Abstract
The uncatalyzed reactions of O2 (S = 1) with organic substrates (S = 0) are thermodynamically favorable but kinetically slow because they are spin-forbidden and the one-electron reduction potential of O2 is unfavorable. In nature, many of these important O2 reactions are catalyzed by metalloenzymes. In the case of mononuclear non-heme iron enzymes, either FeII or FeIII can play the catalytic role in these spin-forbidden reactions. Whereas the ferrous enzymes activate O2 directly for reaction, the ferric enzymes activate the substrate for O2 attack. The enzyme–substrate complex of the ferric intradiol dioxygenases exhibits a low-energy catecholate to FeIII charge transfer transition that provides a mechanism by which both the Fe center and the catecholic substrate are activated for the reaction with O2. In this Perspective, we evaluate how the coupling between this experimentally observed charge transfer and the change in geometry and ligand field of the oxidized metal center along the reaction coordinate can overcome the spin-forbidden nature of the O2 reaction.
Mononuclear non-heme iron enzymes perform a wide range of important biological functions involving O2 in parallel to those of heme enzymes (1–5). Although the uncatalyzed reactions of O2 (S = 1) with organic substrates (S = 0) are thermodynamically favorable, the reactions are kinetically slow because they are spin-forbidden and the one-electron reduction potential of O2 is unfavorable. Most of these enzymes catalyze the O2 reaction by using a high-spin FeII to activate O2 through a redox process that also involves the substrate or an additional cofactor providing the required number of electrons. These ferrous O2-activating enzymes include extradiol dioxygenases, pterin-dependent hydroxylases, α-ketoglutarate-dependent enzymes, and Rieske dioxygenases. However, some mononuclear non-heme iron enzymes possess an oxidized iron active site and use the FeIII to activate substrate for the O2 reaction. These ferric substrate-activating enzymes presently include the lipoxygenases (LO) and intradiol dioxygenases
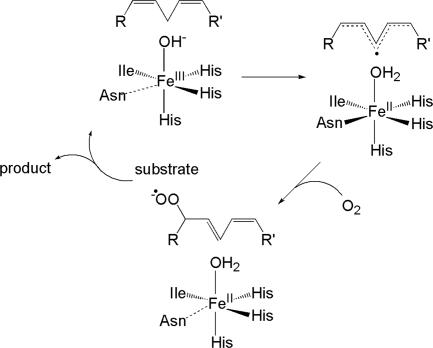
LO employ a relatively straightforward substrate activation mechanism. They catalyze the regio- and stereospecific hydroperoxidation of 1,4-Z,Z-pentadiene-containing polyunsaturated carboxylic acids (6–10). The resting site of LO consists of a ferrous center coordinated to three histidine ligands, a monodentate C-terminal carboxylate of isoleucine, a carbonyl O of asparagine, and a water (11–13). Resting FeIILO is oxidized by the hydroperoxide product to FeIIILO, and the water ligand is deprotonated to hydroxide. This FeIII(OH) species catalyzes the first step of the reaction, namely, hydrogen-atom abstraction from the substrate to form a substrate radical, which is accompanied by the reduction of the FeIII(OH) to FeII(H2O). The substrate bound near this FeII site is activated to a radical species (S = 1/2), and its reaction with O2 (S = 1) is spin-allowed. This step forms a peroxy radical, and then the hydroperoxide product as FeII(H2O) is oxidized to FeIII(OH). This mechanism is summarized in Fig. 1 (14, 15). The driving force for the hydrogen-atom abstraction is related to the strength of the OH bond produced in the abstraction intermediate, which is directly related to the reduction potential of the FeIII/FeII couple (E°) and the pKa of the abstracted proton in the ferrous species. A high reduction potential and a high pKa of the coordinated water in the reduced species increases the hydrogen-atom abstraction driving force and, hence, the catalytic rate (15–17).
Fig. 1.
Mechanism for LO.
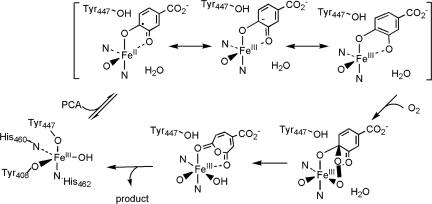
The substrate activation mechanism for intradiol dioxygenases is more complex. These enzymes catalyze the cleavage of molecular oxygen accompanied by insertion of both oxygen atoms between the vicinal hydroxyl groups of the catecholic substrate, resulting in ring cleavage to yield muconic acid derivatives (3–5, 18, 19). The resting state of intradiol dioxygenases contains a high-spin ferric center in a distorted trigonal bipyramidal geometry, with Tyr and His as the axial ligands and Tyr, His, and a hydroxide ligand defining the equatorial plane (20–22). Upon anaerobic substrate binding, the active site shifts to a square pyramidal geometry in which the axial Tyr and equatorial OH− are displaced by the substrate, which binds bidentate in its doubly deprotonated form (23, 24). The open coordination position is trans to the equatorial His, and the substrate binds asymmetric to the FeIII center with the longer bond trans to the equatorial Tyr. EPR and Mössbauer studies have shown that that the iron center remains oxidized and high-spin upon substrate binding (25, 26). Thus direct interaction between the triplet oxygen and the singlet catecholate substrate is still spin-forbidden. Based on different electronic descriptions of the enzyme–substrate (ES) complex, various mechanisms have been proposed for the substrate activation step for the initial O2 attack in the enzymatic reaction (bracket in Fig. 2): (i) FeII-semiquinone character with O2 attacking the iron site; (ii) FeII-semiquinone character with O2 attacking the substrate through radical coupling, and (iii) FeIII-catecholate with strong ketonized-character promoted by lengthening the Fe–Ocatechol bond due to the trans effect of the equatorial Tyr, with O2 directly attacking the substrate through an electrophilic activation (27–33). The net result of the O2 attack is generally thought to be the formation of a peroxy-adduct bridging between the iron site and one of the hydroxylated carbons of the now quinone that requires a spin-forbidden two-electron transfer of the electron pair of the substrate to the triplet O2 molecule (23). It is important to determine the mechanism of substrate activation for O2 reaction in the intradiol dioxygenases both for this class of enzymes and for others where similar mechanisms have been invoked as, for example, in cofactor biogenesis in copper-containing amine oxidases (34, 35).
Fig. 2.
Intradiol dioxygenases mechanism.
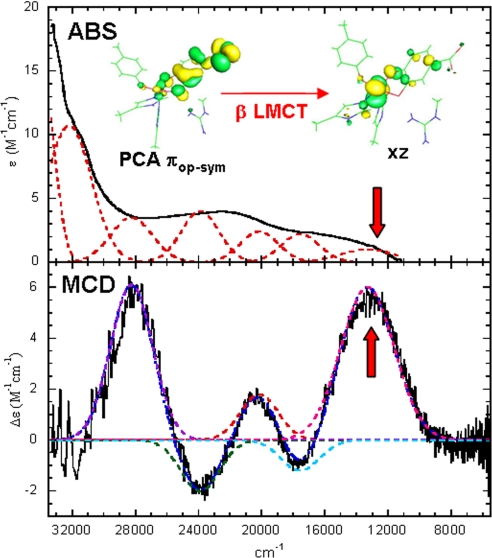
Because the differences among the substrate activation proposals of intradiol dioxygenases (Fig. 2) lie in the electronic structure description of the ES complex, it was essential to determine the nature of this ES complex experimentally, particularly its degree of radical character. In our previous spectroscopic studies on protocatechuate 3,4-dioxygenase (3,4-PCD) with protocatechuate (PCA) bound, we found that the ES complex is best described as a highly covalent FeIII-catecholate complex with the covalency distributed unevenly among the four valence donor orbitals of the catecholate (36). Because the catecholate has not been fully oxidized to a semiquinone and the oxidation state of the iron remains at the FeIII level (S = 5/2), the reaction with triplet oxygen is still formally spin-forbidden. The combined absorption and magnetic circular dichroism spectra of the ES complex shown in Fig. 3 reflect a very low energy β electron charge transfer transition (the high-spin FeIII has five d electrons with α spin) from the PCA substrate to the FeIII center involving the πop-sym (out-of-plane, symmetric) highest occupied molecular orbital, which is the frontier orbital of the catecholate. This low-energy catecholate to FeIII charge transfer (arrow in Fig. 3) provides a mechanism in which both the Fe center and the catecholic substrate are activated for the reaction with O2 (36).
Fig. 3.
Absorption (ABS) and magnetic circular dichroism (MCD) spectra of the ES complex of 3,4-PCD.
In this Perspective, we will consider how this β charge transfer between PCA and the FeIII center and the ligand field of the oxidized metal site in intradiol dioxygenases can overcome the spin-forbidden nature of the two-electron transfer of an electron pair from the highest occupied molecular orbit of the substrate to the triplet O2 molecule. A reaction coordinate leading to the final intradiol-cleaved product also will be considered.
Nature of the ESO2 Complex
A two-electron transfer between PCA and O2 accompanies the formation of the peroxy intermediate bridging between the Fe center and one of the hydroxyl carbons on the catecholic substrate. Direct interaction between the triplet O2 (S = 1) and the singlet catecholic substrate (S = 0) is formally spin-forbidden. However, EPR studies showed that the ES complex contains a high-spin FeIII center with Stot = 5/2 (25, 26). Hence, the accessible spin states for the O2 (S = 1) reaction with the ES complex are 3/2, 5/2, and 7/2.
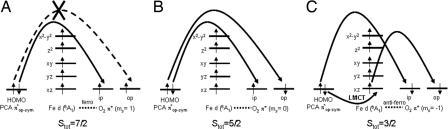
To obtain Stot = 7/2, the O2 molecule has to be in its 3Σg− ground state and ferromagnetically coupled to the high-spin FeIII center for the initial O2 attack (Fig. 4A). This would allow the transfer of a β electron from the occupied PCA πop-sym orbital to the O2 π* orbital either directly or through the Fe center. However, the remaining half-occupied PCA πop-sym orbital has an α spin. Transfer of this α electron to the remaining half-occupied O2 π* orbital to complete the two-electron redox process is therefore restricted. Geometry optimization of ESO2 with Stot = 7/2 shows very weak O2 interactions with both the Fe center and the substrate (Fe–O2 = 2.42 Å, Fe–C3 = 2.78 Å, Fe–C4 = 2.80 Å). These limited bonding interactions with the ES complex suggests that the Stot = 7/2 surface is not a favorable path for the initial O2 reaction.
Fig. 4.
Schematics of electron transfer in the initial O2 reaction of intradiol ctron transfer in the initial O2 reaction of intradiol dioxygenases at various spin states: Stot = 7/2 (A), Stot = 5/2 (B), Stot = 3/2 (C).
For Stot = 5/2, the O2 molecule has to be partially in an excited singlet state (mixed 1Δg and 1Σg+) at the start of the O2 reaction (Fig. 4B). Because the 1Δg and 1Σg+ states are ≈1 eV and 1.6 eV higher in energy than the 3Σg− ground state of O2, the initial O2 reaction with Stot = 5/2 is energetically highly unfavorable.
To obtain Stot = 3/2, the O2 molecule is in its 3Σg− ground state and antiferromagnetically coupled to the high-spin FeIII center for the initial O2 attack (Fig. 4C, d5 Fe has five α spins and triplet O2 has two β spins). Spectroscopic data on the ES complex of 3,4-PCD (Fig. 3) (36) showed that the doubly occupied πop-sym orbital of the catecholic substrate PCA is the highest occupied molecular orbital. The high-lying πop-sym orbital of the substrate can readily donate into the O2 π* orbital for an electrophilic attack by O2. However, because O2 is in its 3Σg− state, only the α electron from the doubly occupied πop-sym orbital can be donated directly to the O2 π* orbital in the formation of a CPCA–O bond. The second electron required to reduce O2 to O22− has to be donated either by the substrate πop-sym orbital, which would require a forbidden spin-flip of the β electron or by an occupied d orbital (dxz) of the FeIII center, which has α spin and can interact with the remaining half-occupied π* orbital of O2 due to their antiferromagnetic coupling. The strong covalent interaction between the PCA πop-sym and dxz orbitals in the β manifold, reflected by the low-energy ligand-to-metal charge transfer transition observed in the spectroscopic data (Fig. 3), results in significant transfer of the β electron from the occupied PCA πop-sym orbital into the unoccupied β dxz orbital. This transfer would compensate the Fe for the donation of an α electron to O2. However, because the dxz orbital is the lowest-energy orbital in the Fe d manifold, the transfer of a β electron from the PCA to Fe and an α electron from Fe to O2 results in an excited intermediate spin state (S = 3/2) on the Fe center (Fig. 4C).
In the following sections, we consider the O2 reaction coordinate leading to the bridging peroxo complex along the Stot = 3/2 surface and explore the geometric changes involved in stabilizing the intermediate spin on the FeIII center. We also will discuss this reaction coordinate in the context of the mechanisms for overcoming the spin-forbidden nature of the O2 reaction in the intradiol dioxygenases.
Reaction Coordinate to Form the Peroxy ESO2 Complex
O2 can interact with the Fe center and the hydroxylated carbon of catecholic substrate either sequentially or in a concerted manner in the formation of the peroxy ESO2 complex. Previous experimental and theoretical studies showed that the ES complex of 3,4-PCD has little FeII character (20, 25, 26, 36); thus, direct interaction of O2 at the iron center as the first step of the reaction is unlikely. To determine whether the substrate is the initial site of O2 attack, we performed density functional theory calculations using an experimentally calibrated methodology developed in ref. 36 on hypothetical GaIII catecholate and semiquinone complexes. These complexes have a similar ligand environment to the enzyme active site. Because GaIII is diamagnetic with the d10 manifold strongly stabilized relative to the valence orbitals of the catecholic substrate, the metal d orbitals cannot participate in an interaction with O2, which allows evaluation of whether triplet oxygen can directly attack the substrate in its two limiting electronic descriptions (catecholate and semiquinone). Geometry optimizations of these GaIII complexes with O2 showed that triplet O2 does not interact with GaIII-catecholate. While a bridging superoxy complex is formed with GaIII-semiquinone, this adduct is 13 kcal/mol higher in energy than the nonbonding species, indicating that the direct attack of O2 on PCA in the ES complex of 3,4-PCD also is unlikely. This result is consistent with previous theoretical studies (32, 37) and suggests that O2 does not attack the iron center or the catecholate of the ES complex in a sequential manner.
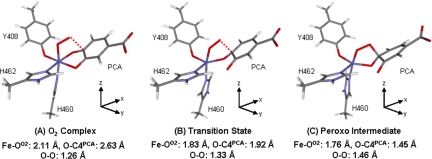
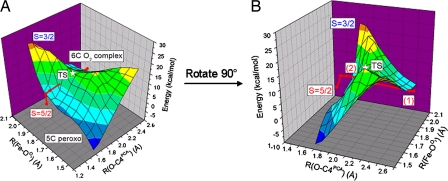
Binding O2 to the ES complex of 3,4-PCD results in a Stot = 3/2 complex with Fe–OO2 = 2.11 Å, O–C3PCA = 2.61 Å and O–C4PCA = 2.63 Å (Fig. 5A). Formation of this O2 complex is exothermic by 3.5 kcal/mol, and molecular orbital analysis shows that O2 interacts with both Fe and PCA in this complex (Fig. 6). This orbital interaction suggests that O2 attacks the iron and the catecholate in a concerted fashion. To form the bridging peroxo intermediate, the distal oxygen has to attack either of the hydroxylated carbons (C3 or C4). Reaction coordinates along the Fe–OO2 bond and either of the O–C3PCA and O–C4PCA bonds for Stot = 3/2 were calculated, and the 2D energy profile along the O–C4PCA reaction coordinate with a double-ξ basis set (6–31G*/3–21G*) in vacuum is shown in Fig. 7. A transition state is located at Fe–OO2 = 1.83 Å and O–C4PCA = 1.92 Å, with an imaginary frequency of −300 cm−1 (Fig. 5B). The vibrational motion of this mode corresponds to the distal O atom moving toward C4PCA and the O3PCA moving away from the Fe center. After the transition state, the peroxo-bridged complex is formed. The peroxy bridged quinone substrate now binds monodentate through O4PCA to form a five-coordinate (5C) Fe center (Fig. 5C). More accurate energies of all of the optimized structures were calculated with a triple-ξ basis set (TZVP), including solvation effects (polarized continuum model with a dielectric constant ε = 4.0) and zero-point and thermal corrections and are reported in this Perspective unless otherwise specified. The transition state for the formation of the peroxo intermediate bridging to C4PCA (Fig. 5B) is 12.2 kcal/mol higher in energy than the O2 complex (Fig. 5A). The overall reaction is slightly exothermic by 1.9 kcal/mol. The barrier of the peroxo formation can be attributed to the geometry change along the reaction coordinate. As the reaction proceeds from the six-coordinate (6C) O2 complex to the transition state, the Fe–O3PCA bond lengthens from 2.05 Å to 2.61 Å while the Fe–OO2 bond shortens from 2.11 Å to 1.83 Å. This leads to a 5C site at the FeIII center. However, because the bond between the distal oxygen and the substrate has not yet formed (O–C4PCA = 1.92 Å), this 5C structure is destabilized relative to the 6C O2 complex. As the O–C4 distance shortens, the bond between the distal oxygen and the substrate forms and the energy of the final peroxo product is stabilized relative to the transition state. Intrinsic reaction coordinate calculations toward both reactant and product directions at the transition state result in structures that look similar to the 6C O2 and the 5C peroxy complexes, respectively. This result confirms the presence of an accessible reaction pathway for the geometric change observed in the enzyme active site.
Fig. 5.
Geometry optimized structures of the O2 complex (A), transition state (B), and peroxo intermediate (C) obtained in the reaction coordinate calculation along Fe–OO2 and O–C4PCA. Their corresponding axes are included with the structures.
Fig. 6.
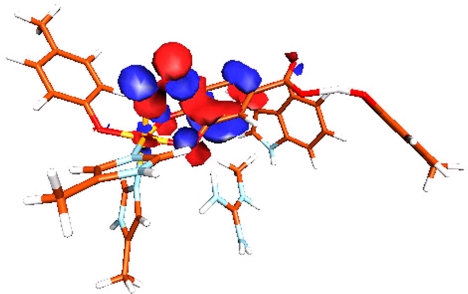
Molecular orbital demonstrating the concerted interactions of Fe and PCA with O2 in the Stot = 3/2 O2 complex.
Fig. 7.
O2 reaction coordinate for 3,4-PCD. (A) Two-dimensional potential energy surface along R(O–C4PCA) and R(Fe–OO2) reaction coordinates for Stot = 3/2 and Stot = 5/2 (red). (B) The two crossing points (1 and 2) between Stot = 3/2 and Stot = 5/2. The energies are obtained with BP86 + 10% Hartree–Fock exchange and 6–31G*/3–21G* basis set in vacuum.
The energy profile along the O–C3PCA reaction coordinate with Stot = 3/2 (data not shown) also leads to the formation a 5C bridging peroxo intermediate, but the monodentate substrate binds through O3PCA instead of O4PCA. The formation of this peroxo intermediate is exothermic by 0.5 kcal/mol. Hence, in terms of the energetics for the formation of the peroxo intermediate, there is no preference in C3 versus C4 attack by the distal oxygen, but the asymmetry of the Fe–OPCA bonds (Fe–O3PCA = 2.47 Å, Fe–O4PCA = 1.97 Å) observed in the crystal structure of the ES complex would facilitate the dissociation of the Fe–O3PCA bond in the attack of O2 at C4PCA, favoring this reaction. In this and the next section, we focus on the coordinate for O2 attack at C4PCA to understand the mechanism for overcoming the spin-forbiddeness of the O2 reaction.
As discussed in the previous section, the initial O2 attack with Stot = 5/2 is unfavorable because the O2 molecule has an excited singlet state character. However, the total energy of the Stot = 5/2 system decreases dramatically as the interaction between the singlet O2 molecule and the ES complex is increased. Upon formation of the O2 complex, the total energy of the Stot = 5/2 system decreases from being 38.3 kcal/mol higher than the Stot = 3/2 complex to only 3.6 kcal/mol. The energy profile along the O–C4PCA reaction coordinate of the Stot = 5/2 O2 complex (Fig. 7, red) shows that the energy barrier for the formation of the 5C peroxo intermediate is only 3.7 kcal/mol. This is significantly lower than the 12.2 kcal/mol observed in the Stot = 3/2 reaction but the total energy of the final 5C peroxo species in the Stot = 5/2 reaction is 4.1 kcal/mol less stable than the Stot = 3/2 product. Thus, the Stot = 5/2 surface intersects with the Stot = 3/2 surface at two different positions (Fig. 7B); the first is near the beginning of the reaction coordinate (Fe–OO2 = 1.95 Å, O–C4PCA = 2.40 Å) and the second is after the Stot = 3/2 transition state (Fe–OO2 = 1.90 Å, O–C4PCA = 1.80 Å). Although Stot = 3/2 is the more favorable reaction path for the initial O2 attack, intersystem crossing from Stot = 3/2 to Stot = 5/2 at the first intersection point of the two potential surfaces could, in principle, favor the overall energetics of the peroxy formation. However, differences in electronic as well as geometric structure between the two isoenergetic structures at the crossing point govern the feasibility of the intersystem crossing between these two spin surfaces. In the section below, we evaluate the viability of this intersystem crossing and present an alternative description of the mechanism for overcoming the spin-forbidden nature of the O2 reaction.
Mechanism for Overcoming the Spin-Forbidden Reaction
For facile spin-crossover from the Stot = 3/2 to Stot = 5/2 surfaces, the two species are required to have similar geometries and energies. Moreover, the electronic structures must be able to spin-orbit couple. Spin-orbit coupling (SOC) is effectively a localized, single-center, one-electron operator and can be written as
The L+S− + L−S+ operator in Eq. 1 performs a spin-flip and this process is accompanied by a change in the orbital due to the associated L+/L− raising/lowering operator. Hence, the two orbitals of opposite spins involved in SOC have to be on the same center with different spatial components. SOC can also proceed through the LzSz operator between microstates having the same Ms for the two different Stot states. In our case, this means coupling between the Ms = 3/2 microstates of the Stot = 3/2 and 5/2 states. This mechanism of SOC is feasible only if the two microstates differ solely in the occupation of two orbitals with the same spin and these two orbitals can couple through Lz.
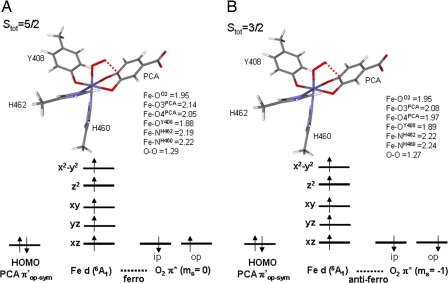
The two isoenergetic structures for Stot = 5/2 and 3/2 at the second crossing point along the O–C4PCA reaction coordinate have very different geometries; hence, intersystem crossing is not feasible. However, as shown in Fig. 8, the two isoenergetic structures for Stot = 5/2 and 3/2 at the first crossing point have similar geometries. It only takes 3.1 kcal/mol to excite the Stot = 3/2 electronic configuration in its optimized structure into a Stot = 5/2 state. Hence, it is energetically feasible for intersystem crossing. Orbital analysis on the Stot = 5/2 structure shows significant overlap between the doubly occupied PCA πop-sym orbital and the O2 molecule (ms = 0) in which the two π* orbitals are occupied by two electrons of opposite spin (β in πip* and α in πop*, with the in-plane (ip) and out-of-plane (op) orbitals defined by the Fe–O–O plane). For the Stot = 3/2 structure, a covalent interaction between the PCA πop-sym and O2 πip* in the α manifold is observed while the O2 πop* orbital remains singly occupied by a β electron. For both spin states, the iron center remains high-spin ferric with strong bonding interactions with PCA. Hence, the major difference in the electronic structure between the two spin states at the crossing point lies in the spin of the electron residing in the singly occupied πop*, with α for Stot = 5/2 and β for Stot = 3/2 (Fig. 8). Although both of these spin orbitals are localized on O2, they have the same spatial component in their wave functions (πop*). Hence, the Stot = 3/2 surface cannot effectively intersystem cross to the Stot = 5/2 surface through the SOC mechanism at this point of the potential energy surface as the orbital angular momentum operators associated with SOC in Eq. 1 require a change in orbital occupation. Thus, the O2 reaction with the ES complex should remain on the Stot = 3/2 surface from the initial attack at the Fe center through the formation of the bridging peroxo complex.
Fig. 8.
Optimized geometry and electronic structures of the ESO2 complex at the crossing point (Fe–O = 1.95 Å, O–C4 = 2.4 Å) of the Stot = 5/2 and Stot = 3/2 O-C4PCA reaction coordinates: Stot = 5/2 (A) and Stot = 3/2 (B).
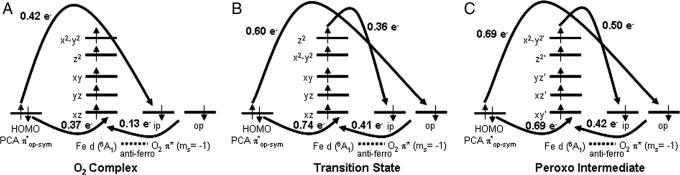
To understand how the spin forbiddeness of the O2 reaction is overcome on the Stot = 3/2 surface, electronic structures at three points along the O–C4 reaction coordinate are considered: (i) the O2 complex, (ii) the transition state, and (iii) the final bridged peroxo intermediate. The amount of charge transfer among the five Fe d orbitals, the PCA πop-sym orbital and the two O2 π* orbitals were quantified by calculating the total percentage of orbital occupancy and assigning the major bonding partner(s) for each of the orbitals in both α and β spins. For example, in the ES complex, the β PCA πop-sym orbital is 61% occupied (summed over all occupied molecular orbitals), and the remaining 39% is contributed in the three unoccupied Fe β dπ orbitals, with 18% on dxz. Thus the Fe β dxz orbital is the major bonding partner of the β PCA πop-sym, and a total of 0.39 e− has been transferred from the β PCA πop-sym to the Fe β dπ manifold. The electron transfers among orbitals for the three different points along the reaction coordinates are summarized in Fig. 9.
Fig. 9.
Schematics of electron transfer at three different points along the O–C4PCA reaction coordinates: O2 complex (A), transition state (B), and peroxo intermediate (C).
O2 Complex [Figs. 5A (Geometry) and 9A (Electronic Structure)].
The Fe–O2 bond is defined as the z axis with the two Fe–OPCA bonds defining the x and y axes. The energy ordering of the Fe d orbitals is as follows: dxz < dyz < dxy < dz2 < dx2 − y2. Because the dihedral angle between the Fe–O–O and O–O–C4 planes is 14.5°, our frontier molecular orbital analysis in ref. 36 predicted that O2 πip* will directly overlap with the PCA πop-sym orbital. Consistent with this model, 0.42 e− is donated directly from the α PCA πop-sym to the α O2 πip*, whereas 0.37 e− is donated through covalent bonding between β PCA πop-sym and Fe β dxz, as observed in our spectroscopic studies of the ES complex (Fig. 3) (36). The small charge transfer from the occupied β O2 π* orbitals to the unoccupied Fe β d orbitals reflects the covalent interactions between O2 and Fe. The net result is that PCA starts to gain more semiquinone character as a total of 0.79 e− is lost from the PCA πop-sym and the electronic description of the Fe–O2 unit is between the two limiting cases, FeII–O2− and FeIII–O2.
Transition State [Figs. 5B (Geometry) and 9B (Electronic Structure)].
Due to the lengthening of the Fe–O3PCA bond and the contraction of the Fe–Operoxide bond, the active site now adopts an intermediate structure between square pyramidal and trigonal bipyramidal. The energy ordering of the Fe d orbitals in the same molecular coordinate system as defined in the O2 complex is as follows: dxz < dyz < dxy < dx2 −y2 < dz2. The dissociation of the Fe–O3PCA bond allows the remaining three equatorial ligands (Tyr-408, His-462, and O4PCA) to reorganize in the plane and leads to the stabilization of the dxz orbital. On the other hand, the destabilization of the dz2 orbital is attributed to the strong Fe–Operoxide bond. Due to the large geometric rearrangement to produce the 5C Fe center, the Fe–O–O–C4PCA dihedral angle increases in magnitude from 14.5° to −35.0°, which allows direct overlap between the α PCA πop-sym and O2 πop* orbitals. The charge transfer pattern in the O2 complex also is observed in the transition state: 0.60 e− from the α PCA πop-sym to the α O2 op*, 0.74 e− from β PCA πop-sym to Fe β dxz and 0.41 e− from the β O2 π* to the Fe β d orbitals. There also is charge transfer of 0.36 e− from the Fe α dz2 orbital to the α O2 πip* orbital at the transition state. These results demonstrate that the two α electrons involved in reducing O2 to O22− come from two different sources, one from the catecholic substrate and the other from the Fe center. Due to the ligand rearrangement, the α electron transfer from the Fe proceed through the good overlap between the highest energy d orbital (dz2) and the O2 πip* orbital. However, this is compensated by the β donation from the PCA to the Fe dπ orbitals and results in an S = 3/2 intermediate spin electronic configuration at the FeIII center. The S = 3/2 is now in its ground state as the distorted trigonal bipyramidal structure around the Fe center stabilizes this electronic configuration. Considering the total e− gain and loss in the O2 π* and PCA πop-sym orbitals, respectively, the O2 has been reduced to a superoxide, while the PCA is at the semiquinone level at the transition state.
Final Peroxo Intermediate [Figs. 5C (Geometry) and 9C (Electronic Structure)].
The active site becomes more trigonal bipyramidal with the axial direction defined by the short Fe–Operoxide bond. The charge transfer pattern of this peroxo intermediate is the same as in the transition state, except that the two-electron redox process between the PCA and the O2 molecule is now complete. The same amount of α and β spins of the PCA πop-sym orbital are transferred to the α O2 πip* and Fe β dxz orbitals, respectively. The α electron transfer from the Fe dz2 orbital to the O2 πip* has increased to 0.50 e−. These results show that three different electron transfer processes (one β and two α) are involved with no spin flip in the formally spin-forbidden, two-electron redox process between the singlet catecholate and the triplet O2.
While the α electron of the singlet substrate can have direct overlap with the triplet O2 molecule, its β counterpart cannot be transferred directly to the O2 molecule without a spin-flip process. The FeIII center in the intradiol dioxygenases thus plays a critical role in overcoming the spin-forbidden nature of the O2 reaction. The strong covalent interaction between the substrate and the iron center, as reflected in the low-energy spectroscopic feature of the ES complex (Fig. 3), allows the transfer of this β electron from the substrate to the metal. With the metal acting as a buffer, an electron of the appropriate spin (α) can be transferred to the O2 molecule to complete the two-electron redox process. However, this would leave the iron center in an excited intermediate spin state (S = 3/2). The ligand field geometry change at the Fe center along the reaction coordinate is key in stabilizing this intermediate spin configuration. As the catecholic substrate is oxidized, the iron loses the Fe–O3PCA bond in the equatorial plane and the bonding interaction between the Fe and O2 is strengthened in the axial direction, producing a trigonal bipyramidal ligand field with a strong axial bond, which destabilizes the dz2 orbital and stabilizes the intermediate Stot = 3/2 spin state on the ferric center.
The Reaction Coordinate
As developed in the previous section, the O2 reaction with the ES complex remains on the Stot = 3/2 surface from the initial attack at the Fe center all of the way through the formation of the bridging peroxo complex. However, experimental data show that the ferric center is high-spin, S = 5/2, at the beginning and end of the catalytic cycle; hence, the 5C Stot = 3/2 peroxo intermediate must return to the Stot = 5/2 reaction surface. Inspection of the 6C O2 complex and the 5C peroxo species shows that, although the three endogenous ligands show very little change in their positions, the OTyr-408–Fe–O4PCA angle has increased from 100.7° to 135.7° as the peroxo is formed and the Fe–O3PCA bond is lost. This geometric change is due to the swinging of the Fe–O4PCA bond away from the trans position of His-462. Such geometric arrangement is very similar to those observed in the crystal structures of enzyme–inhibitor complexes (38). In these inhibitor complexes, Tyr-447 becomes the ligand trans to His-462. Opening the OTyr-408–Fe–O4PCA angle in the peroxy species would allow coordination of a sixth ligand, either Tyr-447 or a solvent water molecule. Kinetics studies of the O2 reaction of both the wild-type enzyme and the Y447H mutant show similar spectroscopic features for the two oxygen intermediates, ESO2 and ESO2*, with the latter being the enzyme–product complex (39). The absence of additional spectral features in the oxygen intermediates of the wild-type enzyme relative to the Y447H mutant strongly suggests the sixth ligand that binds to the 5C peroxo species is a water molecule. In the previous section, we emphasized how the intermediate spin state on the FeIII center is stabilized by a change in coordination number from 6 to 5 and the associated ligand field as the peroxo complex is formed. Thus, we expect the change in ligand field as the sixth ligand binds to the 5C peroxo complex would allow the Stot = 3/2 peroxo complex to return to Stot = 5/2 and complete the catalytic reaction.
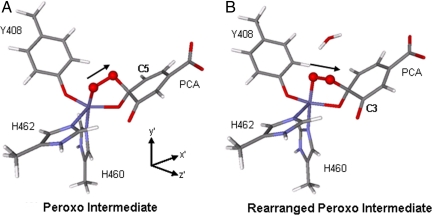
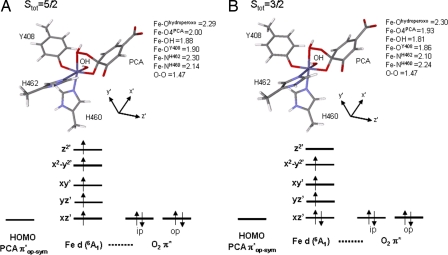
Attempts to bind a water to the open site of the Stot = 3/2 peroxo species lead to a very long Fe–OH2O distance of 3.61 Å, where the water is held weakly to FeIII active site through hydrogen bonding with the catecholic substrate. However, if the water molecule is added to the active site such that it is allowed to hydrogen-bond to the peroxo bridge, an interesting geometric change is observed. The O–O bond vector changes from pointing toward C5PCA, as in the 5C peroxo complex, to pointing toward C3PCA (Fig. 10). Such a rearrangement for the Stot = 5/2 species has been proposed by Siegbahn et al. (37) to be important in producing intradiol-cleaved product. Upon formation of this rearranged 5C peroxo species, proton donation from H2O to the peroxo unit results in OH− binding to the open coordination site. Geometry optimization of the 6C bridging hydroperoxy complex at both Stot = 3/2 and Stot = 5/2 shows that the latter species is now more stable by 2.8 kcal/mol. The geometric and electronic structures of these two bridging hydroperoxy adducts are shown in Fig. 11. Both of the structures are octahedral at the FeIII center, with the Fe–Ohydroperoxo bond lengthened to ≈2.3 Å. The 5C strong axial trigonal bipyramidal stabilization for the intermediate spin we observe for the peroxo species is no longer present. Comparing the electronic structures of the 6C hydroperoxo species in the two different spin states and using the coordinate system in Fig. 11, we can see that SOC between dxz′ and dz2′ in the Stot = 3/2 adduct through Ly would allow for intersystem crossing and the FeIII center can now return to the Stot = 5/2 state. We have considered the energetics for O–O bond cleavage following the formation of this Stot = 5/2 hydroperoxy adduct that lead to the formation of an anhydride intermediate. The results are similar to those reported by Siegbahn et al. (37) and, thus, will not be further discussed in this Perspective.
Fig. 10.
Geometries of peroxo intermediates: optimized peroxo intermediate (A), rearranged peroxo intermediate optimized with hydrogen bonding from water (B). The black arrow indicates the O–O bond vector.
Fig. 11.
Optimized geometry and electronic structures of the 6C hydroperoxo complex for both Stot = 5/2 (A) and Stot = 3/2 (B).
In summary, coordination of the sixth ligand to the 5C Stot = 3/2 peroxo adduct results in a ligand field change that allows the FeIII center to return to the Stot = 5/2 reaction surface and complete the catalytic cycle.
Concluding Comments
We have developed a mechanism in which the high-spin ferric active site of the intradiol dioxygenases can use its coordination environment to tune the ligand field around the metal center to allow a change in spin state from S = 5/2 to S = 3/2 along the reaction coordinate, which involves the transfer of three electrons without a change in their spins. The intermediate S = 3/2 spin state is accessible to the FeIII center upon O2 binding and dissociation of an Fe–Ocatecholate bond. This provides a mechanism for overcoming the spin-forbidden nature of the reaction between the triplet O2 and singlet organic substrates. This 5C Stot = 3/2 peroxo intermediate must return to the Stot = 5/2 surface to complete the reaction and this can be accomplished by coordination of H2O to the open position in the FeIII center. H2O coordination also provides a proton for the O–O bond cleavage and the insertion of an O atom for the intradiol dioxygenation.
Acknowledgments
This research was supported by National Institutes of Health Grants GM40392 (to E.I.S.) and GM24689 (to J.D.L.). M.Y.M.P. thanks the Natural Sciences and Engineering Research Council of Canada for a postgraduate scholarship.
Abbreviations
- LO
lipoxygenase
- ES
enzyme–substrate
- 3,4-PCD
protocatechuate 3,4-dioxygenase
- PCA
protocatechuate
- 5C
5-coordinate
- 6C
6-coordinate
- SOC
spin-orbit coupling.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Feig AL, Lippard SJ. Chem Rev. 1994;94:759–805. [Google Scholar]
- 2.Hegg EL, Que L., Jr Eur J Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- 3.Lipscomb JD, Orville AM. Metal Ions Biol Syst. 1992;28:243–298. [Google Scholar]
- 4.Que L, Jr, Ho RYN. Chem Rev. 1996;96:2607–2624. doi: 10.1021/cr960039f. [DOI] [PubMed] [Google Scholar]
- 5.Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 6.Gardner HW. Biochim Biophys Acta. 1991;1084:221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- 7.Siedow JN. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:145–188. [Google Scholar]
- 8.Yamamoto S. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 9.Ford-Hutchinson AW, Gresser M, Young RN. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- 10.Kühn H, Borngräber S. In: Lipoxygenases and Their Metabolites. Nigam S, Pace-Asciak CR, editors. New York: Plenum; 1999. pp. 5–28. [Google Scholar]
- 11.Boyington JC, Gaffney BJ, Amzel LM. Science. 1993;260:1482–1486. doi: 10.1126/science.8502991. [DOI] [PubMed] [Google Scholar]
- 12.Minor W, Steczko J, Stec B, Otwinowski Z, Bolin JT, Walter R, Axelrod B. Biochemistry. 1996;35:10687–10701. doi: 10.1021/bi960576u. [DOI] [PubMed] [Google Scholar]
- 13.Tomchick DR, Phan P, Cymborowski M, Minor W, Holman TR. Biochemistry. 2001;40:7509–7517. doi: 10.1021/bi002893d. [DOI] [PubMed] [Google Scholar]
- 14.Nelson MJ, Seitz SP. Curr Opin Struct Biol. 1994;4:878–884. doi: 10.1016/0959-440x(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 15.Solomon EI, Zhou J, Neese F, Pavel EG. Chem Biol. 1997;4:795–808. doi: 10.1016/s1074-5521(97)90113-7. [DOI] [PubMed] [Google Scholar]
- 16.Holman TR, Zhou J, Solomon EI. J Am Chem Soc. 1998;120:12564–12572. [Google Scholar]
- 17.Gardner KA, Mayer JM. Science. 1995;269:1849–1851. doi: 10.1126/science.7569922. [DOI] [PubMed] [Google Scholar]
- 18.Bugg TDH, Winfield CJ. Nat Prod Rep. 1998:513–530. [Google Scholar]
- 19.Bugg TDH, Lin G. Chem Commun. 2001:941–952. [Google Scholar]
- 20.True AE, Orville AM, Pearce LL, Lipscomb JD, Que L., Jr Biochemistry. 1990;29:10847–10854. doi: 10.1021/bi00500a019. [DOI] [PubMed] [Google Scholar]
- 21.Ohlendorf DH, Orville AM, Lipscomb JD. J Mol Biol. 1994;244:586–608. doi: 10.1006/jmbi.1994.1754. [DOI] [PubMed] [Google Scholar]
- 22.Davis MI, Orville AM, Neese F, Zaleski JM, Lipscomb JD, Solomon EI. J Am Chem Soc. 2002;124:602–614. doi: 10.1021/ja011945z. [DOI] [PubMed] [Google Scholar]
- 23.Orville AM, Lipscomb JD, Ohlendorf DH. Biochemistry. 1997;36:10052–10066. doi: 10.1021/bi970469f. [DOI] [PubMed] [Google Scholar]
- 24.Horsman GP, Jirasek A, Vaillancourt FH, Barbosa CJ, Jarzecki AA, Xu CL, Mekmouche Y, Spiro TG, Lipscomb JD, Blades MW, et al. J Am Chem Soc. 2005;127:16882–16891. doi: 10.1021/ja053800o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Que L, Jr, Lipscomb JD, Zimmerman R, Münck E, Orme-Johnson NR, Orme-Johnson WH. Biochim Biophys Acta. 1976;452:320–334. doi: 10.1016/0005-2744(76)90182-0. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker JW, Lipscomb JD, Kent TA, Münck E. J Biol Chem. 1984;259:4466–4475. [PubMed] [Google Scholar]
- 27.Que L, Jr, Lipscomb JD, Munck E, Wood JM. Biochim Biophys Acta. 1977;485:60–74. doi: 10.1016/0005-2744(77)90193-0. [DOI] [PubMed] [Google Scholar]
- 28.Cox DD, Que L., Jr J Am Chem Soc. 1988;110:8085–8092. [Google Scholar]
- 29.Jang HG, Cox DD, Que L., Jr J Am Chem Soc. 1991;113:9200–9204. [Google Scholar]
- 30.Lipscomb JD, Orville AM, Frazee RW, Dolbeare KB, Elango N, Ohlendorf DH. In: Spectroscopic Methods in Bioinorganic Chemistry. Solomon EI, Hodgson KO, editors. Washington, DC: Am Chem Soc; 1998. pp. 387–402. [Google Scholar]
- 31.Funabiki T, Konishi T, Kobayashi S, Mizoguchi A, Takano M, Yoshida S. Chem Lett. 1987:719–722. [Google Scholar]
- 32.Funabiki T, Yamazaki T. J Mol Catal A. 1999;150:37–47. [Google Scholar]
- 33.Hitomi Y, Yoshida M, Higuchi M, Minami H, Tanaka T, Funabiki T. J Inorg Biochem. 2005;99:755–763. doi: 10.1016/j.jinorgbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Okajima T, Kishishita S, Yoshimura M, Kawamori A, Tanizawa K, Yamaguchi H. Nat Struct Biol. 2002;9:591–596. doi: 10.1038/nsb824. [DOI] [PubMed] [Google Scholar]
- 35.Dove JE, Schwartz B, Williams NK, Klinman JP. Biochemistry. 2000;39:3690–3698. doi: 10.1021/bi992225w. [DOI] [PubMed] [Google Scholar]
- 36.Pau MYM, Davis MI, Orville AM, Lipscomb JD, Solomon EI. J Am Chem Soc. 2007;129:1944–1958. doi: 10.1021/ja065671x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borowski T, Siegbahn PEM. J Am Chem Soc. 2006;128:12941–12953. doi: 10.1021/ja0641251. [DOI] [PubMed] [Google Scholar]
- 38.Orville AM, Elango N, Lipscomb JD, Ohlendorf DH. Biochemistry. 1997;36:10039–10051. doi: 10.1021/bi970468n. [DOI] [PubMed] [Google Scholar]
- 39.Frazee RW, Orville AM, Dolbeare KB, Yu H, Ohlendorf DH, Lipscomb JD. Biochemistry. 1998;37:2131–2144. doi: 10.1021/bi972047b. [DOI] [PubMed] [Google Scholar]