 14-3-3 binding site in Cdc25C strongly compromises G2 checkpoint control (Peng et al., 1997; Kumagai et al., 1998). For example, Xenopus egg extracts containing unreplicated or damaged DNA and the Cdc25–S287A mutant that lacks a 14-3-3 binding site enter mitosis at approximately the same time as control extracts in which a checkpoint has not been triggered (Kumagai et al., 1998). This observation indicates that the checkpoint response is strongly compromised when 14-3-3 proteins cannot bind to Cdc25, although it is not known whether checkpoint control is totally abolished under these circumstances. In Xchk1-depleted extracts, the checkpoint defect is less severe, most probably at least in part because another kinase in egg extracts can also phosphorylate Ser-287 of Cdc25.
Intriguingly, the amount of 14-3-3 bound to Cdc25 is the same in the absence and presence of unreplicated/damaged DNA (Kumagai et al., 1998). Furthermore, as shown here, 14-3-3 proteins remain bound to Cdc25 in Xchk1-depleted extracts, despite the fact that checkpoint control is clearly disrupted in such extracts. These observations might appear to be inconsistent with the concept that binding of 14-3-3 to Cdc25 causes extracts containing unreplicated/damaged DNA to arrest in interphase. The affinity of 14-3-3 for a target with a single binding site should allow dynamic binding and dissociation of these molecules (Yaffe et al., 1997). Thus, it will be critical to determine the rate of phosphorylation and dephosphorylation of Ser-287 on Cdc25 in the absence and presence of unreplicated/ damaged DNA. On the other hand, it seems possible that, although binding of 14-3-3 to Cdc25 may be important for proper checkpoint control, this interaction may not be the whole explanation for checkpoint regulation. In this regard, it is intriguing that Xchk1 phosphorylates Cdc25 in vitro at sites that apparently do not confer 14-3-3 binding. It will be important to determine whether these phosphorylations occur at sites on Cdc25 that are phosphorylated in vivo and, if so, to determine their role in the regulation of Cdc25. In addition to the possibility that Xchk1 could regulate Cdc25 by phosphorylating sites distinct from Ser-287, Xchk1 could possess an additional target(s) besides Cdc25. For example, O'Connell et al. (1997) have proposed that Wee1 is a target of Chk1 in fission yeast.
Xchk1 undergoes a marked phosphorylation in the presence of either unreplicated or damaged DNA. This finding could mean that Xchk1 responds to both the replication and damage checkpoints in Xenopus egg extracts. However, it is possible that some of the DNA in aphidicolin-treated nuclei may undergo damage. Likewise, it is quite likely that sites of damage in UV-treated nuclei would impede the progression of replication forks. Nonetheless, the phosphorylation of Xchk1 correlates well with the cell cycle delay that is imposed when unreplicated or damaged DNA is present. In principle, the checkpoint-dependent phosphorylation of Xchk1 could elicit increased kinase activity toward Cdc25, but technical difficulties in isolating Xchk1 in its fully phosphorylated form have thus far precluded an answer to this question. Alternatively, this phosphorylation could potentiate the action of Xchk1 by some other mechanism such as controlling its intracellular localization or protein–protein interactions. Finally, it is possible that the hyperphosphorylation of Xchk1 is not causally responsible for Xchk1-mediated cell cycle delay.
The checkpoint-specific phosphorylation of Xchk1 appears to involve both autophosphorylation and the action of a distinct kinase. The kinase-negative Xchk1–N135A mutant clearly becomes phosphorylated in Xchk1-depleted extracts containing unreplicated or damaged DNA, indicating that this phosphorylation cannot be due to either inter- or intramolecular autophosphorylation. However, the wild-type Xchk1 protein is more extensively phosphorylated in the presence of unreplicated or damaged DNA in both control and Xchk1-depleted extracts, suggesting that autophosphorylation may also be involved. The kinase(s) that phosphorylates Xchk1 is unknown, but studies in fission yeast have implicated the protein kinase Rad3 in the regulation of Chk1 (al-Khodairy et al., 1994; Bentley et al., 1996; Walworth and Bernards, 1996). Rad3 is a member of the DNA-dependent protein kinase family with homologues known as ATM and ATR in mammals (Paulovich and Hartwell, 1995; Keegan et al., 1996; Carr, 1997; Flaggs et al., 1997; Westphal, 1997; Cliby et al., 1998). Xenopus homologues of ATM and ATR have not been described, but these proteins could potentially be involved either directly or indirectly in the checkpoint-associated phosphorylation of Xchk1.
In conclusion, we have shown that Xchk1 phosphorylates Ser-287 of Xenopus Cdc25, thereby allowing 14-3-3 proteins to associate with this activator of Cdc2. Xchk1 appears to play an important role in mitotic timing in egg extracts: Xchk1-depleted extracts are compromised in their checkpoint responsiveness and exogenous addition of active Xchk1 can delay mitosis substantially even in the absence of unreplicated or damaged DNA. Nonetheless, Xchk1 does not account fully for the phosphorylation of Cdc25 on Ser-287, since binding of 14-3-3 to Cdc25 is not diminished in Xchk1-depleted extracts. The checkpoint response of egg extracts to unreplicated DNA appears to involve both Xchk1-dependent (caffeine-sensitive) and Xchk1-independent (caffeine-insensitive) pathways. The nature of the caffeine-insensitive, Xchk1-independent pathway remains to be determined. In principle, it could involve either the Ser-287–specific kinase that is distinct from Xchk1 or some other facet of Cdc2 regulation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-43974) and a postdoctoral fellowship from the Damon Runyon-Walter Winchell Cancer Research Fund to S.X. Wang. W.G. Dunphy is an investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper
- GST
glutathione-S-transferase
- HBS
Hepes-buffered saline
- MPF
M phase–promoting factor
- Xchk1
Xenopus Chk1
Footnotes
We are very grateful to W. Sullivan (University of California, Santa Cruz, CA) for providing the sequence of Drosophila Grapes. We thank our colleagues for comments on the manuscript.
Address all correspondence to W.G. Dunphy, Division of Biology, 216-76, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, CA 91125. Tel.: (626) 395-8433. Fax: (626) 449-0679. E-mail: dunphy@cco.caltech.edu
References
- al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Lehmann AR, Carr AM. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley NJ, Holtzman DA, Flaggs G, Keegan KS, DeMaggio A, Ford JC, Hoekstra M, Carr AM. The Schizosaccharomyces pomberad3 checkpoint gene. EMBO (Eur Mol Biol Organ) J. 1996;15:6641–6651. [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Carr AM. Checkpoints take the next step. Science. 1996;271:314–315. doi: 10.1126/science.271.5247.314. [DOI] [PubMed] [Google Scholar]
- Carr AM. Control of cell cycle arrest by the Mec1sc/Rad3sp DNA structure checkpoint pathway. Curr Opin Genet Dev. 1997;7:93–98. doi: 10.1016/s0959-437x(97)80115-3. [DOI] [PubMed] [Google Scholar]
- Carr AM, Moudjou M, Bentley NJ, Hagan IM. The chk1 pathway is required to prevent mitosis following cell-cycle arrest at ‘start'. Curr Biol. 1995;5:1179–1190. doi: 10.1016/s0960-9822(95)00234-x. [DOI] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO (Eur Mol Biol Organ) J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. . Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Flaggs G, Plug AW, Dunks KM, Mundt KE, Ford JC, Quiggle MRE, Taylor EM, Westphal CH, Ashley T, Hoekstra MF, Carr AM. Atm-dependent interactions of a mammalian chk1 homolog with meiotic chromosomes. Curr Biol. 1997;7:977–986. doi: 10.1016/s0960-9822(06)00417-9. [DOI] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophilagrapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Francesconi S, Grenon M, Bouvier D, Baldacci G. p56(chk1) protein kinase is required for the DNA replication checkpoint at 37°C in fission yeast. EMBO (Eur Mol Biol Organ) J. 1997;16:1332–1341. doi: 10.1093/emboj/16.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO (Eur Mol Biol Organ) J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1(+)- like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Phosphorylation and activation of the XenopusCdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol Biol Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopuscdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan KS, Holtzman DA, Plug AW, Christenson ER, Brainerd EE, Flaggs G, Bentley NJ, Taylor EM, Meyn MS, Moss SB, Carr AM, Ashley T, Hoekstra MF. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopusextracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Control of the Cdc2/cyclin B complex in Xenopusegg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 Proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopusegg extracts. Mol Biol Cell. 1998;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a XenopusWee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray AW. The genetics of cell cycle checkpoints. Curr Opin Genet Dev. 1995;5:5–11. doi: 10.1016/s0959-437x(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO (Eur Mol Biol Organ) J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, Atherton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Nat Acad Sci USA. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiaein response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, Wu Z, Stephenson MT, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Poon RY, Chau MS, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of crb2, a protein with BRCT motif, with cut5 and chk1. Genes Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Desany BA, Jones WJ, Liu Q, Wang B, Elledge SJ. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Schlegel R, Pardee AB. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986;232:1264–1266. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophilamidblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia colias fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Strausfeld U, Labbe JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Doree M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Sun Z, Fay DS, Marini F, Foiani M, Stern DF. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 1996;10:395–406. doi: 10.1101/gad.10.4.395. [DOI] [PubMed] [Google Scholar]
- Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- Walworth NC, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO (Eur Mol Biol Organ) J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A DNA damage checkpoint meets the cell cycle engine. Science. 1997;277:1450–1451. doi: 10.1126/science.277.5331.1450. [DOI] [PubMed] [Google Scholar]
- Westphal CH. Atm displays its many talents. Curr Biol. 1997;7:R789–R792. doi: 10.1016/s0960-9822(06)00406-4. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]