Abstract
VEGF is the best characterized mediator of tumor angiogenesis. Anti-VEGF agents have recently demonstrated impressive efficacy in human cancer trials, but the optimal dosing of such agents must still be determined empirically, because biomarkers to guide dosing have yet to be established. The widely accepted (but unverified) assumption that VEGF production is quite low in normal adults led to the notion that increased systemic VEGF levels might quantitatively reflect tumor mass and angiogenic activity. We describe an approach to determine host and tumor production of VEGF, using a high-affinity and long-lived VEGF antagonist now in clinical trials, the VEGF Trap. Unlike antibody complexes that are usually rapidly cleared, the VEGF Trap forms inert complexes with tissue- and tumor-derived VEGF that remain stably in the systemic circulation, where they are readily assayable, providing unprecedented capability to accurately measure VEGF production. We report that VEGF production is surprisingly high in non-tumor-bearing rodents and humans, challenging the notion that systemic VEGF levels can serve as a sensitive surrogate for tumor load; tumor VEGF contribution becomes significant only with very large tumor loads. These findings have the important corollary that anti-VEGF therapies must be sufficiently dosed to avoid diversion by host-derived VEGF. We further show that our assay can indicate when VEGF is optimally blocked; such biomarkers to guide dosing do not exist for other anti-VEGF agents. Based on this assay, VEGF Trap doses currently being assessed in clinical trials are in the efficacious range.
Keywords: aflibercept, angiogenesis, tumor, endothelial cell
VEGF is critical in many settings of physiological and pathological angiogenesis (1). In particular, high VEGF expression is characteristic of many types of cancers (1), suggesting that it might be an attractive target for therapeutic intervention aimed at preventing tumors from recruiting the blood supply that they need to survive (2). The first attempts at validating this particular approach were taken by Ferrara and colleagues (3), who demonstrated that a murine anti-human VEGF antibody suppressed the growth of human tumor cell lines implanted in nude mice. This led to the generation of a humanized monoclonal antibody, bevacizumab (Avastin; Genentech, South San Francisco, CA), which yielded impressive results in a controlled clinical trial in patients with metastatic renal cell cancer (4, 5). At doses of 3 and 10 mg/kg, bevacizumab treatment resulted in a significant prolongation in time to tumor progression compared with placebo, although the increased efficacy of the higher dose in this study suggested that the maximally efficacious dose may not yet have been attained (4, 5). Bevacizumab was subsequently granted FDA approval based on the demonstration that it significantly improved the progression-free and overall survival in patients with metastatic colorectal cancer when given in combination with irinotecan 5-FU/LV chemotherapy (6). Several other drugs designed to block VEGF signaling have since been developed and recently approved [BAY 43–9006 (sorafenib) and SU11248 (sunitinib)] or are proceeding through clinical trials [PTK787 (vatalanib), ZD6474 (zactima), ZD6126, SU5416 (semaxanib), and AG-013736] (7–9).
As new anti-VEGF agents proceed through the clinic, it would be very useful to have biomarkers that could either identify patients whose tumors depend most on VEGF or that could guide dosing by indicating when optimal VEGF blockade has been achieved. Unfortunately, accepted biomarkers do not currently exist for VEGF blockade and are few and far between for other targeted agents, such as epidermal growth factor receptor for colon cancer, Kit for gastrointestinal stromal tumor, and HER2/NEU for breast cancer (10). VEGF itself has been suggested as a candidate biomarker for guiding the application of anti-VEGF therapies. It is widely assumed that VEGF production is quite low in healthy adults in the absence of active angiogenesis. Were that the case, blood levels of VEGF in cancer patients might provide a useful index of tumor VEGF production (11, 12). However, because VEGF is rapidly cleared from the systemic circulation (having a half-life of only minutes), the sensitivity of assays measuring VEGF in the peripheral blood leads to a wide variability for blood levels of VEGF in published reports. Furthermore, VEGF is present at substantial levels within platelets and released upon their lysis such that preparation of peripheral blood samples that avoid contamination from platelet-derived VEGF becomes difficult. These limitations are reflected in the disparate values reported for circulating VEGF levels in cancer patients, which range from 0.04 to 1 ng/ml, calling into question the utility of plasma VEGF levels as a useful biomarker for guiding anti-angiogenic therapy (11, 13–19).
VEGF Trap is a fully human soluble decoy receptor protein that consists of a fusion of the second Ig domain of human VEGF receptor (VEGFR) 1 and the third Ig domain of human VEGFR2 with the constant region (Fc) of human Ig IgG1 (20). The VEGF Trap was engineered to have optimized pharmacokinetic properties and a very high affinity for all isoforms of VEGF-A (<1 pM), as well as placental growth factor, a closely related angiogenic factor (20). VEGF Trap has shown robust antitumor effects in numerous mouse models of cancer and is now in clinical trials (21, ‡, §, ¶, ‖). Here, we show that—unlike VEGF antibodies that tend to form multimeric immune complexes that are rapidly cleared from the circulation and can form immune complex deposits in tissues—the VEGF Trap forms a stable and inert 1:1 complex with VEGF. This VEGF–VEGF Trap complex has a long plasma half-life and can readily be measured in the systemic circulation, thus affording a reliable way to measure the rates of VEGF production in both tumor-bearing and non-tumor-bearing adult animals and humans. This unique ability to capture and thus precisely measure total VEGF levels, regardless of whether the VEGF comes from tumor or normal host tissues, allows for the unprecedented opportunity to accurately determine tumor and host VEGF production rates. Surprisingly, we find that total body VEGF production rates are quite high in normal adult rodents and humans, with the fractional contribution made by tumors being comparatively small. This finding has the important implication that therapies directed toward neutralizing VEGF produced by tumors must be provided in sufficient amounts so as to avoid being largely consumed by the significant levels of VEGF produced by the rest of the body. Toward this end, measurement of VEGF Trap complex allows the identification of VEGF Trap doses required to completely capture and block tumor-derived VEGF, providing a useful guide for optimizing angiogenic blockade; such assays do not exist for other anti-VEGF agents. Based on this assay, we report that VEGF Trap doses currently being assessed in clinical trials appear to be in the efficacious range.
Results
VEGF Trap Forms an Inert Complex with VEGF That Remains Stably in the Circulation.
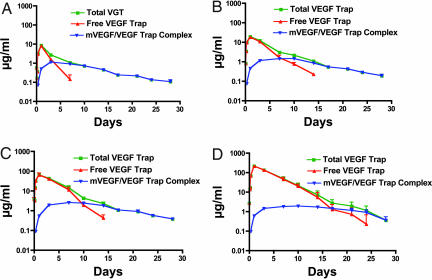
Initial studies to determine the clearance rate of VEGF Trap revealed that it could form stable detectable complexes with endogenous VEGF in normal adult mice. After single injections of increasing amounts of VEGF Trap, we measured total VEGF Trap, uncomplexed/unbound or “free” VEGF Trap, and VEGF Trap–mouse VEGF “complex” at various times after injection (Fig. 1 A–D represent increasing amounts of injected VEGF Trap). Because no exogenous VEGF was provided, complexes represent the association of VEGF Trap with endogenous murine VEGF. As expected, total VEGF Trap levels increased proportional to dose (determined by combining free VEGF Trap levels with complex levels) (Fig. 1, see green curves). Somewhat unexpectedly, substantial levels of VEGF Trap complexed with mouse VEGF accumulated rapidly (Fig. 1, see blue curves). At all doses of VEGF Trap tested, maximal levels of complex (≈1–2 μg/ml) were attained within 24–48 h of injection and sustained at this level for at least several days. Consistent with conversion of free VEGF Trap into complexed VEGF Trap, most of the injected VEGF Trap is initially found in the free, unbound form, but after reaching peak levels (≈24 h after injection) free VEGF Trap in the circulation declines progressively (Fig. 1, note that red curves, corresponding to free VEGF Trap, initially overlap at early time points with green curves, representing total VEGF Trap, but then drop, as is most obvious at the lowest dose). Levels of free VEGF Trap decline because of a “consumption” (binding VEGF, thus being converted to complex) and clearance, which occurs at an identical rate for free and bound Trap. Thus, as long as free VEGF Trap remains in excess of bound, maximal steady-state levels of complex are maintained in the circulation. VEGF Trap is also able to bind placental growth factor with high affinity and is capable of forming stable circulating placental growth factor–VEGF Trap complexes in vivo with the same profile as VEGF–VEGF Trap complex, albeit at ≈10-fold lower levels (data not shown).
Fig. 1.
s.c. injection of VEGF Trap into SCID mice at different doses reveals different levels of circulating free VEGF Trap but similar levels of circulating mouse VEGF–VEGF Trap complex. At all doses ranging from 1 mg/kg (A) to 25 mg/kg (D), a steady-state level of VEGF–VEGF Trap complex is achieved, which plateaus at ≈1 μg/ml. Dose-dependent levels of free VEGF Trap are observed as follows: 1 mg/kg to 10 μg/ml Cmax falling below complex levels at 4 days (A); 2.5 mg/kg to 20 μg/ml Cmax falling below complex levels at 7 days (B); 10 mg/kg to 80 μg/ml Cmax falling below complex levels at 9 days (C); and 25 mg/kg to 200 μg/ml Cmax falling below complex levels at 17 days (D). The half-life of VEGF Trap is ≈2 days at doses >2.5 mg/kg. (n = 6 for each dose.)
In separate experiments, the bioavailability of VEGF Trap and the efficiency of VEGF capture were determined by injecting s.c. [supporting information (SI) Fig. 7A] or i.v. (SI Fig. 7B) preformed complexes of the Trap and its VEGF target, or both agents separately. The results show that the bioavailability of s.c. (SQ) injected complex was essentially identical to that of i.v. injected complex, indicating that negligible complex was depositing within tissues. Moreover, whether the VEGF Trap was injected as a preformed complex with VEGF (single bolus) or the Trap and its target were injected separately, similar levels of complex were rapidly noted in the circulation, indicating that the Trap efficiently captures its target and brings it into the systemic circulation. In addition, VEGF Trap is also capable of sequestering VEGF already bound in target tissues as shown by injecting VEGF before VEGF Trap (SI Fig. 7). Thus, VEGF Trap efficiently captures and forms inert complexes with VEGF that enter and remain stably in the circulation, readily accessible for measurement.
Although VEGF Trap Forms a 1:1 Complex with VEGF, VEGF Antibodies Form Heterogeneous, Multimeric Immune Complexes with VEGF.
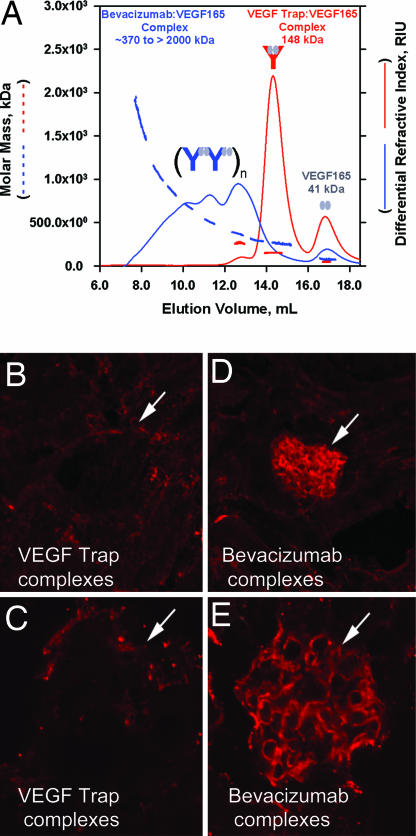
The above findings suggested that VEGF Trap might behave very differently than VEGF antibodies, because antibodies commonly form multimeric immune complexes that rapidly deposit in tissues and thus are rapidly cleared from the circulation. Because immune complexes rapidly disappear, the amount of captured ligand cannot be determined from levels of bound or unbound antibodies remaining in the circulation. To demonstrate directly that the VEGF Trap behaves in a fundamentally different way than antibodies, we compared VEGF Trap complex formation and clearance with that of a well characterized VEGF antibody, bevacizumab (Avastin). As predicted, size exclusion chromatography (SEC) of a preformed VEGF Trap–VEGF165 complex revealed a single major homogenous peak, with an approximate molecular mass (as judged by comparison to molecular mass standards, data not shown) of ≈150 kDa corresponding to that expected of a 1:1 complex between VEGF Trap (≈110 kDa) and VEGF165 (≈40 kDa) (Fig. 2A, solid red line); a minor peak of free excess VEGF165 was also seen, as was a small shoulder of higher molecular mass. The molecular masses of the peaks were confirmed by using coupled multiangled laser light scattering (MALLS) (dashed red lines in Fig. 2A). In contrast, SEC of preformed bevacizumab–VEGF165 complexes revealed a heterogeneous mixture corresponding to very high molecular masses (Fig. 2A, solid blue line) in addition to the small peak of free excess VEGF165. The purity of free VEGF Trap, bevacizumab, and VEGF was >97%, as determined by SEC (data not shown). Coupled MALLS analysis revealed molecular masses of the heterogeneous mixture ranging from 370 kDa (corresponding to a multimer consisting of two bevacizumab molecules, each with a molecular mass of ≈145 kDa, and two VEGF165 molecules, each with a molecular mass of ≈40kDa) to >2,000 kDa (corresponding to much larger multimers) (Fig. 2A, dashed blue line). Consistent with the apparent tendency of bevacizumab to form multimeric immune complexes with VEGF, preformed bevacizumab–VEGF165 complexes rapidly disappeared from the circulation when injection intravenously, as would be expected for multimeric immune complexes (SI Fig. 8; note that the levels of Bevacizumab when complexed with VEGF rapidly drop compared with the levels of free Bevacizumab that remain much higher), and in contrast to what was described above with VEGF Trap complexes that remain stably in the circulation. Because immune complexes can often be cleared by depositing in the renal glomeruli, we further explored apparent differences in the clearance of bevacizumab–VEGF and VEGF Trap–VEGF complexes by performing immunostaining in the kidney. After i.v. administration, renal glomeruli stained strongly for bevacizumab–VEGF complexes (Fig. 2 D and E) but not for VEGF Trap–VEGF complexes (Fig. 2 B and C). Current evidence indicates that, as a class, pharmacological agents that block VEGF signaling may produce mechanism-based effects on kidney function. Deposition of immune complexes as noted for bevacizumab/VEGF in the renal glomeruli could further accentuate renal toxicity in a nonspecific and non-class-dependent manner.
Fig. 2.
The molar masses of VEGF Trap–VEGF and bevacizumab–VEGF complexes were determined by MALLS coupled to SEC. (A) Using a 1:2 molar ratio of VEGF Trap to VEGF165, discrete peaks were observed at ≈17 ml for VEGF (41 kDa) and ≈14.5 ml for VEGF Trap–VEGF complex (148 kDa) with SEC (red line) and MALLS (dashed red line). In contrast, a 1:2 molar ratio of bevacizumab to VEGF165 revealed a heterogeneous multimeric complex that ranged in molar mass from ≈370 kDa to >2,000 kDa (SEC, solid blue line; MALLS, dashed blue line). (B–E) One milligram of a preformed complex of VEGF Trap and VEGF165 (B and C) or bevacizumab and VEGF165 (D and E) were injected into the left ventricle of 2- to 3-month-old C57bl6 mice. After 10 min, mice were killed, and their kidneys were processed for immunocytochemistry, using an anti human Fc reporter antibody to the human Fc moiety present on both VEGF Trap and bevacizumab. Significant staining was observed in the glomeruli of bevacizumab/VEGF treated mice but not in the glomeruli of VEGF Trap/VEGF treated mice (white arrows).
VEGF Trap Complex Formation Reveals Unexpectedly High Production of Endogenous VEGF in Normal Adult Mice.
As shown above, VEGF antibodies form immune complexes that rapidly deposit in tissues and thus do not allow for easy ascertainment of the amount of complex formed. In contrast, VEGF Trap forms inert complexes with VEGF that remain stably in the circulation and are thus readily accessible for measurement. In fact, the above findings demonstrate that, if VEGF Trap is present at sufficient levels so as to be in excess of Trap bound in complexes, the steady-state levels of VEGF Trap complex in the circulation reflect the total amount of VEGF produced. Daily production rates of VEGF can be calculated by assuming that steady-state levels of VEGF Trap–VEGF complex reflect a balance between production of VEGF leading to formation of complex, and clearance of the resulting complex. Based on experimentally determined values for the steady-state levels of complex and its clearance (see Materials and Methods), we estimate that mice produce ≈0.065 μg of VEGF per day per ml of the volume of distribution, or ≈0.006 μg per gram of tissue per day. Because VEGF is active at picomolar levels, this at first seems to be a surprisingly high level of production for a normal adult animal (see below for comparison to tumor production rates). However, it should be noted that in the absence of VEGF Trap, any VEGF that enters the systemic circulation is rapidly cleared. For this reason, among others noted above, it has not proven possible to consistently and reliably measure systemic VEGF levels, preventing accurate estimation of VEGF production rates in normal adult animals.
Tumor-Derived VEGF Represents a Minority of Total Body VEGF Under Conditions of Minimal Tumor Burden.
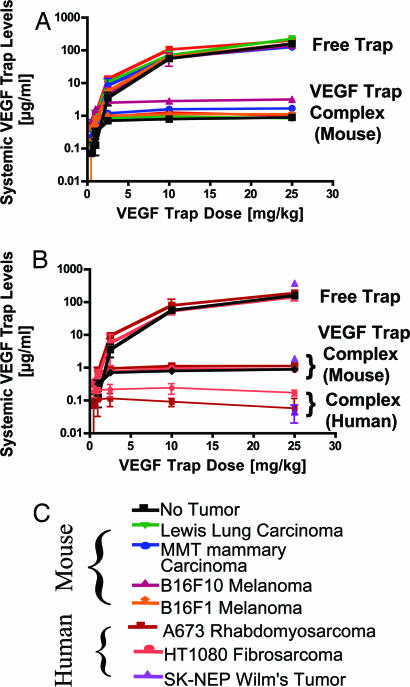
Next, we compared the total body production rate of VEGF, as determined above, with tumor production rates of VEGF. Toward this end, we implanted mice with tumors, allowed these tumors to grow to 0.5–3% of total body weight (average mouse weight, ≈25 g) and measured levels of VEGF Trap complex in these mice to compare them to complex levels found in healthy, non-tumor-bearing mice. Surprisingly, in mice bearing four different types of rodent tumors, the total levels of complex were not markedly different from those seen in non-tumor-bearing mice (1–2 μg/ml; see Fig. 3A and compare with Fig. 1). This finding implies that tumor-derived VEGF represented only a small proportion of total body VEGF or circulating bioavailable VEGF in these mice.
Fig. 3.
In mice bearing tumors <3% body weight, the tumor pool of VEGF production is modest compared with endogenous mouse tissue VEGF production. (A and B) Mouse (A) or human (B) tumors were allowed to grow to ≈100 mm3, and then VEGF Trap was administered twice per week for 1–2 weeks at 0.5, 1, 2.5, 10, and 25 mg/kg. At the termination of the experiment, free VEGF Trap, mouse, and human complex levels were measured in serum. In all cases, regardless of terminal tumor volume, levels of circulating mouse complex were ≈1 μg/ml, whereas human complex levels in the mice bearing human tumors were ≈0.1 μg/ml. Free Trap levels increased incrementally, with the dose levels rising above complex levels at the 2.5 mg/kg dose and reaching ≈100 μg/ml at the 25 mg/kg dose. (n = 6 for each dose). (C) Legend of mouse and human tumor types used.
To further validate this unanticipated finding, we analyzed VEGF Trap complex levels in mice bearing human tumors, where it is possible to distinguish complexes formed with endogenous mouse VEGF with those formed with human VEGF derived from the implanted tumors by analyzing human VEGF–VEGF Trap complex levels in mouse serum. The levels of mouse-derived complexes (Fig. 3B) in these animals were equivalent to those of non-tumor-bearing mice (Fig. 2, above) and mice bearing rodent-derived tumors (Fig. 3A). In contrast, the levels of VEGF Trap complexed with tumor-derived human VEGF were an order of magnitude lower (0.08–0.2 μg/ml) (Fig. 3B). This result was seen in mice bearing tumors of three different human cell lines (SK-NEP, A673, and HT1080). Together, these studies demonstrate that normal total body production of VEGF eclipses the production from tumors that may weight as much as 3% of body weight (mouse weight ranges from 23 to 29 g). Thus, it is unlikely that total levels of free VEGF in the systemic circulation would provide a sensitive index of tumor burden, even if accurate measurement of unbound VEGF in blood samples were readily achievable. Moreover, the above findings suggest that therapeutic compounds designed to bind and inactivate tumor-derived VEGF would have to be provided at sufficient levels to avoid being diverted by significant levels of VEGF normally produced by the rest of the body.
VEGF Trap Complex Levels Provide Guidance on When Efficacious VEGF Blockade Is Achieved.
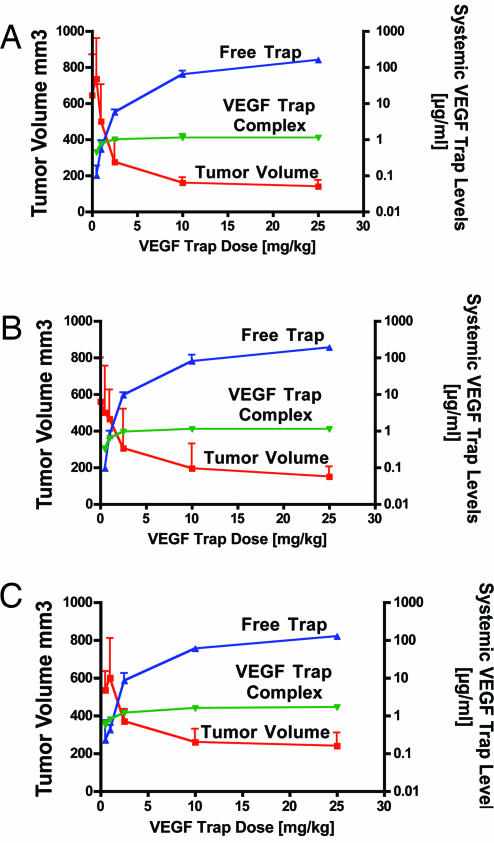
Based on the results above, it is evident that drugs that bind and neutralize VEGF must engage significant levels of VEGF derived from normal tissues, in addition to that originating from tumors. Therefore, we reasoned that measurements of VEGF Trap complex might provide a useful guide to when the dose of VEGF Trap sufficient to substantially neutralize both host and tumor-derived VEGF had been achieved. Indeed, for three different tumors [B16F1 mouse melanoma (Fig. 4A); A673 human rhabdomyosarcoma (Fig. 4B); and MMT mouse mammary carcinoma (Fig. 4C)], increasing the VEGF Trap dose resulted in progressive, marked improvements in anti-tumor efficacy until a dose at which free VEGF Trap substantially exceeded maximal steady-state levels of complex was reached (Fig. 4). For all three tumor types, this was achieved at a dose of 2.5 mg/kg VEGF Trap given twice weekly: at this dose, free VEGF Trap (blue curve) is severalfold the level of complex (green curve), and past this point further dose escalation yields only modest incremental increases in complex levels (green curve) and in anti-tumor efficacy (red curve). In other tumor types, such as U87 glioblastoma, higher levels of VEGF Trap are required to achieve maximal efficacy (22).
Fig. 4.
VEGF Trap Complex provides guidance on when optimal VEGF blockade is achieved for antitumor purposes. In mice bearing B16F1 mouse melanoma tumors (A), A673 human rhabdomyosarcoma (B), and MMT mouse mammary carcinoma tumors (C) grown to ≈100 mm3 before treatment, increasing the dose of VEGF Trap from 0.5 mg/kg twice per week to 25 mg/kg twice per week results in a steady-state of mouse complex at ≈1 μg/ml at 1–2.5 mg/kg and free circulating VEGF Trap levels of ≈10 μg/ml at the 2.5 mg/kg dose, rising to ≈100 μg/ml at the 25 mg/kg dose. Tumors remain quite large at the 0.5 and 1 mg/kg doses but begin to show a significant lack of growth at the 2.5 mg/kg dose, where free Trap levels rise above steady-state complex levels (n = 6 for each dose). Tumors were treated with VEGF Trap from 6–13 (B16F1), 4–13 (MMT), and 12–18 (A673) days after implantation.
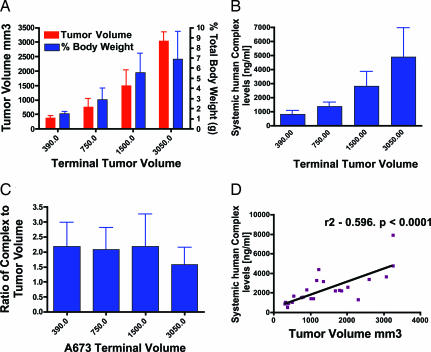
Human VEGF/VEGF Trap Complex Levels Are Directly Related to Tumor Size.
The finding that conventionally sized s.c. tumors in mice produced <10% the amount of total body VEGF prompted us to determine whether there is a consistent relationship between tumor size and VEGF production levels. Human tumors (A673 rhabdomyosarcoma) were implanted into mice and allowed to grow to various sizes before injecting VEGF Trap. In this case, we could define a clear linear relationship between tumor size (Fig. 5A) and complex levels (Fig. 5B, note that the assay reflects levels of complexes containing only human VEGF to specifically detect only tumor-derived complex). The amount of complex per unit weight of tumor was similar across different-sized tumors (Fig. 5C), indicating that tumors maintained their rates of VEGF production as they grew. Linear regression analysis confirmed that there was a very strong correlation between A673 tumor volume and circulating human VEGF complex (Fig. 5D).
Fig. 5.
Human VEGF–VEGF Trap complex levels are directly related to tumor size. Human A673 rhabdomyosarcoma tumors were grown in mice to ≈100, ≈300, ≈500, and ≈750 mm3, at which point they were treated with a single bolus of 25 mg/kg. VEGF Trap, tumor volume, and human complex levels were measured after 2 weeks (n = 6). (A) Increasing tumor volume equates with an increase in tumor burden. (B) Increasing human tumor burden is reflected in an increase in circulating human VEGF–VEGF Trap complex. (C) The ratio of human VEGF–VEGF Trap complex to tumor volume remains steady at ≈2-fold. (D) Linear regression analysis comparing systemic levels of human VEGF–VEGF Trap to tumor volume reveals that increasing tumor volume directly correlates with increasing complex levels. (P < 0.0001.)
At these larger tumor sizes, the amount of complex (ranging from ≈0.8 to 5 μg/ml) contributed by the tumor matched or even exceeded that contributed by the rest of the body, confirming that tumors do indeed make substantially more VEGF per cell than does the average cell in the normal adult host. For example, in the largest tumors (weighing ≈10% of the total mass of the mouse, Fig. 5A), the tumor-derived human VEGF–VEGF Trap complex levels (≈5 μg/ml, Fig. 5B) were ≈3-fold above the levels of murine VEGF–VEGF Trap complex, indicating that the tumors made ≈30 times the amount of VEGF per unit of weight compared with normal, adult tissues.
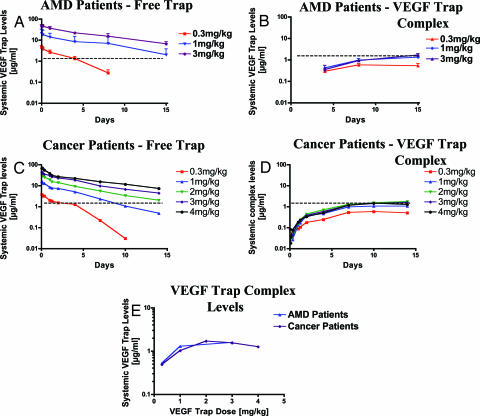
VEGF Trap Complex Formation in Human Subjects With and Without Cancer.
Very large tumors that substantially contribute to VEGF Trap complex formation in mice are generally not seen in the human patient. This in turn suggests that it is unlikely that most tumors in human patients become large enough to make a readily detectable contribution to total body VEGF production. To determine whether or not this was indeed the case, we studied VEGF Trap complex formation in non-cancer patients [patients suffering from age-related macular degeneration (AMD)] and then compared these results with complex formation in cancer patients. In the AMD patients, the lowest dose of VEGF Trap tested (0.3 mg/kg, i.v.) was insufficient to neutralize all VEGF, as evidenced by the levels of free Trap quickly falling below those of bound VEGF Trap, and bound VEGF Trap did not approach the maximal steady-state levels seen with higher doses (Fig. 6 A and B). However, doses of 1.0 and 3.0 mg/kg (i.v.) maintained substantial free Trap levels throughout the dosing period (Fig. 6A), and maximal complex levels were attained, as evidenced by equivalent levels of complex being generated at the two higher doses (≈1–2 μg/ml, see Fig. 6B). In cancer patients with advanced solid tumors or non-Hodgkin's lymphoma, remarkably similar results were obtained. That is, similar doses of VEGF Trap were required to saturate VEGF binding and complex formation (Fig. 6 C–E). In addition, the maximal steady-state levels of VEGF–VEGF Trap complex were similar to those seen in non-cancer patients (Fig. 6 B, D, and E). These findings indicate that, consistent with our findings in mice, endogenous VEGF production in adult human subjects is quite high, whether or not the individuals harbor tumors (Fig. 6E).
Fig. 6.
Circulating free VEGF Trap and human VEGF–VEGF Trap complex levels are very similar in the plasmas of AMD and cancer patients. (A and B) Patients with AMD received a single i.v. bolus of VEGF Trap at 0.3, 1, or 3 mg/kg, and free VEGF Trap and complex levels were measured at 2 and 4 h and 1, 4, 8, and 15 days (n = 7, 0.3 mg/kg; n = 7, 1 mg/kg; n = 5, 3 mg/kg). (C and D) Patients with cancer received a single i.v. bolus of VEGF Trap at 0.3, 1, 2, 3, or 4 mg/kg, and free VEGF Trap and complex levels were measured at 1, 2, 4, and 8 h and 1, 2, 4, 7, 10, and 14 days (n = 3, 0.3 mg/kg; n = 7, 1 mg/kg; n = 6, 2 mg/kg; n = 5, 3 mg/kg; n = 7, 4 mg/kg). (E) Complex levels in AMD patients at 15 days and cancer patients at 14 days were plotted against the different doses revealing an almost exact overlap. Dotted lines denote the steady-state circulating levels of VEGF–VEGF Trap complex in AMD and cancer patients.
Using the same approach as was used for the mouse (see Materials and Methods), human production rates of VEGF in humans were found to be ≈0.0025 μg per gram of tissue per day, which is remarkably similar to that calculated for mice (see above). If our findings in animal models continue to be predictive, these VEGF Trap levels achieved in ongoing clinical studies should be in the efficacious range.
Discussion
At present, there are a number of anti-angiogenic agents targeting the VEGF pathway that are proceeding through clinical trials or already approved for the treatment of cancer (9). One major challenge is the lack of objective measures to guide dosing to determine when sufficient blockade has been achieved or to inform pharmacological response to these drugs. VEGF itself has been suggested as a potential biomarker for the above purposes, based on the assumption that VEGF in the peripheral circulation was primarily derived from the tumor and therefore accurately reflected tumor burden (19). However, to date it has proven difficult to accurately measure systemic levels of VEGF, correlate these levels with tumor burden, or use them as a guide to dosing (11, 12). Here, we describe the use of the VEGF Trap, a potent VEGF antagonist that forms a stable, inert complex with VEGF, as an index that allows for the accurate assessment of VEGF production rates. In addition, this unique property of the VEGF Trap allows accurate assessment of the amounts of VEGF made by a resident tumor compared with the rest of the body. Furthermore, in animals, this approach has been shown to provide a useful guide to selecting dosing regimens that substantially block available VEGF. This has not been possible with anti-VEGF antibodies, as VEGF–antibody complexes are rapidly cleared.
We find unexpectedly high levels of VEGF production in the normal adult setting, where it has long been assumed that, in the absence of ongoing angiogenesis, VEGF production rates would be quite low (11, 12). However, the unexpectedly high rates of VEGF production in non-tumor-bearing adult mice and humans is consistent with the recent realization that VEGF likely plays an ongoing role in the “quiescent” vasculature of normal adults (23). For example, treating normal adult mice and monkeys with VEGF antagonists can increase hematocrit (a measure of the proportion of the blood volume occupied by red blood cells) (24). Similarly, VEGF antagonists can also increase blood pressure (25), indicating that VEGF is involved in regulating vascular tone in the adult (26). We make the unexpected observation that constitutive VEGF production by normal adult tissues is sufficient to mask the lower levels made by most tumors, making it difficult to use peripheral levels of VEGF as a reliable indicator of tumor burden. However, in mice, VEGF production by tumors is clearly related to tumor size, and, when tumors become quite large, the VEGF Trap complex assays readily detect the tumors' VEGF contribution.
Our observations are consistent with recent studies by Bocci et al. (27), which reported that plasma VEGF levels are normally very low or undetectable, but are rapidly increased upon treatment with blocking VEGFR2 antibodies. In these experiments, the observed acute increase in circulating VEGF was not associated with increased VEGF expression in normal tissues, or the tumors, but reflected displacement of VEGF from VEGF receptors. It was also noted that maximal VEGF release occurred at antibody doses that produced near optimal anti-tumor effects, suggesting that maximal VEGF receptor blockade was attained. By extension, the induced increases in plasma VEGF could be used to guide dosing of anti-VEGFR antibodies.
The findings reported by Bocci et al. also support the notion that, in normal adult tissues, there is substantial basal production of VEGF, which is locally sequestered and thus not readily measured in the periphery unless it is dislodged. However, measurement of VEGF in the circulation after its displacement by anti-VEGFR antibodies cannot account for VEGF sequestered by binding to sites other than VEGFR1 or VEGFR2 (e.g., neuropilins or heparin) and thus cannot be used to calculate total VEGF production rates in host or tumors. Studies with the VEGF Trap, which also displaces tissue bound VEGF, extend these findings by precisely determining and comparing host and tumor production rates of VEGF. We also show that the observations made in mice seem to also apply to humans and that the levels of VEGF Trap complexed to VEGF can serve as a sensitive guide for the effective dosing of this particular therapeutic candidate. By extension, determination of the dose required to achieve maximum levels of circulating complexes involving a blocker and its target could serve as a useful guide for the dosing of any therapeutic agent that forms long-lived inert circulating complexes with its target.
The sustained circulating levels of VEGF complex observed after VEGF Trap administration is not seen with VEGF-blocking. Unlike the VEGF Trap, which forms an inert 1:1 complex with VEGF that retains the same circulating half-life as unbound VEGF Trap, antibodies to VEGF form heterogeneous multimeric complexes with their antigens, which are cleared much more rapidly than the unbound antibodies. Thus, such immune complexes are not accessible for assays in the systemic circulation, and it is not possible to use systemic levels of such complexes as a guide to VEGF production or to having achieved efficacious antibody levels. Moreover, the formation of such immune complexes could produce undesirable off-mechanism effects. For example, Meyer et al. report that bevacizumab forms immune complexes with VEGF that can induce platelet aggregation, which they suggest “might be a possible cause for unexpected arterial thromboembolic events in clinical trials.”**In addition, immune complexes can deposit in tissues, including the kidney, potentially contributing to renal damage; consistent with this, we show that VEGF antibodies complexed to VEGF have a much higher propensity to deposit in kidney glomeruli compared with VEGF Trap complexes. Further consistent with this, Gerber et al. have reported “anti-VEGF (antibody) deposition in glomeruli” with complement C3 staining and glomerulosclerosis, “which was generally more severe in animals treated with high-affinity mAbs,” to VEGF (28). Thus, VEGF Trap, which does not form multimeric immune complexes but instead forms inert 1:1 complexes with VEGF, may not share the same adverse effect profile as anti-VEGF antibodies that can form immune complexes.
In summary, our studies show that assays of free and bound VEGF Trap can serve as useful indicators for the proportion of bioavailable VEGF that is bound and neutralized at a given dose of VEGF Trap. In mice, the majority of endogenous VEGF is captured at doses that result in maximal, steady-state levels of VEGF Trap–VEGF complex, at which point near-optimal efficacy is typically attained. Use of this assay in cancer patients might similarly allow for rapid determination of dosing regimens that are likely to be efficacious. Importantly, application of these assays in early stage clinical trials in patients indicates that the doses currently being evaluated in ongoing clinical studies are in the efficacious range (25, 29–32, ¶).
Materials and Methods
ELISAs.
Free VEGF Trap and Complex Measurement.
Levels of free VEGF Trap were measured by using a functional ELISA, which uses VEGF165 as the capture and an antibody to the IgG2 domain of VEGFR1 as the report. Mouse VEGF–VEGF Trap complex is measured by using an antibody to mouse VEGF as the capture and the same antibody as above as the report. Human VEGF–VEGF Trap complex is measured by using an antibody to human VEGF as the capture and an antibody to human Fc as the report.
MALLS Coupled to SEC.
A multiangle laser light scattering instrument was coupled to a size exclusion column to measure the molar mass and aggregation of VEGF165 bound to VEGF Trap or bevacizumab.
Immunocytochemistry of VEGF Trap/VEGF and Bevacizumab–VEGF Complex Deposition in Kidney Glomeruli.
Preformed VEGF165–VEGF Trap or VEGF165–bevacizumab complexes were injected into the left ventricles of C57bl6 mice, and deposition in the kidney was determined immunocytochemically.
Calculation of VEGF Production Rates Based on Steady-State VEGF–VEGF Trap Complex Levels in Mouse and Man.
Endogenous VEGF production rates were determined by using the following equation: Complex production rate [μg/day per ml of volume of distribution(ml-d)] = 0.5 × Css μg/ml per t½ days = 0.5 × Css/t½ μg/ml-d. Because VEGF accounts for 1/4 of the mass of the complex, the VEGF production rate (μg per day per ml of volume of distribution) = 0.25 × (0.5 × Css μg/ml per t½ days) = 0.125 × Css/t½ μg/ml-d.
Tumor Implantation.
Tumor cell lines were implanted s.c. into the right flank of 7- to 9-week-old male SCID/CB17 mice, and serum samples were taken at termination of the experiment. To assess the relationship between tumor volume and human complex levels, A673 tumors were grown in mice to different sizes, at which point the mice were treated with a single bolus of 25 mg/kg VEGF Trap, and tumor volume and mouse and human complex levels measured over a 2-week period.
Human Clinical Trials.
Clinical trial design for the studies presented herein are available in refs. 25, 29, and 31–34.
Data Analysis.
Linear regression analysis comparing circulating human VEGF–VEGF Trap complex levels with tumor volume was done by using the data analysis package in GraphPad Prism. Pharmacokinetic analyses were done by using the WinNonlin PK/PD modeling and analysis package (Pharsight, Mountain View, CA). Molar masses of proteins and their complexes were determined by using ASTRA software (Wyatt Technology, Santa Barbara, CA) as described in ref. 35.
Additional Details.
For a more detailed description of the methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the following Regeneron colleagues: Alain Thibaut, Robert Terifay, Jesse M. Cedarbaum, Chris Daly, Ella Ioffe, Thomas Daly, Douglas McDonald, Nicholas Gale, Samuel Davis, and Len Schleifer and our Sanofi-Aventis colleagues Paul Juniewicz, Marie-Christine Bissery, Friedheml Bladt, and Marielle Chiron for valuable scientific and editorial input.
Abbreviations
- AMD
age-related macular degeneration
- MALLS
multiangled laser light scattering
- SEC
size exclusion chromatography
- VEGFR
VEGF receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708865104/DC1.
Rixe, O., Verslype, C., Meric, J. B., Tejpar, S., Bloch, J., Crabbe, M., Khayat, D., Furfine, E. S., Assadourian, S., Van Cutsem, E. (2006) J. Clin. Oncol. 24:13161 (abstr.).
Mulay, M., Limentani, S. A., Carroll, M., Furfine, E. S., Cohen, D. P., Rosen, L. S. (2006) J. Clin. Oncol. 24:13061 (abstr.).
Tew, W. P., Colombo, N., Ray-Coquard, I., Oza, A., del Campo, J., Scambia, G., Spriggs, D. (2007) J. Clin. Oncol. 25:5508 (abstr.).
Massarelli, E., Miller, V.A., Leighl, N., Rosen, P., Albain, K., Hart, L., Melnyk, O., Sternas, L., Akerman, J., Herbst, R. S. (2007) J. Clin. Oncol. 25:7627 (abstr.).
Meyer, T., Robson, T., Amirkhosravi, A., Langer, F., Desai, H., Amaya, M., Elias, P., Francis, J. L. (2007) Am. Soc. Hematol. 108:1091 (abstr.).
References
- 1.Ferrara N. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JC. Clin Cancer Res. 2004;10:6367S–6370S. doi: 10.1158/1078-0432.CCR-050006. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Mass RD, Sarkar S, Holden SN, Hurwitz H. J Clin Oncol. 2005;23:249S–249S. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Oates AJ, Holden S, Koeppen H. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 9.Quesada AR, Munoz-Chapuli R, Medina MA. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 10.Baker M. Nat Biotechnol. 2005;23:297–304. doi: 10.1038/nbt0305-297. [DOI] [PubMed] [Google Scholar]
- 11.Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, Jinno K, Kotani Y. Eur J Cancer. 1998;34:2041–2045. doi: 10.1016/s0959-8049(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 13.El-Houseini ME, Abdel-Azim SA, El-Desouky GI, Abdel-Hady S, El-Hamad MF, Kamel AM. J Egypt Natl Canc Inst. 2004;16:57–61. [PubMed] [Google Scholar]
- 14.Ascierto PA, Leonardi E, Ottaiano A, Napolitano M, Scala S, Castello G. Anticancer Res. 2004;24:4255–4258. [PubMed] [Google Scholar]
- 15.Shimanuki Y, Takahashi K, Cui R, Hori S, Takahashi F, Miyamoto H, Fukurchi Y. Lung. 2005;183:29–42. doi: 10.1007/s00408-004-2521-4. [DOI] [PubMed] [Google Scholar]
- 16.Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, et al. Cancer Res. 2000;60:2898–2905. [PubMed] [Google Scholar]
- 17.Jimeno A, Daw NC, Amador ML, Cusatis G, Kulesza P, Krailo M, Ingle AM, Blaney SM, Adamson P, Hidalgo M. Pediatr Blood Cancer. 2007;49:352–357. doi: 10.1002/pbc.20753. [DOI] [PubMed] [Google Scholar]
- 18.Shaked Y, Bocci G, Munoz R, Man S, Ebos JM, Hicklin DJ, Bertolini F, D'Amato R, Kerbel RS. Curr Cancer Drug Targets. 2005;5:551–559. doi: 10.2174/156800905774574020. [DOI] [PubMed] [Google Scholar]
- 19.Bremnes RM, Camps C, Sirera R. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et al. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ, Yancopoulos GD. Cold Spring Harbor Symp Quant Biol. 2005;70:411–418. doi: 10.1101/sqb.2005.70.052. [DOI] [PubMed] [Google Scholar]
- 22.Wachsberger PR, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, Yancopoulos GD, Dicker AP. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, et al. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 24.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, et al. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, Cedarbaum JM, Campochiaro PA. Ophthalmology. 2006;113:1522.e1–1522.e14. doi: 10.1016/j.ophtha.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, et al. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, et al. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 28.Gerber H-P, Wu X, Yu L, Wiesmann C, Liang XH, Lee CV, Fuh G, Olsson C, Damico L, Xie D, et al. Proc Natl Acad Sci USA. 2007;104:3478–3483. doi: 10.1073/pnas.0611492104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konner J, Dupont J. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–S85. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 30.Shah SM, Tatlipinar S, Quinlan E, Sung JU, Tabandeh H, Nguyen QD, Fahmy AS, Zimmer-Galler I, Symons RC, Cedarbaum JM, et al. Invest Ophthalmol Vis Sci. 2006;47:5460–5468. doi: 10.1167/iovs.06-0012. [DOI] [PubMed] [Google Scholar]
- 31.Dupont J, Schwartz J, Koutcher J, Spriggs D, Gordon M, Mendelson D, Murren J, Lucarelli A, Cedarbaum J. Proc Am Soc Clin Oncol. 2004;22:3009. [Google Scholar]
- 32.Dupont J, Rothenberg ML, Spriggs DR, Cedarbaum JM, Furfine ES, Cohen DP, Dancy I, Lee H, Cooper W, Lockhart AC. Proc Am Soc Clin Oncol. 2005;23:3029. [Google Scholar]
- 33.Tew WP, Colombo N, Ray-Coquard I, Oza A, del Campo J, Scambia G, Spriggs D. Am Soc Clin Oncol. 2007;25:5508. [Google Scholar]
- 34.Massarelli E, Miller VA, Leighl N, Rosen P, Albain K, Hart L, Melnyk O, Sternas L, Akerman J, Herbst RS. Am Soc Clin Oncol. 2007;25:7627. [Google Scholar]
- 35.Wen J, Arakawa T, Philo JS. Anal Biochem. 1996;240:155–166. doi: 10.1006/abio.1996.0345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.