Abstract
AnkyrinG (−/−) neurons fail to concentrate voltage-sensitive sodium channels and neurofascin at their axon proximal segments, suggesting that ankyrinG is a key component of a structural pathway involved in assembly of specialized membrane domains at axon proximal segments and possibly nodes of Ranvier (Zhou, D., S. Lambert, D.L. Malen, S. Carpenter, L. Boland, and V. Bennett, manuscript submitted for publication). This paper addresses the mechanism for restriction of 270-kD ankyrinG to axon proximal segments by evaluation of localization of GFP-tagged ankyrinG constructs transfected into cultured dorsal root ganglion neurons, as well as measurements of fluorescence recovery after photobleaching of neurofascin– GFP-tagged ankyrinG complexes in nonneuronal cells. A conclusion is that multiple ankyrinG-specific domains, in addition to the conserved membrane-binding domain, contribute to restriction of ankyrinG to the axonal plasma membrane in dorsal root ganglion neurons. The ankyrinG-specific spectrin-binding and tail domains are capable of binding directly to sites on the plasma membrane of neuronal cell bodies and axon proximal segments, and presumably have yet to be identified docking sites. The serine-rich domain, which is present only in 480- and 270-kD ankyrinG polypeptides, contributes to restriction of ankyrinG to axon proximal segments as well as limiting lateral diffusion of ankyrinG–neurofascin complexes. The membrane-binding, spectrin-binding, and tail domains of ankyrinG also contribute to limiting the lateral mobility of ankyrinG–neurofascin complexes. AnkyrinG thus functions as an integrated mechanism involving cooperation among multiple domains heretofore regarded as modular units. This complex behavior explains ability of ankyrinB and ankyrinG to sort to distinct sites in neurons and the fact that these ankyrins do not compensate for each other in ankyrin gene knockouts in mice.
Keywords: ankyrin, axon initial segment, cell polarity, membrane dynamics, spectrin
Clustering of voltage-gated sodium channels at the axon proximal segments and nodes of Ranvier is believed to be essential for production and propagation of action potentials in myelinated axons. The mechanisms responsible for concentrating sodium channels in these specialized membrane domains in axons are not established, but are likely to involve specialized members of the ankyrin family of spectrin-binding proteins. Ankyrins are membrane-associated proteins that bind to certain ion channels, including the voltage-sensitive sodium channel, and have the potential to couple these channels to the spectrin–actin network (Srinivasan et al., 1988; Bennett and Gilligan, 1993; Bennett et al., 1997). Ankyrins also bind through their conserved membrane-binding domains to cell adhesion molecules of the L1 cell adhesion molecule (CAM) family (Davis et al., 1993, 1996; Davis and Bennett, 1994; Garver et al., 1997), and have been proposed to form lateral complexes involving L1 CAMs and ion channels (Michaely and Bennett, 1995a ,b; Lambert et al., 1997). Ankyrins are concentrated at physiological sites with a high density of voltage-sensitive sodium channels including nodes of Ranvier, axon proximal segments, and postsynaptic folds of the neuromuscular junction (Flucher and Daniels, 1989; Kordeli et al., 1990). The isoforms of ankyrin colocalized with voltage-sensitive sodium channels at nodes of Ranvier and axon proximal segments have been identified as 480/270-kD alternatively spliced variants of ankyrinG (Kordeli et al., 1995). An ankyrinG polypeptide also has been identified at the neuromuscular junction (Wood and Slater, 1998). In addition to ankyrinG, isoforms of neurofascin and NrCAM, which are members of the L1 CAM family, have been identified at nodes of Ranvier and axon proximal segments (Davis et al., 1996).
480/270-kD ankyrinG colocalizes with sodium channel clusters in amyelinated regions of peripheral nerves of the dystrophic mouse (Deerinck et al., 1997), and in axons of cultured retinal ganglion neurons in the absence of direct contact with glial cells (Kaplan et al., 1997). These results imply targeting of channels and ankyrin to sites defined by intrinsic polarity to the axon, independent of cell–cell contact, although soluble factors released by glial cells may play a role (Kaplan et al., 1997). 480/270-kD ankyrinG also colocalized in the developing sciatic nerve in clusters containing sodium channels, neurofascin, and NrCAM (Lambert et al., 1997). The earliest event observed in myelinating nerve, preceding expression of myelin-associated glycoprotein (MAG) by Schwann cells, was clustering of neurofascin and NrCAM, which were followed by ankyrin and sodium channels. These observations led to the proposal that extracellular signals, perhaps related to the soluble factor described by Kaplan et al. (1997), induce clustering of neurofascin and NrCAM, which in turn recruit ankyrinG and sodium channels (Lambert et al., 1997).
Mice deficient in cerebellar ankyrinG polypeptides have recently been developed by targeted gene disruption (Zhou, D., S. Lambert, P. Melen, S. Carpenter, L. Boland, and V. Bennett, manuscript submitted for publication). Cerebellar neurons of these ankyrinG (−/−) mice fail to concentrate sodium channels as well as neurofascin at axon proximal segments. These findings demonstrate that 480/270-kD ankyrinG is required for clustering of both sodium channels and neurofascin at axon proximal segments. Neurofascin and L1 CAMs therefore are not sufficient to provide the initial targeting signal for ankyrinG at axon proximal segments, in contrast to expectations from observations of development of the node of Ranvier (Lambert et al., 1997).
This paper addresses the mechanism for restriction of 270-kD ankyrinG to axon proximal segments of cultured dorsal root ganglion (DRG)1 neurons. A conclusion is that multiple ankyrinG-specific domains, in addition to the conserved membrane-binding domain, cooperate in targeting and restriction of ankyrinG to axon proximal segments. Moreover, measurements of fluorescence recovery after photobleaching (FRAP) of green fluorescent protein (GFP)- tagged ankyrin constructs cotransfected with neurofascin in a nonneuronal cell line demonstrate that the membrane-binding, spectrin-binding, tail and serine-rich domains each contribute to immobilization of 270-kD ankyrinG–neurofascin complexes in the plasma membrane. These results provide strong evidence that association between the ankyrinG membrane-binding domain and neurofascin is not sufficient to restrict ankyrinG to axon proximal segments, and that other ankyrinG domains and yet to be defined protein interactions are also involved.
Materials and Methods
DRG Culture
The culture of embryonic DRG was modified from methods previously described (Kleitman et al., 1991). In brief, DRGs were obtained from E15 rat embryos and plated on laminin-coated dishes after dissociation with 0.02% collagenase and 0.25% trypsin-EDTA. The medium was changed from normal medium (10% FBS in DME, 100 ng/ml NGF) to the defined medium (1× N1 additive [Sigma Chemical Co., St. Louis, MO], 100 ng/ml NGF, 10 μM FdU and 10 μM uridine in DME) 24 h after plating. The culture was kept in the defined medium for 2 d before changing back to normal medium for an additional 2 d. The antimitotic cycle was repeated once to kill most of dividing nonneuronal cells. After 8 d in culture, DRG neurons are ready for immunofluorescence and gene gun transfection.
Preparation of cDNA Constructs
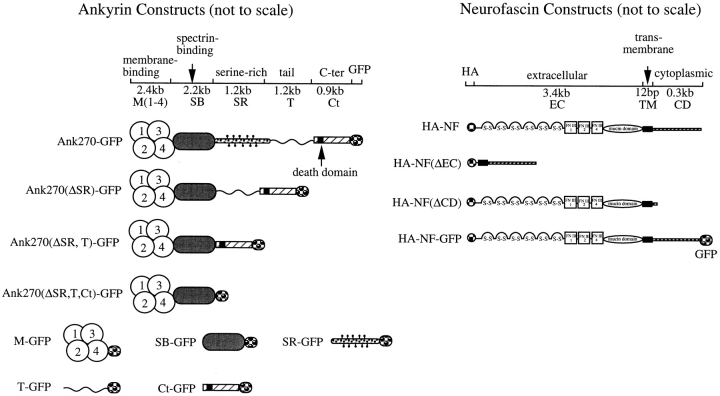
Assembly of the full-length cDNA for 270-kD ankyrinG (Ank270) was achieved by ligating the first half of membrane-binding domain, which was isolated from adult rat brain 5′-stretch plus cDNA library (Clontech Laboratories Inc., Palo Alto, CA) and confirmed by DNA sequencing, with construct M-Sb-Sr-T-C (Zhang and Bennett, 1996) through the EcoRI-NsiI sites. The COOH-terminal domain of 270-kD ankyrinG was PCR amplified and introduced into the SalI site of pEGFP-N1 vector (Clontech Laboratories Inc.), while keeping in-frame with the downstream EGFP protein (Ank-Ct). The full-length 270-kD ankyrinG with GFP tagged at its COOH terminus (see Fig. 2, Ank270–GFP) was prepared by ligating the EcoRI-EcoRV fragment of construct Ank270 into the EcoRI-EcoRV sites of construct Ank-Ct. The cDNA construct for 190-kD ankyrinG (see Fig. 2, Ank270[ΔSR,T]–GFP) was prepared similarly except using construct M-Sb-C (Zhang and Bennett, 1996) instead of M-Sb-Sr-T-C. The cDNA construct lacking the first half of the membrane-binding domain (corresponding to the amino acids 0–393 of human ankyrinG [Kordeli et al., 1995]) was prepared by putting the EcoRV fragment of M-Sb-Sr-T-C (Zhang and Bennett, 1996) into the corresponding site of the construct Ank-Ct (see Fig. 2, Ank270[ΔM1,2]–GFP). The construct containing only the membrane-binding and the spectrin binding domain (see Fig. 2, Ank270[ΔSR,T,Ct]–GFP) was obtained by replacing the EcoRV-NotI fragment of construct Ank270[ΔSR,T]–GFP with a PCR-amplified EGFP sequence. The construct expressing the membrane-binding domain was made by introducing a PCR-amplified sequence into the EcoRI-SalI sites of pEGFP-N1 vector while keeping in-frame translation of the downstream EGFP (M–GFP). Constructs expressing the serine-rich (SR-GFP), spectrin-binding (SB-GFP), tail (T-GFP), and COOH-terminal (Ct-GFP) domains were prepared similarly. To make a construct with deletion of the serine-rich domain (see Fig. 2, Ank270[ΔSR]–GFP), a PCR-amplified fragment of the tail domain of the 270-kD ankyrinG was introduced into the EcoRV site of construct Ank270(ΔSR,T)–GFP.
Figure 2.
Schematic diagram of cDNA constructs used in transfection experiments. Preparation of cDNA constructs of ankyrin and neurofascin is described in Materials and Methods. Ank270–GFP represents the full-length 270-kD ankyrinG with GFP tag at its COOH terminus. Construct Ank270(ΔSR)–GFP has a deleted serine-rich domain and Ank270(ΔM1,2)–GFP lacks the first half of the membrane-binding domain. Construct Ank270(ΔSR,T)– GFP is a 190-kD natural isoform of ankyrinG without the serine-rich and tail domains. Deletion of the COOH-terminal domain from construct Ank270(ΔSR,T)–GFP results in construct Ank270(ΔSR,T,Ct)–GFP. M-GFP, SB-GFP, SR-GFP, T-GFP, and Ct-GFP represent the membrane-binding domain, the spectrin-binding domain, the serine-rich domain, the tail domain, and the COOH-terminal domain, respectively. In the neurofascin construct family, construct HA–NF contains the full-length of neurofascin with HA-epitope at the NH2 terminus. Constructs HA– NF(ΔEC) and HA–NF(ΔCD) have the deletion of the extracellular domain and the cytoplasmic domain respectively. HA–NF–GFP contains a GFP tag at the COOH terminus of the full-length neurofascin.
The full-length rat neurofascin cDNA with a hemagglutinin (HA) epitope at the NH2 terminus (Garver et al., 1997) was cut out by HindIII-NotI from PBluescript KS vector (Stratagene, La Jolla, CA), and subcloned into the corresponding sites of pEGFP-N1 vector (Clontech Laboratories Inc.). The resultant construct (see HA–NF in Fig. 2) does not contain the EGFP sequence. The cytoplasmic domain-deleted neurofascin (see HA–NF[ΔCD] in Fig. 2) was made by replacing the ScaI-NotI fragment of construct HA–NF with a PCR-amplified fragment containing the sequence of the ScaI-ApaI fragment with a stop codon right after the ApaI site. The extracellular domain-truncated neurofascin (see Fig. 2, HA–NF[ΔEC]) was prepared through two steps. First, the 5′-untranslated region of neurofascin with the start codon, the signal peptide, and the HA tag was PCR amplified and subcloned into the BglII-HindIII sites of pEGFP-N1 vector. Then the transmembrane and cytoplasmic domains were introduced into the HindIII-NotI sites of the first-step construct. Construct with the full-length neurofascin tagged with EGFP at its COOH terminus was obtained also by two steps (see HA–NF–GFP in Fig. 2). The HindIII-ApaI fragment of neurofascin in pBluescript KS vector was transferred into the corresponding sites of pEGFP-N3 vector (Clontech Laboratories Inc.) so as to keep in-frame reading of EGFP with the introduced neurofascin fragment. Then a PCR-amplified cytoplasmic domain of neurofascin was introduced into the ApaI site of the above construct.
cDNA Transfection and Immunofluorescence
DRG cultures were transfected using Helios™ Gene Gun system following the manufacturer's instructions. 150–180 psi pressure was used to deliver cDNA-coated microcarriers into DRG cultures. 24 h after transfection, DRG cultures were fixed in 2% paraformaldehyde and then subjected to 0.5% Triton X-100 permeabilization before being stained with a polyclonal antibody specific for GFP (Clontech Laboratories Inc.). Immunofluorescence for endogenous proteins was performed similarly.
Human kidney 293 cells were cultured in 10% FBS and DME (GIBCO BRL, Gaithersburg, MD) and transfected by Lipofectamine according to the manufacturer's protocol (GIBCO BRL). 24 h after transfection, cultures were either subject to photobleaching (see below) or fixed in 2% paraformaldehyde and then stained with a monoclonal antibody specific for the HA epitope of neurofascin (Berkeley Antibody Co., Richmond, CA).
Chicken polyclonal antibody against 270/480-kD ankyrinG (Zhang and Bennett, 1996) and rabbit polyclonal antibodies against ankyrinB (Chan et al., 1993), brain spectrin (Davis and Bennett, 1983), and neurofascin (Davis et al., 1996) were described previously. TRITC-conjugated goat anti–mouse and goat anti–rabbit, and FITC-conjugated goat anti–rabbit antibodies were purchased from Rockland (Gilbertsville, PA). FITC-conjugated donkey anti–chicken was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Fluorescence Recovery Measurement after Photobleaching
Photobleaching of the cytoplasm-localized Ank270–GFP was performed essentially as described, using an Odyssey confocal imaging system (Garver et al., 1997). Photobleaching of the plasma membrane-localized transfected proteins was performed in a Zeiss LSM 410 laser confocal system coupled with an UV laser (Carl Zeiss Inc., Thornwood, NY). A selected region enclosing the plasma membrane to be studied was photobleached using the UV laser beam for 60 ms. The radii of photobleached regions range from 1 to 3 μm. The fluorescence recovery inside the photobleached area was immediately recorded by a home-designed program containing several ‘Time Series' with different time intervals. The recorded data was analyzed using NIH Image software (Bethesda, MD). The recovered fraction was calculated as (Ie −Ii)/(Io −Ii). Io, the average intensity of the selected region before photobleaching; Ii, the average intensity of the selected region immediately after photobleaching; and Ie, the average intensity of the selected region after 50 s of recovery. The recovery rate was calculated as w 2/t, where w is the radius of the photobleached region, and t is the time in seconds required for the average intensity of the selected region to recover to the value of (Ie + Ii)/2.
Results
Native AnkyrinG and Neurofascin but Not AnkyrinB and Spectrin Are Restricted to Axon Proximal Segments of DRG Neurons
480/270-kD ankyrinG (Fig. 1, A, D, and G) and neurofascin (Fig. 1 H) are concentrated at the axolemma of axon proximal segments of cultured embryonic DRG neurons. AnkyrinG also is associated with the plasma membrane in cell bodies of neurons, although at a lower level than in the axon proximal segment (Fig. 1, A, D, and G). AnkyrinB, in contrast, is distributed along the length of axons and is present in only low levels in axon proximal segments (Fig. 1 B). AnkyrinB also exhibits only minimal staining in the cell body (Fig. 1 B). Spectrin is distributed along axons in a pattern resembling ankyrinB (Fig. 1 E). These results demonstrate that cultured DRG neurons have a polarized distribution of native ankyrinG and neurofascin in axon proximal segments similar to localization of these proteins in sections of intact brain tissue (Kordeli et al., 1995; Davis et al., 1996).
Figure 1.
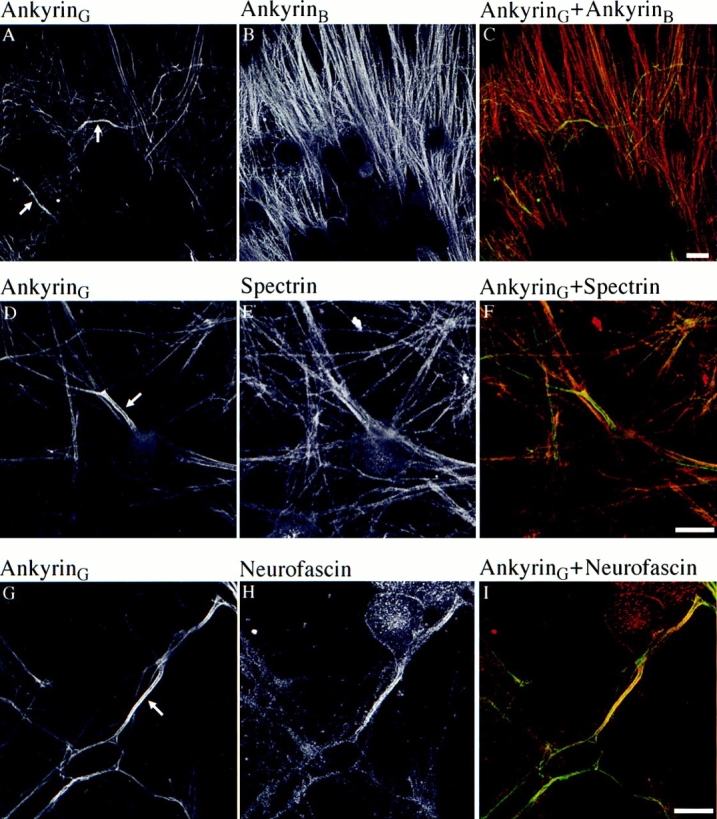
Distribution of 270/ 480-kD native ankyrinG, ankyrinB, spectrin, and neurofascin in cultured DRG neurons. 270/480-kD ankyrinG (A) and ankyrinB (B) are double labeled in 9-d-old embryonic DRG culture. (C) Composite image of A and B. AnkyrinG is concentrated at the proximal segment (A, arrows), whereas ankyrinB is more evenly distributed in axons (B). Spectrin (E), compared with double-labeled ankyrinG (D, arrow, proximal segment), also lacks polarized distribution at the proximal segment. (F) Composite image of D and E. Neurofascin (H), a member of L1 CAM family of cell adhesion molecules, is concentrated and colocalized with ankyrinG at the proximal segment (G, arrow). (I) Composite image of G and H. The green channel (FITC) corresponds to ankyrinG stain whereas the red channel (TRITC) represents other proteins. Bar, 25 μm.
AnkyrinG and ankyrinB both bind to neurofascin with high affinity through their membrane-binding domains, which share 74% amino acid identity (Davis et al., 1993; Davis and Bennett, 1994; Kordeli et al., 1995; Zhang et al., 1998). Thus, enrichment of neurofascin and ankyrinG, but not ankyrinB, at axon proximal segments of DRG neurons (Fig. 1, C and I ) suggests a mechanism independent of neurofascin for targeting ankyrinG specifically to the axolemma of axon proximal segments. The targeting protein(s) are not currently known spectrin polypeptides, based on lack of concentration of spectrin at axon proximal segments (Fig. 1 F).
Unique Serine-rich and Tail Domains of 270-kD AnkyrinG Contribute to Restriction of AnkyrinG at Axon Proximal Segments
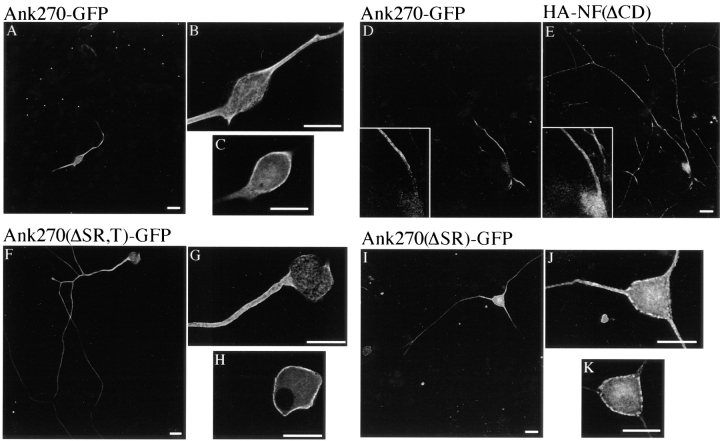
AnkyrinG is distinguished from ankyrinB by a unique O-GlcNAc–glycosylated serine-rich domain and a highly divergent tail domain (Kordeli et al., 1995; Zhang and Bennett, 1996). In addition, ankyrinG and ankyrinB differ in sequence in portions of the spectrin-binding domain and in the COOH-terminal domain. To identify the roles of the serine-rich and tail domains in targeting 480/270-kD ankyrinG to axon proximal segments, we transfected DRG neurons with cDNAs, each with a COOH-terminal GFP tag, encoding 270-kD ankyrinG and its variants lacking the serine-rich and/or tail domains (Fig. 2). The distribution of transfected 270-kD ankyrinG (Fig. 2, Ank270–GFP) is visualized by immunostaining of the COOH-terminal GFP tag, and is highly restricted to the axolemma of the proximal segment in a pattern similar to native 480/270-kD ankyrinG (Fig. 3, A and B). The intensity of immunofluorescence attenuates abruptly beyond the proximal segment (Fig. 3 A and Fig. 4, A and B), which is usually tens of micrometers in length in DRG neurons (Hsieh et al., 1994). Double labeling of cultures cotransfected with GFP-tagged 270-kD ankyrinG (Fig. 3 D) and HA-tagged cytoplasmic domain-truncated neurofascin, HA–NF(ΔCD) (Fig. 2 and Fig. 3 E), indicates the polarized distribution of the transfected ankyrinG is not due to incomplete extension or partial optical sectioning of axons since the full length of the axon is well displayed by mutant neurofascin (Fig. 3 E). The relatively even distribution of cytoplasmic domain-truncated neurofascin along the length of the axon also suggests that the extracellular and transmembrane domains of neurofascin are not sufficient to achieve polarized localization at the proximal segment. Some GFP-tagged ankyrinG is also targeted to areas of the plasma membrane of the cell body, at levels approximating those in the proximal segments (Fig. 3 C). This behavior is in contrast to the distribution of native ankyrinG and could represent a consequence of high levels of expression of the transfected ankyrinG.
Figure 3.
The unique serine-rich and tail domain of ankyrinG contribute to restriction of 270-kD ankyrinG at the axon proximal segment. The cDNAs of 270-kD ankyrinG and its variants lacking the serine-rich and/or tail domains are transfected into 8-d-old DRG culture using Helios™ Gene Gun system. Expression of transfected proteins is visualized by immunostaining of the GFP tag. The transfected 270-kD ankyrinG (Ank270-GFP) is highly restricted to the proximal segment (A). High zoom recording reveals plasma membrane stain of the transfected 270-kD ankyrinG at the proximal segment (B) and the cell body (C). The restricted location of transfected Ank270–GFP (D) is displayed against the full length of the transfected axon which is revealed by double labeling of the cotransfected cytoplasmic domain-deleted neurofascin (E). The transfected 190-kD ankyrin (Ank270[ΔSR,T]–GFP) is also localized at the plasma membrane of the proximal segment (G) and the cell body (H). However, a significant amount of the transfected Ank270(ΔSR,T)–GFP is distributed beyond the proximal segment into the axon (F). Insertion of the tail domain into Ank270(ΔSR,T)– GFP (Ank270[ΔSR]–GFP) improves but does not totally restore restriction of the transfected ankyrin to the proximal part of the axon (I). J and K show membrane localization of Ank270(ΔSR)–GFP at the proximal segment and the cell body respectively. Bar, 25 μm.
Figure 4.
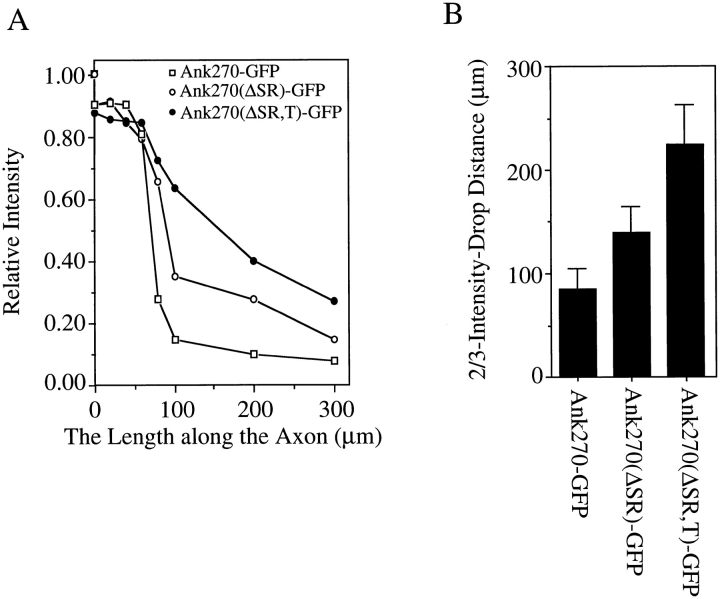
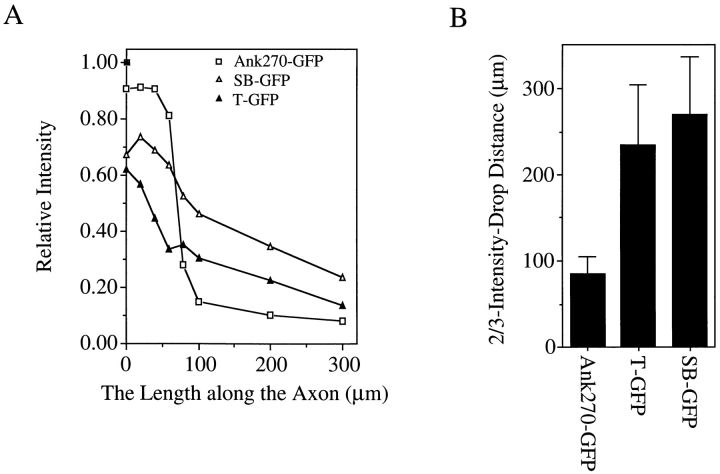
The immunofluorescence intensity profile of transfected ankyrins along the length of axons. The immunofluorescence intensity of the GFP tag of transfected ankyrins is measured using Carl Zeiss LSM measurement program. The distance is calculated from the starting region of the proximal segment. The fluorescence intensity is normalized to the intensity at the plasma membrane of the cell body. (A) Measurement of the intensity drop along the length of the axon. (B) Two-thirds of the intensity-drop distance (the distance for the intensity of immunofluorescence to drop to one-third of the intensity at the starting region of the proximal segment). Data in B represent the mean ± SEM for five transfected neurons.
Transfected 190-kD ankyrinG (refer to Fig. 2, Ank270 [ΔSR,T]–GFP), which lacks the unique serine-rich and tail domains of 270-kD ankyrinG (Kordeli et al., 1995), is also located at the axolemma of the proximal segment (Fig. 3, F and G). However, 190-kD ankyrinG is much less restricted, and distributes well beyond the proximal segment into the axon (Fig. 3 F and Fig. 4, A and B). The distance required for the intensity of immunofluorescence to drop to one-third of the intensity at the proximal segment is more than twice as long for 190-kD ankyrinG than for 270-kD ankyrinG (Fig. 4 B). Insertion of the tail domain into 190-kD ankyrinG (refer to Fig. 2, Ank270[ΔSR–GFP]) improves, but does not completely restore, the restriction of the transfected ankyrinG to the proximal area of the axon (Fig. 3 I and Fig. 4, A and B). Ank270(ΔSR)–GFP distributes in a punctate pattern different from that of 270-kD ankyrinG and 190-kD ankyrinG (Fig. 3, J and K). These data suggest that the unique serine-rich domain and tail domain of 270/480-kD ankyrinG contribute to restriction of ankyrinG at axon proximal segments.
Spectrin-binding and Tail Domains of AnkyrinG Contain Specific Plasma Membrane-binding Sites
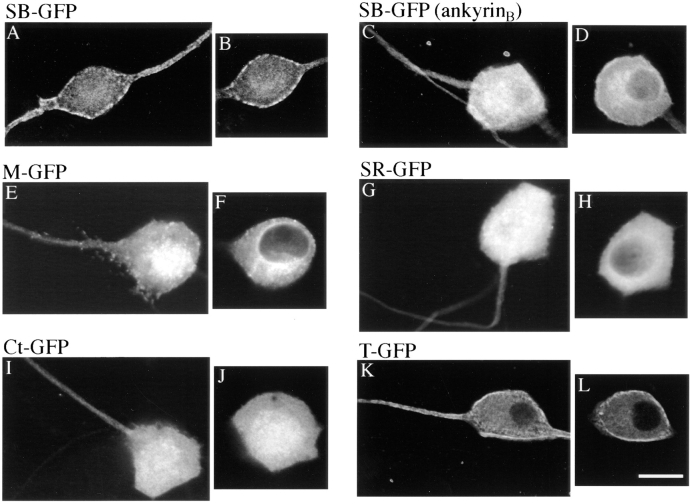
To examine which domain(s) are sufficient for restriction of ankyrinG at the axolemma of axon proximal segments, different domains of 270-kD ankyrinG were individually introduced into DRG neurons. The membrane-binding domain (Fig. 5, E and F), the serine-rich domain (Fig. 5, G and H) and the COOH-terminal domain (Fig. 5, I and J) are predominantly distributed throughout the cytoplasm of the cell body and the axon, but are not concentrated at the axolemma. The failure in recruiting the membrane-binding domain to the plasma membrane might be a consequence of over-expression and does not exclude the possibility of a limited number of binding sites. The spectrin-binding domain (Fig. 5, A and B) and the tail domain (Fig. 5, K and L), on the other hand, were targeted to the axolemma of the proximal segment as well as the plasma membrane of the cell body. The spectrin-binding domain also distributes in a punctate pattern similar to that of Ank270(ΔSR)–GFP (Fig. 5, A and B). However, neither the spectrin-binding nor tail domains are restricted to the proximal segment as the case with transfected 270-kD ankyrinG, and their intensity-drop profiles along axons are similar to that of 190-kD ankyrinG (Fig. 6, A and B). These data suggest an important role for plasma membrane-binding sites in the spectrin-binding and the tail domain for restriction of ankyrinG at axon proximal segments. The restrictive effect of the serine-rich domain (refer to Fig. 3 I and see Fig. 5, G and H) seems unrelated to targeting to the plasma membrane, at least as resolved by these techniques.
Figure 5.
The spectrin-binding domain and the tail domain of ankyrinG contain binding sites to the axolemma of axon proximal segments. Different domains of 270-kD ankyrinG tagged with GFP at their COOH termini were individually transfected into DRG neurons. Expression of transfected proteins is examined by immunostaining of the GFP tag. The spectrin-binding domain SB-GFP (A and B) and the tail domain T-GFP (K and L) are targeted to the plasma membrane of the proximal segment and the cell body. The membrane-binding domain M-GFP (E and F), COOH-terminal domain Ct-GFP (I and J), and the serine-rich domain SR-GFP (G and H), however, are predominantly localized in the cytoplasm of the axon and the cell body. The spectrin-binding domain of ankyrinB (SB-GFP [ankyrinB]) is not targeted to the axolemma of the proximal segment (C), although a small fraction is distributed at the membrane of the cell body (D). Bar, 25 μm.
Figure 6.
The immunofluorescence intensity profile of transfected spectrin-binding and tail domains of ankyrinG in axons. (A) Immunofluorescence profile. (B) the two-thirds intensity-drop distance of transfected spectrin-binding and tail domains. Data represent the mean ± SEM for five transfected neurons.
The transfected spectrin-binding domain of ankyrinB is not targeted to the axolemma and is distributed in the axoplasm and the cytoplasm of the cell body (Fig. 5, C and D). The spectrin-binding domain of ankyrinG thus contains targeting information lacking in the spectrin-binding domain of ankyrinB, and could contribute to specific restriction of ankyrinG to axon proximal segments.
AnkyrinG Domains Cooperate in Restricting the Lateral Mobility of AnkyrinG –Neurofascin Complexes
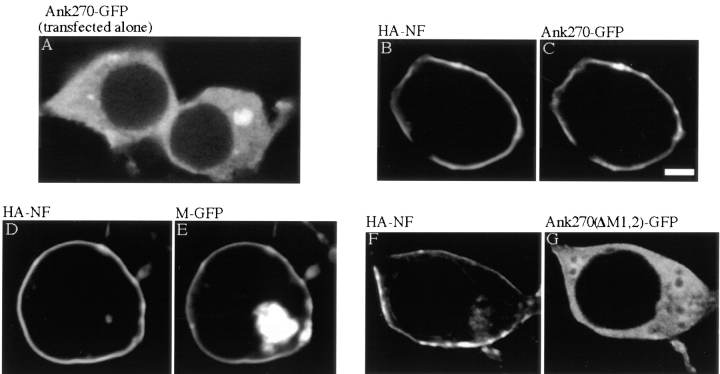
The serine-rich domain and possibly other domains of 270-kD ankyrinG could contribute to restriction of ankyrinG to axon proximal segments through interactions limiting lateral mobility in addition to binding to membrane-targeting sites. Activity of ankyrinG domains in immobilizing ankyrinG–neurofascin complexes was evaluated in a nonneuronal cell line (293 cells from human embryonic kidney) cotransfected with GFP-tagged ankyrinG and HA-tagged neurofascin (Fig. 7). 270-kD ankyrinG transfected alone is located throughout the cytoplasm of 293 cells (Fig. 7 A). However, 270-kD ankyrin cotransfected with neurofascin is recruited to the plasma membrane (Fig. 7, B and C). Targeting of transfected ankyrinG in 293 cells to the plasma membrane is solely governed by interactions between the membrane-binding domain and cotransfected neurofascin, since the membrane-binding domain of ankyrinG alone is sufficient for membrane recruitment by co transfected neurofascin (Fig. 7, D and E). Moreover, deletion of the first half of the membrane-binding domain abolishes recruitment of ankyrinG to the plasma membrane even though the COOH-terminal, tail, serine-rich, and spectrin-binding domains are still present (Fig. 7, F and G). The structural requirements for activity of neurofascin in recruiting ankyrinG are explored in a separate study (Zhang et al., 1998).
Figure 7.
Association of 270-kD ankyrinG to the plasma membrane of human kidney 293 cells requires the membrane-binding domain and cotransfected neurofascin. Transfected 270-kD ankyrinG (Ank270-GFP) alone visualized by the GFP fluorescence distributes throughout the cytoplasm of 293 cells (A). Cotransfection with neurofascin (HA–NF) recruits Ank270–GFP to the plasma membrane (C). (B) Double labeling of cotransfected neurofascin by immunostaining of the HA epitope. The membrane-binding domain of ankyrin (M-GFP) is sufficient to be recruited to the plasma membrane (E) by cotransfected neurofascin (D). Deletion of the first half of the membrane-binding domain (Ank270(ΔM1,2)–GFP) abolishes the membrane-recruitment by cotransfected neurofascin (F and G). Bar, 3 μm.
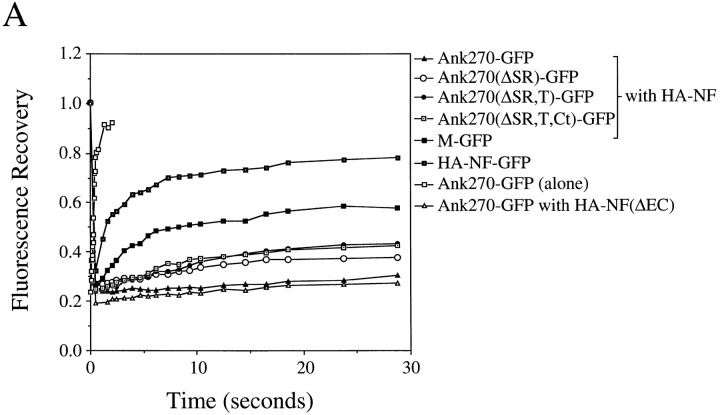
Dynamic behavior of GFP-tagged ankyrinG–neurofascin complexes were examined by measurements of FRAP of the GFP signal (Fig. 8). Dynamic parameters were first determined for GFP-tagged 270-kD ankyrinG (Ank270– GFP) alone, which was localized in the cytoplasm, and for GFP-tagged neurofascin (HA–NF–GFP) alone. Cytoplasmic 270-kD ankyrinG exhibits rapid and nearly complete recovery after photobleaching (91% recovery at a rate of 1.1 × 10−7 cm2/s), consistent with the diffusion rate of a protein in solution (Fig. 8, A and B). GFP-tagged neurofascin (HA–NF–GFP) transfected in the absence of cotransfected ankyrinG exhibited a 72% recovery at a rate of 5.6 × 10 cm2/s (Fig. 8, A and C). This rate is comparable to other membrane proteins that are freely diffusing in plasma membranes (Jacobson et al., 1987; Zhang et al., 1991). In contrast, plasma membrane-associated 270-kD ankyrinG (Ank270–GFP cotransfected with HA–NF) displays a greatly reduced rate (4 × 10−10 cm2/s) as well as extent (10%) of FRAP (Fig. 8, A, B, and C). This dramatic decrease of membrane lateral mobility is not due to lateral association of the extracellular domain of neurofascin with itself or with other membrane-spanning proteins, since deletion of the extracellular domain of neurofascin has little effect on membrane dynamic behavior of cotransfected 270-kD ankyrinG (Ank270–GFP with HA–NF[ΔEC] in Fig. 8, A and B). Thus, 270-kD ankyrinG provides the major contribution to immobilization of ankyrin-neurofascin complexes in the plane of the plasma membrane.
Figure 8.
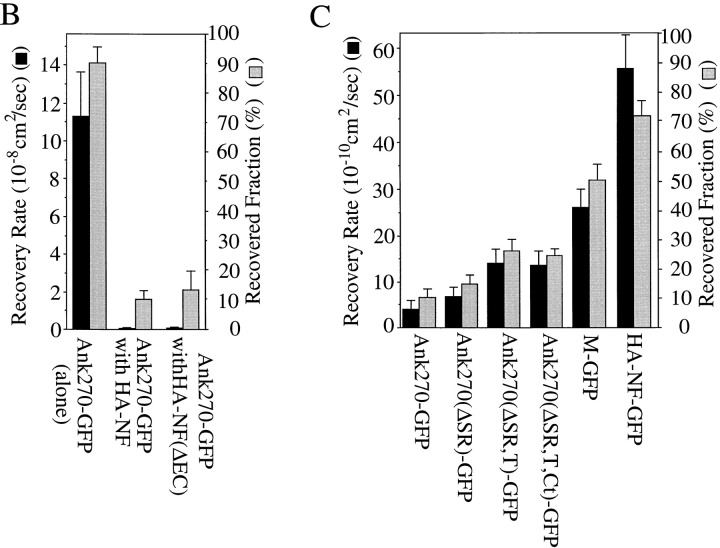
Multiple domains of 270-kD ankyrinG contribute to immobilization of ankyrinG at the plasma membrane of human kidney 293 cells. (A) FRAP plasma membrane-localized 270-kD ankyrinG and its variants with deleted domains. Ankyrins with the membrane-binding domain are capable of being recruited to the plasma membrane by cotransfected neurofascin HA–NF or the extracellular domain-truncated neurofascin HA–NF(ΔEC) (Zhang et al., 1998). Dynamics of membrane-associated ankyrins are evaluated by fluorescence recovery measurement after photobleaching the GFP signals. Also included are the FRAP of cytoplasm-located 270-kD ankyrinG and membrane-associated GFP-neurofascin (HA–NF–GFP). (B) Comparison of recovery rate (the time taken to achieve half of the 50-s recovery) and recovered fraction (the fraction recovered after 50 s) between the cytoplasm-localized ankyrin and the membrane-associated ankyrin. (C) Recovery rate and recovered fraction of membrane-associated ankyrins. HA–NF–GFP is measured as a reference for freely diffusing membrane proteins. Data in B and C represent the mean ± SEM for six experiments.
The role of ankyrinG domains in immobilizing neurofascin were evaluated using a range of ankyrinG constructs (refer to Fig. 2, and see Fig. 8, A and C). The lateral mobility of the membrane-localized complexes formed by cotransfected neurofascin (HA–NF) and the membrane-binding domain of ankyrinG (M-GFP) exhibits more than a 50% reduction of rate of recovery (2.6 × 10−9 cm2/s) and a 30% decrease of extent of recovery (51%) compared with behavior of GFP neurofascin (HA–NF–GFP) alone. Addition of the spectrin-binding domain to the membrane-binding domain (Ank270[ΔSR,T,Ct]–GFP) results in another 50% reduction in the rate of recovery (1.4 × 10−9 cm2/s) and a decrease in extent of recovery to ∼25% (refer to Fig. 4). Subsequent addition of the COOH-terminal domain (Ank270[ΔSR,T]–GFP), however, had no apparent effect. Addition of the tail domain (Ank270[ΔSR]– GFP) further decreased the rate (7 × 10−10 cm2/s) and the extent (16%) of recovery. Finally, addition of the serine-rich domain (Ank270–GFP) had another reduction in rate to 4 × 10 cm2/s and extent of recovery to 10 percent.
Discussion
The current paradigm for organization of ankyrin–membrane protein complexes derives from the erythrocyte membrane, and envisions monomeric ankyrin forming lateral complexes between integral proteins via the multivalent membrane-binding domain and spectrin via the spectrin-binding domain (Bennett, 1990; Michaely and Bennett, 1995a ,b). This study provides evidence that a simple erythrocyte-based model is not adequate to explain ankyrinG organization at axon proximal segments. The spectrin-binding, serine-rich, and tail domains all contribute to restriction of ankyrinG to the axonal plasma membrane in DRG neurons. Multiple ankyrinG domains also contribute to limiting the lateral mobility of ankyrin–neurofascin complexes in a nonneuronal cell. The ankyrinG-specific spectrin-binding and tail domains are capable of binding directly to sites on the plasma membrane of axon proximal segments and neuronal cell bodies, and presumably have yet to be identified docking sites. The serine-rich domain, which is present only in 480- and 270-kD ankyrinG polypeptides, contributes to restriction of ankyrinG to axon proximal segments as well as limiting lateral diffusion of ankyrinG–neurofascin complexes. AnkyrinG thus functions as an integrated mechanism involving cooperation among multiple domains heretofore regarded as modular units. This complex behavior explains the ability of ankyrinB and ankyrinG to sort to distinct sites in neurons and the fact that these ankyrins do not compensate for each other in ankyrin gene knockouts in mice (Zhou, D., S. Lambert, P. Malen, S. Carpenter, L. Boland, and V. Bennett, manuscript submitted for publication) (Scotland, P., D. Zhou, H. Benveniste, and V. Bennett, manuscript submitted for publication).
The mechanism for restriction of dynamic behavior of neurofascin by ankyrinG could result from self-association of ankyrinG domains and/or low affinity interaction with membrane or cytoskeletal proteins. For example, ankyrin associates with micromolar affinity with microtubules (Bennett and Davis, 1981). Association of transfected ankyrinG with endogenous spectrin does not seem likely, since ankyrinG remained in the cytoplasm in the absence of cotransfected neurofascin, whereas spectrin in these cells is primarily localized at the plasma membrane (Zhang et al., 1998). As an alternative to specific protein interactions, ankyrinG could be nonspecifically trapped in the membrane-apposed cytoskeletal meshwork, according to the membrane-skeleton fence model (Kusumi et al., 1993; Jacobson et al., 1995). Further analysis will be required to differentiate these possibilities.
Functions attributed so far to the spectrin-binding domain of ankyrin have been association with spectrin (Bennett, 1978; Davis and Bennett, 1984a ,b), and contact with the Na+/K+ ATPase (Davis and Bennett, 1990). Disruption of the spectrin network abolishes the polarized distribution of ankyrin as well as Na+/K+-ATPase at cell–cell contact sites in cultured cells (Hu et al., 1995). However, antibody against total brain spectrin revealed no enrichment of spectrin at axon proximal segments of DRG neurons and thus, known spectrins are unlikely to provide a sufficient mechanism to specifically enrich ankyrinG at the sites. A similar lack of correlation between spectrin and ankyrinG has been reported at nodes of Ranvier in sciatic nerves, where spectrin is continuously distributed along plasma membranes of axons and the myelinating Schwann cell (Lambert et al., 1997). Since different spectrin isoforms have different affinities for ankyrins (Howe et al., 1985; Davis and Bennett, 1984a ), one explanation of these results is the existence of a novel isoform of spectrin which is targeted to axon proximal segments and nodes of Ranvier, but is not identified by our current antibody. Alternatively, a novel ankyrinG-binding protein unrelated to spectrin could be targeted to axon proximal segments. In addition, a yet to be identified protein at the axolemma of axon proximal segments provides a binding site for the tail domain of 270-kD ankyrinG.
The traditional interactions between membrane proteins and ankyrin mediated by the membrane-binding domain of ankyrin may not provide the initial step in restriction of ankyrin at the axolemma of axon proximal segments. Instead, enrichment of ankyrin, which could be achieved through combined influences of the spectrin-binding, tail, and serine-rich domains, may precede and direct assembly of membrane proteins at the sites. Failure of transfected mutant neurofascin with a deleted cytoplasmic domain to be concentrated at axon proximal segments (Fig. 3 E) favors the possibility that the ankyrin-binding activity, which is located in the cytoplasmic domain of neurofascin (Davis and Bennett, 1994) (Zhang, X., J.D. Davis, S. Carpenter, and V. Bennett, manuscript in preparation), is required for restriction of neurofascin at these sites. Moreover, the disrupted assembly of neurofascin as well as sodium channels at axon proximal segments in ankyrinG (−/−) mice further supports the role of ankyrin in directing membrane proteins to axon proximal segments (Zhou, D., S. Lambert, P. Malen, S. Carpenter, L. Boland, and V. Bennett, manuscript submitted for publication).
The potential defining role of ankyrin in clustering of membrane proteins at axon proximal segments is inconsistent with conclusions from analysis of morphogenesis of nodes of Ranvier, suggesting that clusters of neurofascin/ NrCAM provide sites for subsequent assembly of ankyrin and sodium channels (Lambert et al., 1997). The disparity could be attributed to the potentially different mechanisms used in establishment of axon proximal segments and nodes of Ranvier, despite similarities in their molecular organization (Kordeli et al., 1995; Davis et al., 1996; Bennett et al., 1997) and ultrastructure (Waxman, 1984). Clustering of ankyrin or sodium channels at axon proximal segments does not require the presence of glial cells and thus is most likely determined by an intrinsic polarity of neurons (Fig. 3, A, D, and G) (Kaplan et al., 1997). In contrast, clustering of ankyrin and sodium channels in the axonal region beyond the proximal segment, which is regarded as an early event for assembly of nodes of Ranvier, requires signals from glial cells, although whether these signals are diffusable or require direct glia–axon contact has been debated (Salzer, 1997; Dugandzija-Novakovic et al., 1995; Vabnick et al., 1996, 1997; Deerinck et al., 1997; Kaplan et al., 1997). Clustering of neurofascin/NrCAM at the initial sites of nodes of Ranvier provides a potential mechanism to bridge glial cell signals to intracellular determinants, such as clustering of ankyrin, during the early development of nodes of Ranvier. On the other hand, the unique position of axon proximal segments might be sufficient to provide a cue to polarize the molecular composition at this region characterized by concentration of ankyrin and subsequent recruitment of ankyrin-binding membrane proteins.
In carefully timed neuronal Schwann cell cocultures, we have observed that clustering of ankyrin precedes clustering of sodium channels. In addition, each MAG-positive myelinating Schwann cell has two ankyrin clusters at each end of this Schwann cell. These ankyrin clusters are covered by processes of the myelinating Schwann cell, suggesting axon-Schwann cell contact defines clustering as well as the clustering sites of ankyrin (Zhang, 1998). These results indicate that ankyrin, as at axon proximal segments, is a candidate to direct assembly of sodium channels at the precursor sites of nodes of Ranvier, although the role of glial cells may differ between nodes and proximal segments.
This paper suggests a complicated and cooperative scenario for establishment of the specialized membrane domain of axonal proximal segments, which may involve not only interactions between membrane proteins and ankyrin but also ankyrin and other yet to be identified proteins which precede ankyrin at these sites. Isoforms of ankyrinG also are targeted to sites of cell–cell contact in epithelial tissues (Peters et al., 1995), and proteins similar to those of axon proximal segments could also be required for epithelial polarity. A challenge for future work will be to fully elucidate the structural pathway leading to assembly of ankyrin-based membrane domains.
Abbreviations used in this paper
- CAM
cell adhesion molecule
- DRG
dorsal root ganglion
- FRAP
fluorescence recovery after photobleaching
- GFP
green fluorescent protein
- HA
hemagglutinin
Footnotes
L. Davis (Duke University, HHMI, Durham, NC) is gratefully acknowledged for contributions to culture of DRG neurons. H. Wilson (Duke University) is thanked for advice on transfection with the Helios Gene Gun.
This research was supported in part by a grant from the National Institutes of Health (DK 29808).
Address all correspondence to V. Bennett, Howard Hughes Medical Institute and Departments of Cell Biology and Biochemistry, Duke University Medical Center, Durham, North Carolina 27710. Tel.: (919) 684-3538. Fax: (919) 684-3590. E-mail: benne012@mc.duke.edu
References
- Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978;253:2292–2299. [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Bennett V, Davis J. Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc Natl Acad Sci USA. 1981;78:7550–7554. doi: 10.1073/pnas.78.12.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lambert S, Davis JQ, Zhang X. Molecular architecture of the specialized axonal membrane at the node of Ranvier. Soc Gen Physiol Ser. 1997;52:107–120. [PubMed] [Google Scholar]
- Chan W, Kordeli E, Bennett V. 440-kD ankyrin-B: structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J Cell Biol. 1993;123:1463–1473. doi: 10.1083/jcb.123.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Brain spectrin. Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem. 1983;258:7757–7766. [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Brain ankyrin-purification of a 72,000 M rspectrin-binding domain. J Biol Chem. 1984a;259:1874–1881. [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem. 1984b;259:13550–13559. [PubMed] [Google Scholar]
- Davis JQ, Bennett V. The anion exchanger and Na+K(+)-ATPase interact with distinct sites on ankyrin in in vitro assays. J Biol Chem. 1990;265:17252–17256. [PubMed] [Google Scholar]
- Davis JQ, McLaughlin T, Bennett V. Ankyrin-binding proteins related to nervous system cell adhesion molecules: candidates to provide transmembrane and intercellular connections in adult brain. J Cell Biol. 1993;232:121–133. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin-binding activity shared by the neurofascin/L1/NrCam family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Davis J, Lambert S, Bennett V. Molecular composition of the node of Ranvier: Identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain−) and NrCAm at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerinck TJ, Levinson SR, Bennett GV, Ellisman MH. Clustering of voltage-sensitive sodium channels on axons is independent of direct Schwann cell contact in the dystrophic mouse. J Neurosci. 1997;17:5080–5088. doi: 10.1523/JNEUROSCI.17-13-05080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci. 1995;15:492–503. doi: 10.1523/JNEUROSCI.15-01-00492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B, Daniels M. Membrane proteins in the neuromuscular junction. Distribution of Na channels and ankyrin is complementary to acetylcholine receptors and the 43-kD protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Garver T, Davis J, Ren Q, Tuvia S, Bennett V. Tyrosin phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Sacramone LM, Mooseker MS, Morrow JS. Mechanisms of cytoskeletal regulation: modulation of membrane affinity in avian brush border and erythrocyte spectrins. J Cell Biol. 1985;101:1379–1385. doi: 10.1083/jcb.101.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh ST, Kidd GJ, Crawford TO, Xu Z, Lin WM, Trapp BD, Cleveland DW, Griffin JW. Regional modulation of neurofilament organization by myelination in normal axons. J Neurosci. 1994;14:6392–6401. doi: 10.1523/JNEUROSCI.14-11-06392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu RJ, Moorthy S, Bennett V. Expression of functional domains of beta G-spectrin disrupts epithelial morphology in cultured cells. J Cell Biol. 1995;128:1069–1080. doi: 10.1083/jcb.128.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Ishihara A, Inman R. Lateral diffusion of proteins in membrane. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Sheets ED, Simson R. Revisiting the fluid mosaic model of membranes. Science. 1995;268:1441–1442. doi: 10.1126/science.7770769. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Kleitman, N., P. Wood, and R.P. Bunge. 1991. Tissue culture methods for the study of myelination. In Culturing Nerve Cells. G. Banker and K. Goslin, editors. The MIT Press, Cambridge, MA. 337–377.
- Kordeli E, Davis J, Trapp B, Bennett V. An isoform of ankyrin is localized at nodes of Ranvier in myelinated axons of central and peripheral nerves. J Cell Biol. 1990;110:1341–1352. doi: 10.1083/jcb.110.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG: a new ankyrin gene with neural-specific isoforms localized at the axonal proximal segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem. 1995a;270:22050–22057. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- Michaely P, Bennett V. Mechanism for binding site diversity on ankyrin: comparison of binding sites on ankyrin for neurofascin and the C1−/ HCO3 −anion exchanger. J Biol Chem. 1995b;270:31298–31302. doi: 10.1074/jbc.270.52.31298. [DOI] [PubMed] [Google Scholar]
- Peters LL, John KM, Lu FM, Eicher EM, Higgins A, Yialamas M, Turtzo LC, Otsuka A, Lux SE. Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J Cell Biol. 1995;130:313–321. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Clustering sodium channels at the node of Ranvier: close encounters of the axon-glia kind. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Novakovic SD, Levinson SR, Schachner M, Shrager P. The clustering of axonal sodium channels during development of the peripheral nervous system. J Neurosci. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Messing A, Chiu SY, Levinson SR, Schachner M, Roder J, Li C, Novakovic S, Shrager P. Sodium channel distribution in axons of hypomyelinated and MAG null mutant mice. J Neurosci Res. 1997;50:321–336. doi: 10.1002/(SICI)1097-4547(19971015)50:2<321::AID-JNR20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Waxman, S.G. 1984. Nodelike membrane at extranodal sites: comparative morphology and physiology. In The Node of Ranvier. J.C. Zagoren and S. Fedoroff, editors. Academic Press, Inc. Orlando, FL. 311–351.
- Wood SJ, Slater CR. β-Spectrin is colocalized with voltage-gated sodium channels and ankyrinGat the adult rat neuromuscular junction. J Cell Biol. 1998;140:675–684. doi: 10.1083/jcb.140.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Crise B, Su B, Hou Y, Rose JK, Bothwell A, Jacobson K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol-linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 1991;115:75–84. doi: 10.1083/jcb.115.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bennett V. Identification of O-linked N-acetylglucosamine modification of ankyrinGisoforms targeted to nodes of Ranvier. J Biol Chem. 1996;271:31391–31398. doi: 10.1074/jbc.271.49.31391. [DOI] [PubMed] [Google Scholar]
- Zhang, X. 1998. Structural and functional analysis of 270/480 kDa ankyrinG and its interaction with neurofascin. Ph.D. thesis. Duke University, Durham, NC. 132–162.
- Zhang, X., J. Davis, S. Carpenter, and V. Bennett. 1998. Structural requirements for association of neurofascin with ankyrin. J. Biol. Chem. 273:In press. [DOI] [PubMed]