Abstract
Zinc is an essential trace element and catalytic/structural component used by many metalloenzymes and transcription factors. Recent studies indicate a possible correlation of zinc levels with the cancer risk; however, the exact role of zinc and zinc transporters in cancer progression is unknown. We have observed that a zinc transporter, ZIP4 (SLC39A4), was substantially overexpressed in 16 of 17 (94%) clinical pancreatic adenocarcinoma specimens compared with the surrounding normal tissues, and ZIP4 mRNA expression was significantly higher in human pancreatic cancer cells than human pancreatic ductal epithelium (HPDE) cells. This indicates that aberrant ZIP4 up-regulation may contribute to the pancreatic cancer pathogenesis and progression. We studied the effects of ZIP4 overexpression in pancreatic cancer cell proliferation in vitro and pancreatic cancer progression in vivo. We found that forced expression of ZIP4 increased intracellular zinc levels, increased cell proliferation by 2-fold in vitro, and significantly increased tumor volume by 13-fold in the nude mice model with s.c. xenograft compared with the control cells. In the orthotopic nude mice model, overexpression of ZIP4 not only increased the primary tumor weight (7.2-fold), it also increased the peritoneal dissemination and ascites incidence. Moreover, increased cell proliferation and higher zinc content were also observed in the tumor tissues that overexpressed ZIP4. These data reveal an important outcome of aberrant ZIP4 expression in contributing to pancreatic cancer pathogenesis and progression. It may suggest a therapeutic strategy whereby ZIP4 is targeted to control pancreatic cancer growth.
Keywords: cell proliferation, tumor progression, zinc uptake
Pancreatic cancer is the fourth leading cause of cancer-related deaths in North America. Although some progress has been made in surgery, chemotherapy, and radiation therapy in recent decades, the incidence of pancreatic cancer still remains equal to the mortality rate (1–3). Survival statistics are poor because there are no reliable tests for early diagnosis and no effective therapies for the metastatic form of pancreatic cancer. The only curative treatment for pancreatic cancer is surgical resection. However, eighty percent of pancreatic adenocarcinomas are not resectable in the patients with clinical symptoms (3, 4). Clearly, there is a pressing need to understand more about pancreatic cancer pathogenesis and to develop an effective treatment for pancreatic cancer. It must be beneficial for therapeutics of pancreatic cancer to target genes that are either more specifically expressed in pancreatic cancer or genes that are involved in multiple pathways, such as cellular metabolism or nutritional uptake. Despite the implication in many malignant cells, the roles of altered cellular metabolism or the interaction between genetics and the environment as an essential factor in cancers, especially in pancreatic cancer, has been largely ignored (5, 6).
Zinc is an essential trace element and catalytic/structural component used by many metalloenzymes and transcription factors that contain zinc-finger motifs (7, 8). Zinc deficiency in animals leads to growth retardation, decreased food intake, impaired DNA synthesis, immune system dysfunction, and severe dermatitis (9). Zinc availability is also important for tumor growth and progression because zinc is a critical component for many enzymes such as carbonic anhydrase and matrix metalloproteinases (MMPs), which are involved in hypoxia, angiogenesis, cell proliferation, and metastasis of cancer (10, 11). High zinc concentrations are toxic to the cells, therefore, cells have evolved a complex system to maintain the balance of zinc uptake, intracellular storage, and efflux (12, 13). Two solute-linked carrier (SLC) gene families were identified in zinc transport, SLC30, which encodes for zinc transporter (ZnT) proteins, and SLC39, which encodes for Zrt-, Irt-like proteins (ZIP) (13–15). They appear to have opposite roles in cellular zinc homeostasis. ZnT transporters reduce intracellular zinc availability by promoting zinc efflux from cells or into intracellular vesicles, whereas ZIP transporters increase intracellular zinc availability by promoting extracellular zinc uptake and vesicular zinc release into the cytoplasm. Both ZnT and ZIP transporter families exhibit unique tissue-specific expression, differential responsiveness to dietary zinc deficiency and excess, and differential responsiveness to physiologic stimuli via hormones and cytokines (16). In a recent study, low levels of ZnT1 have been observed in mammary gland tumor cells. The zinc concentration in these cells is also higher than that in normal cells, which suggests that zinc transport is misregulated in these proliferating tumor cells, and zinc availability might be essential for tumor cell growth (17). In another study, ZIP6 (also known as LIV-1), a breast cancer-associated protein, which belongs to a new subfamily of ZIP transporters, has been found to be associated with estrogen-positive breast cancer and metastasis to lymph nodes (18). Similarly, Kagara et al. (19) found that zinc and the transporter ZIP10 were involved in invasive behavior of breast cancer cells. Those studies suggest a positive correlation between zinc or zinc importers and cancer progression.
ZIP4, encoded by the SLC39A4 gene, plays an important role in maintaining the cellular zinc level by uptaking dietary zinc into intestinal epithelial cells and releasing zinc from vesicular compartments (7, 12, 20). Mutations in the SLC39A4 gene are thought to be the reason for a genetic disorder of zinc-deficiency acrodermatitis enteropathica (AE) (8, 21). Recently, we found in molecular profiling studies that ZIP4 mRNA levels were markedly higher in human pancreatic cancer (22). In light of the importance of zinc in maintaining activities of many enzymes, signaling pathways, and transcription factors, we hypothesized that aberrant ZIP4 expression may contribute to pancreatic cancer tumorigenesis. It is of great interest to investigate the role of ZIP4 in cancers, especially in pancreatic cancer, in which very little is known about the effects of the zinc level and zinc transporters in pancreatic cancer progression. In this study, we examined the expression levels of ZIP4 in human pancreatic cancer tissues and cell lines and the functional contributions of ZIP4 to cancer growth in vitro and in vivo. This study has identified a previously uncharacterized factor in pancreatic malignancy, thereby suggesting a target for cancer therapy.
Results
ZIP4 Is Overexpressed in Human Pancreatic Cancer Tissue Specimens and Cell Lines.
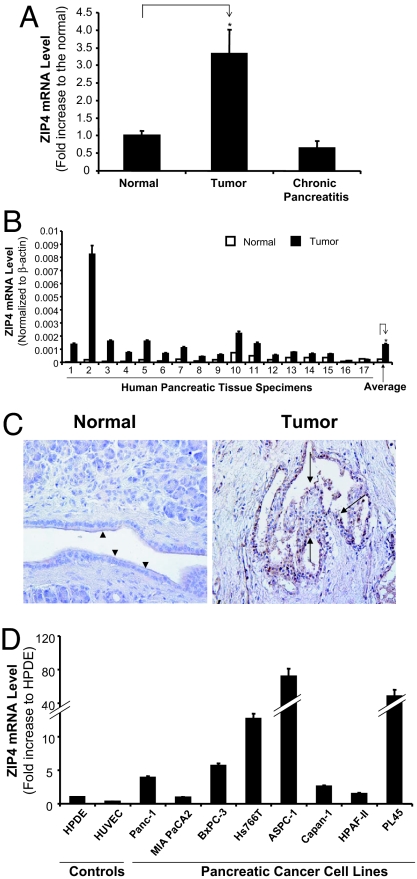
ZIP4 (SLC39A4) mRNA was found by microarray to be overexpressed in human pancreatic cancer tissue samples compared with normal and pancreatitis samples (Fig. 1A). To confirm the microarray data, we examined ZIP4 expression in 17 pairs of human pancreatic cancer tissues with the surrounding normal tissues and five pancreatitis tissues. We also examined the ZIP4 expression in eight human pancreatic cancer cell lines. ZIP4 mRNA was substantially overexpressed in 16 of 17 (94%) clinical pancreatic-adenocarcinoma samples compared with that in their surrounding normal tissues (Fig. 1B and Table 1). In all five pancreatitis tissues, ZIP4 mRNA was as low as that in the surrounding normal tissues (data not shown). Overall, the average mRNA expression in 17 pancreatic cancer tissues was 5.5 times that in the surrounding normal tissues. The tumor samples also showed strong immunoreactivity to human ZIP4 Ab (Fig. 1C). ZIP4 expression was differentially higher in seven human pancreatic cancer cell lines (Panc-1, BxPC-3, Hs766T, ASPC-1, Capan-1, HPAF-II, and PL45) compared with that in human pancreatic ductal epithelium (HPDE) cells, but the expression of ZIP4 in MIA PaCa-2 cells was similar to that in HPDE cells (Fig. 1D). Thus, high expression of ZIP4 in the majority of pancreatic cancer tissue specimens and cell lines suggests that this zinc transporter may contribute to cancer growth.
Fig. 1.
ZIP4 expression in human pancreatic cancer tissue specimens and cell lines. (A) cDNA microarray analysis was done by using Affymetrix chips containing 6,800 genes. Microdissected samples from pancreatic adenocarcinoma (10), chronic pancreatitis (5), and normal pancreas (5) were analyzed. *, P < 0.05. (B) Validation of microarray results of ZIP4 mRNA levels in 17 patients. Total RNA was extracted from tissues, and the mRNA levels for ZIP4 were analyzed by real-time PCR and normalized to that of the house keeping gene, β-actin. Relative mRNA level is presented as fold increase for both tumor and normal samples. All data shown are the means ± SD of three separate experiments. *, P < 0.05. (C) Immunohistochemical staining of ZIP4 expression in human pancreatic cancer tissue and its normal surrounding tissue. Dark brown color represents positive staining of ZIP4. Arrowheads, normal ductal epithelial cells; arrows, tumor epithelial cells. (D) Expression of ZIP4 in human pancreatic cancer cell lines by real-time RT-PCR analysis. The mRNA levels of ZIP4 in eight human pancreatic cancer cell lines were examined by real-time RT-PCR as above. All data shown are the means ± SD of three separate experiments.
Table 1.
Human ZIP4 mRNA levels in 17 pairs of human adenocarcinoma tissues
| Patient no. | mRNA* (normal tissues) | mRNA* (tumor tissues) | Fold increase (tumor/normal) |
|---|---|---|---|
| 1 | 2.5E-05 | 1.4E-03 | 53.6 |
| 2 | 2.2E-04 | 8.2E-03 | 38.2 |
| 3 | 7.8E-05 | 1.6E-03 | 20.4 |
| 4 | 5.5E-05 | 7.4E-04 | 13.5 |
| 5 | 2.1E-04 | 1.6E-03 | 7.5 |
| 6 | 1.0E-04 | 6.6E-04 | 6.5 |
| 7 | 2.4E-04 | 1.1E-03 | 4.5 |
| 8 | 1.1E-04 | 4.4E-04 | 3.9 |
| 9 | 1.9E-04 | 5.8E-04 | 3.1 |
| 10 | 7.5E-04 | 2.2E-03 | 2.9 |
| 11 | 4.9E-04 | 1.4E-03 | 2.9 |
| 12 | 2.1E-04 | 5.4E-04 | 2.6 |
| 13 | 3.5E-04 | 7.5E-04 | 2.1 |
| 14 | 3.6E-04 | 6.4E-04 | 1.8 |
| 15 | 3.8E-04 | 6.2E-04 | 1.6 |
| 16 | 8.2E-05 | 1.2E-04 | 1.5 |
| 17 | 2.7E-04 | 2.2E-04 | 0.8 |
*Relative ZIP4 mRNA levels was normalized to that of β-actin and presented as 2̂(Ct[β-actin] − Ct[ZIP4]).
Overexpression of ZIP4 Increases the Proliferation of Pancreatic Cancer Cells.
To study the potential functions of ZIP4 in pancreatic cancer, three stably overexpressing ZIP4 cell lines were established in MIA PaCa-2 cells (MIA-ZIP4) by using a retrovirus vector (pBabe, Clontech). Parental MIA PaCa-2 cells express less ZIP4 than other pancreatic cancer cell lines (shown in Fig. 1D). Stable cells containing empty vectors (MIA-V) were also established in MIA PaCa-2 cells as controls. Overexpression of ZIP4 in all three MIA-ZIP4 cells was confirmed when compared with MIA-V controls by real-time PCR and Western blotting. ZIP4 overexpression in a representative MIA-ZIP4 stable cell line is shown in Fig. 2 A and B (124-fold increase in mRNA). MIA-ZIP4 cells accumulated more zinc than MIA-V cells by 73% (P < 0.05, Fig. 2C), indicating that the overexpressed ZIP4 protein was fully functional in transporting zinc ion into the cells. MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] assay showed that overexpression of ZIP4 in MIA-ZIP4 cells was associated with increased cell proliferation by 101% on day 5, compared with that in MIA-V (P < 0.01, Fig. 2D) under a serum-starvation condition but not in the presence of serum. These results indicate that ZIP4 plays an important role in cell proliferation in these pancreatic cancer cells.
Fig. 2.
Effect of ZIP4 on MIA CaPa-2 cell proliferation. (A) Overexpression of ZIP4 mRNA in MIA PaCa-2 cells. mRNA levels in MIA-V and MIA-ZIP4 cells were examined with real-time RT-PCR. Human ZIP4 mRNA levels were normalized to that of human β-actin. ZIP4 mRNA levels in MIA-ZIP4 cells were significantly higher than that in MIA-V cells. *, P < 0.01. (B) Overexpression of ZIP4 protein in MIA PaCa-2 cells detected by Western blot. Specific anti-ZIP4 Ab (1:500) was used to probe the protein bands. (C) Zinc concentration in MIA PaCa-2 cells. MIA-V and MIA-ZIP4 cells were treated with 4 μM TPEN for 1 h at 37°C and then incubated with DMEM in the presence of 10 μM ZnCl2 for 5 min before collection. The zinc concentration was examined by ICPMS. *, P < 0.05. (D) MTS assay. MIA-V and MIA-ZIP4 cells were seeded in 96-well plates (2 × 103 cells per well) and serum-starved for 24 h before examining the cell proliferation. Absorbance at 490 nm was recorded daily until day 5 after starvation. Data were expressed as the means ± SD of triplicate values. *, P < 0.05. (E) Zinc-dependent assay. MIA-V and MIA-ZIP4 cells in 96-well plates (2 × 103 cells per well) were treated with 4 μM TPEN for 1 h at 37°C and then incubated with DMEM in the presence of 0, 1, 5, 25, 50, and 125 μM ZnCl2. Absorbance at 490 nm was recorded at day 1 after treatment. Data were expressed as the means ± SD of triplicate values. *, P < 0.05.
We also found a dose-dependent increase of cell proliferation when low concentrations of ZnCl2 were added (in the range of 1–25 μM) to MIA-ZIP4 cells. No significant increase of cell proliferation was found in MIA-V cells upon addition of ZnCl2. However, when a higher concentration of ZnCl2 was added (≈50 μM) to MIA-ZIP4 cells, cell proliferation was dramatically decreased, probably because of the toxicity of zinc. In contrast, MIA-V cells were less sensitive to zinc than MIA-ZIP4 cells, probably because MIA-V cells take up less zinc than MIA-ZIP4 cells. When exogenous ZnCl2 exceeds 50 μM, both MIA-V and MIA-ZIP4 cells were killed (Fig. 2E).
ZIP4 Promotes Pancreatic Cancer Growth in the Nude Mouse Model of s.c. Xenograft.
We further analyzed the role of ZIP4 on tumor growth in vivo using an immunodeficient nude mouse model. MIA-ZIP4 cells showed a dramatic increase (13-fold) in tumor volume after 6 weeks compared with MIA-V control cells in the s.c. tumor model (P < 0.01, Fig. 3A). MIA-ZIP4 cells also significantly increased tumor weight by 30-fold after 6 weeks compared with MIA-V control cells. Tumors from s.c.-injected mice were removed and processed for immunohistochemical analysis. The stability of ZIP4 overexpression in s.c. tumors from the MIA-ZIP4 group was confirmed by real-time RT-PCR. Furthermore, s.c. tumors from MIA-ZIP4-injected mice showed much increased cell proliferation as indicated by the strong positive staining of Ki67, a marker for cell proliferation, compared with that of the MIA-V mice (Fig. 3B). Further analysis of the s.c. tumors for zinc concentration with ICPMS indicated that, overall, 62% more zinc was accumulated in the tumors from the mice implanted with MIA-ZIP4 cells than the tumors from the mice implanted with MIA-V cells (P < 0.05, Fig. 3C), indicating that the overexpressed ZIP4 absorbed more zinc, and an increased amount of zinc is necessary for pancreatic cancer progression.
Fig. 3.
Effects of ZIP4 on pancreatic cancer growth in the nude mouse model of s.c. xenograft. (A) MIA-ZIP4 or MIA-V cells (3 × 106) were s.c. inoculated into the right flank of nude mice (n = 10 per treatment group). Tumor size was measured weekly for 6 weeks. Tumor volume was calculated by the formula: tumor volume [mm3] = (length [mm]) × (width [mm])2 × 0.52. *, P < 0.01. A representative s.c. tumor mass from each group was shown in the Insets. (B) The s.c. tumors were removed and processed for immunohistochemistry analysis. A monoclonal Ab against Ki67 was used to stain the tissue slides from MIA-V and MIA-ZIP4 groups. s.c. tumors from MIA-ZIP4 group showed much increased cell proliferation by Ki67 staining compared with that of the MIA-V mice. (C) Zinc concentration in nude mouse s.c. tumors. The s.c. tumors were removed and homogenized for zinc detection with ICPMS. The average zinc concentration in the MIA-V group and MIA-ZIP4 group was presented. *, P < 0.05.
ZIP4 Enhances Pancreatic Cancer Progression in the Nude Mouse Model of Orthotopic Xenograft.
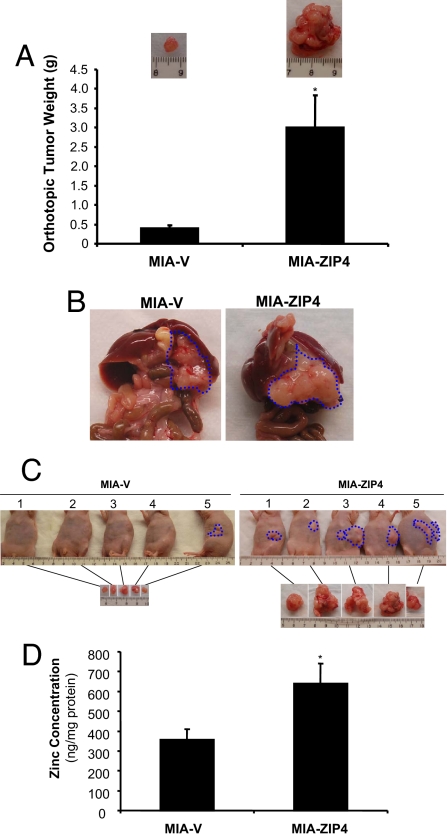
MIA-ZIP4 cells significantly increased tumor weight by 7.2-fold after 7 weeks compared with MIA-V control cells in the orthotopic model (P < 0.01, Fig. 4 A–C). Furthermore, mice given injections of MIA-ZIP4 cells showed jaundice (20%), multiple peritoneal dissemination (100%), and severe abdominal ascitic fluid (40%), whereas only 20% of MIA-V control mice showed mild peritoneal dissemination but no other symptoms (Fig. 4C and Table 2). Further analysis of the primary pancreatic tumors for zinc concentration showed that, overall, 80% more zinc was detected in the tumors from the mice implanted with MIA-ZIP4 cells than the tumors from the mice implanted with MIA-V cells (P < 0.05, Fig. 4D). Those results indicate that ZIP4 could be a malignant factor that significantly contributes to pancreatic cancer progression in vivo.
Fig. 4.
Effects of ZIP4 on pancreatic tumor progression in the nude mouse model of orthotopic xenograft. (A) Tumor weight. MIA-ZIP4 or MIA-V cells (3 × 106) were orthotopically inoculated into the pancreas of nude mice (n = 5 per treatment group). Tumor weight was measured after the mice were euthanized at 7 weeks. *, P < 0.01. A representative orthotopic tumor mass from each group was shown in the Insets. (B) Primary tumors. The orthotopic primary tumors were removed, and a representative picture from each group was shown. (C) Picture of gross appearance. Pictures of the gross appearance and the primary tumors of each group were shown. (D) Zinc concentration in nude mouse primary pancreas tumors. The orthotopic pancreas tumors were removed and homogenized for zinc detection with ICPMS. The average zinc concentration in the MIA-V group and MIA-ZIP4 group was presented. *, P < 0.05.
Table 2.
Orthotopic implantation of pancreatic cancer cells in nude mice at euthanization
| MIA-V (n = 5) | MIA-ZIP4 (n = 5) | |
|---|---|---|
| No. of mice with jaundice | 0 | 1 |
| No. of mice with peritoneal dissemination | 1 | 5 |
| No. of mice with ascites | 0 | 2 |
Discussion
Nutrient uptake and cellular metabolism play essential roles in normal cell cycle and function; therefore, alteration of these events is often associated with cancer. Several studies indicated that zinc transport and metabolism were associated with cancer progression, especially in breast cancer (19, 23). However, the molecular mechanism of how zinc transporters regulate cancer growth remains unclear, and there are no reports available on zinc transporters and human pancreatic cancer. In this study, we have shown that the overexpression of ZIP4, a zinc importer, was significantly increased in most human pancreatic cancer cell lines and surgical specimens of human adenocarcinoma. Forced overexpression of ZIP4 increased the proliferation of pancreatic cancer cells, and significantly increased tumor growth in both s.c. and orthotopic xenografts of the nude mouse models. Such evidence that zinc transport plays a critical role in pancreatic cancer progression is previously undescribed.
Previous studies on zinc concentration in serum and tumor tissues in cancer patients were contradictory. Several reports indicated that levels of zinc in the serum and malignant tissues decreased in liver and prostate cancers (5, 24). However, in breast cancer patients, the zinc levels were decreased in the serum but increased in tumor tissues (23–25). This apparent difference between those studies may be due to different tissue and organ sites, which have different mechanisms for zinc uptake (16). Very few studies have been done in connecting zinc metabolism and zinc transporters with cancer progression. In a recent screening of clinical breast cancer samples for ZIP10 mRNA expression, it was found that ZIP10 was associated with the metastasis of breast cancer to the lymph node, and expression of ZIP10 mRNA was higher in the invasive and metastatic breast cancer cell lines MDA-MB-231 and MDA-MB-435S than in less metastatic breast cancer cell lines MCF7, T47D, ZR75–1, and ZR75–30 (19). Our data have shown that ZIP4 mRNA was significantly increased in 16 of 17 pancreatic cancer specimens and 7 of 8 pancreatic cancer cell lines compared with that in surrounding normal tissues and control HPDE cells, respectively. ZIP4 mRNA expression in pancreatic cancer tissues was also higher than that in chronic pancreatitis tissues. These results indicate that ZIP4 may be a promising marker in pancreatic cancer diagnosis. The overexpressed ZIP4 may provide an increased zinc supply to the fast growing tumor cells, in which the zinc availability is limited. Because ZIP4 functions at the plasma membrane (12, 15, 20), other proteins or cellular factors may be involved in delivering zinc to its target proteins, which need zinc as an essential component, such as zinc-finger proteins and MMPs. In this regard, it has been shown that murine pancreatic metallothionein, an indicator of zinc homeostasis, is extremely sensitive to zinc availability (26). Further studies are warranted in elucidating the zinc delivery pathway and the effects of zinc storage and release on target protein regulation in pancreatic cancer, which will help us to understand the role of zinc metabolism in pancreatic cancer progression.
Zinc plays an important role in cell growth and proliferation. Zinc deficiency is associated with diverse disorders, such as impaired immune response, growth retardation, delayed wound healing, retarded skeletal development, and osteoporosis (9, 27). Previous studies have suggested a direct effect of zinc on both proliferation and differentiation of osteoblast-like cells. It has been indicated that zinc regulates cell proliferation through several different mechanisms. It is essential to enzyme systems that influence cell division and proliferation. Depleting zinc from the extracellular milieu results in decreased activity of deoxythymidine kinase and reduced levels of adenosine(5′)tetraphosphate(5′)-adenosine. Therefore, zinc may directly regulate DNA synthesis through these systems. Zinc also influences hormonal regulation of cell division. For example, the pituitary growth hormone (GH)–insulin-like growth factor-I (IGF1) axis is responsive to zinc status. Zinc appears to be essential for IGF1 induction during cell proliferation (9, 17). Based on experiments in which the timing of the zinc requirement for DNA synthesis in cultured cells was examined, Chesters and Boyne (28) hypothesized that zinc was required for the accumulation and maintenance of a protein that mediated the entry of cells into S phase. Those studies suggest that zinc plays an essential role in cell proliferation and growth, possibly through influencing the DNA synthesis and cell cycle. However, a high concentration of zinc is toxic to the cells, and causes apoptosis (29). Therefore, cells must have a homeostatic mechanism to maintain the intracellular zinc level within a narrow physiologic range through activity of the zinc transporters.
Our study demonstrates that overexpressed ZIP4 increases pancreatic cancer cell proliferation under serum starvation conditions. We also found a dose-dependent increase of cell proliferation when a low concentration of exogenous ZnCl2 was added (<20 μM) to MIA-ZIP4 cells. No significant increase of cell proliferation was found in MIA-V cells upon addition of ZnCl2 at this range. However, higher concentrations of ZnCl2 (>50 μM) caused dramatically decreased cell proliferation in MIA-ZIP4 cells, whereas MIA-V cells were less sensitive to zinc toxicity than MIA-ZIP4 cells. Those results indicated that zinc stimulated cell proliferation in human pancreatic cancer cells at relatively low concentrations. Overexpressed ZIP4 in pancreatic cancer cells MIA PaCa-2 (MIA-ZIP4) is functional because these cells take up more zinc than MIA-V control cells. Thus, MIA-ZIP4 cells may have better growth potential than MIA-V because of increased intracellular zinc. However, detailed molecular mechanisms are unknown and warrant further investigation. Relevant to these observations is the up-regulation of ZIP4 expression and plasma membrane localization in intestinal epithelial cells during zinc restriction (12, 20). The negative responsiveness of mouse ZIP4 (mZIP4) to zinc availability in some cell types may prevent the cytotoxicity observed in the MIA-ZIP4 cells upon addition of zinc, where the ZIP4 promoter is not involved.
Zinc and zinc transporter ZIP10 have also been suggested to be associated with metastatic phenotype of breast cancer cells. Depletion of intracellular zinc and silencing of ZIP10 in invasive breast cancer cells caused a decrease in the migratory activity of these cells, which suggested a positive correlation between zinc import and cancer progression (19). Similarly, ZIP6 (LIV-1) has been associated with estrogen-positive, metastatic breast cancer (18). ZIP4, ZIP6, and ZIP10 are homologs of the nine-member LZT subfamily of ZIP transporters (16). Consequently, our study showing that overexpression of ZIP4 significantly enhanced pancreatic cancer progression in both s.c. and orthotopic xenografts of nude mice is of great interest. Overexpression of ZIP4 not only increased the primary tumor size in the orthotopic nude mouse model but also increased the incidence of peritoneal dissemination and ascites in the mice. Further analysis of the s.c. and orthotopic pancreas tumors indicated that more zinc was accumulated in the tumors from the mice implanted with MIA-ZIP4 cells than the tumors from the mice implanted with MIA-V cells. Inside the solid tumor, zinc availability is limited; therefore, overexpression of ZIP4 may provide more zinc for tumor-related proteins that may require zinc, and therefore, support the tumor growth. This study of the ZIP4 function in human pancreatic cancer provides strong evidence that overexpression of ZIP4 is associated with enhanced cell proliferation and tumor growth, thereby indicating that ZIP4 plays a critical role in human pancreatic cancer progression.
Different types of parental pancreatic cancer cell lines expressed various levels of endogenous ZIP4, and we did not see a correlation between the in vitro growth and ZIP4 expression levels in these cells. The reasons for this are not completely understood. The in vivo data from ZIP4 overexpression stable cell lines (MIA-ZIP4) suggest that ZIP4 plays a critical role in tumor progression, likely by providing zinc to tumor-related proteins where zinc is limited in the solid tumor.
In summary, zinc and zinc transporter ZIP4 may be promising markers for pancreatic cancer. Therapies targeting ZIP4 have potential clinical significance in both human pancreatic cancer and other cancers with high expression of ZIP4.
Materials and Methods
Cells, Chemicals, and Human Tissue Specimens.
Human pancreatic cancer cell lines, Panc-1, MIA PaCa-2, BxPC-3, Hs766T, ASPC-1, Capan-1, HPAF-II, and PL45, were purchased from the American Type Culture Collection (ATCC). The HPDE cells were provided as a generous gift from Dr. Ming-Sound Tsao (Ontario Cancer Institute, Toronto, ON, Canada) (30, 31). All cells were cultured as described (32, 33). The human ZIP4 (hZIP4) antibody was generated in rabbits against a KLH-conjugated 14-aa synthetic peptide and affinity-purified basically as described (20). Other chemicals were from Sigma. Human pancreatic adenocarcinoma specimens were collected from patients who underwent surgery according to an approved human protocol (H-16215) at Baylor College of Medicine.
ZIP4 mRNA Detection.
Sample preparation and microarray analysis (Affymetrix) were previously described (22). The ZIP4 mRNA was analyzed by real-time RT-PCR as described (32, 33). Briefly, real-time PCR was performed with total RNA by using the SYBR supermix kit (Bio-Rad). PCR included the following components: 100 nM each primer, diluted cDNA templates and iQ SYBR green supermix, and running for 40 cycles at 95°C for 20 sec and 60°C for 1 min. PCR efficiency was examined by serially diluting the template cDNA, and the melting-curve data were collected to check PCR specificity. Each cDNA sample was run as triplicates, and the corresponding no-reverse transcriptase (RT) mRNA sample was included as a negative control. The β-actin primer was included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of the β-actin mRNA. The amount of PCR products was measured by threshold cycle (Ct) values. The relative mRNA level was presented as unit values of 2̂[Ct(β-actin) − Ct(gene of interest)]. The primer sequences for human ZIP4 gene (SLC39A4) are: sense 5′ATGTCAGGAGCGGGTCTTGC3′; and antisense 5′ GCTGCTGTGCTGCTGGAAC 3′.
Immunohistochemical Staining.
Human pancreatic adenocarcinoma and surrounding normal tissues were collected and processed into 5-μm slices. Fixed tissue slides were incubated in 0.3% hydrogen peroxide solution to quench endogenous peroxidase activity for 15 min and were subsequently washed with PBS. The slides were then incubated in blocking buffer for 30 min at room temperature before adding anti-hZIP4 antibody and incubated for 60 min at room temperature. The rabbit polyclonal anti-hZIP4 antibody was prepared basically as described (20). After washing with PBS, the section was incubated with biotinylated secondary antibody for 30 min. An avidin–biotin reaction using peroxidase enzyme was used for protein detection (ABC kit; Vector Laboratories). Immune complexes were detected with diaminobenzidine (DAB) under a phase-contrast microscope. Mouse s.c. tumors were collected and processed into 5-μm slices. Fixed tissue slides were incubated with anti-Ki67 antibody (Biodesign International) for 30 min at 4°C, before DAB visualization, and the sections were then mounted and observed under a phase-contrast microscope.
Stable Cell Line Selection.
ZIP4 overexpression cells were selected in MIA PaCa-2 cells with retrovirus vector pBabe (Clontech), following manufacturer's instructions. Briefly, full-length human ZIP4 cDNA (BC062625) was cloned into pBabe vector, and the recombinant plasmid was cotransfected into 293T cells with plasmids PegPam3 and RDF (containing RD114 envelope). Viral supernatants were collected and transduced to the target cells. Stable cell lines expressing ZIP4 (MIA-ZIP4) or empty vector (MIA-V) were selected by adding 0.5 μg/ml puromycin into the medium. Three individual lines were selected for each stable cell.
Western Blot Analysis.
MIA-V and MIA-ZIP4 cells were lysed with ice-cold lysis buffer (20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and protease inhibitor mixture) for 30 min on ice. Cell lysates were then collected after centrifugation at 13,000 × g for 5 min at 4°C. Sixty micrograms of lysate protein was loaded, and total cellular protein was separated with 15% SDS/PAGE and then transblotted overnight at 4°C onto Hybond-P PVDF membrane (Amersham Biosciences). The membrane was probed with anti-ZIP4 (1:500) or anti-β-actin (1:3,000) antibody at room temperature for 1 h and then washed three times with 0.1% Tween 20-TBS and incubated in a horseradish peroxidase-linked secondary antibody (1:2,000) for 1 h at room temperature. The membrane was washed three times with 0.1% Tween 20-TBS, and the immunoreactive bands were detected by using ECL plus reagent kit.
Cell Proliferation Assay.
Cell proliferation was analyzed with the MTS assay. Stable MIA PaCa-2 cells were seeded in 96-well plates (2 × 103 cells per well), and serum-starved (0% FBS) for 24 h. Cell growth was assessed 1, 2, 3, and 5 days after starvation. For zinc-dependent assay, MIA-V and MIA-ZIP4 cells were treated with a membrane-permeable metal chelator, N,N,N′,N-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN; Sigma) at 4 μM for 1 h at 37°C. Cells were washed with PBS to remove excess TPEN because TPEN is toxic to cells with long-time incubation. Cells were then incubated with DMEM in the presence of 0, 1, 5, 25, 50, and 125 μM ZnCl2. Cell growth was assessed 1 day after treatment. Twenty microliters of MTS reagent mixed with 100 μl of growth medium was added to each well and incubated at 37°C for 2 h. Absorbance was recorded at 490 nm with an EL-800 universal microplate reader (Bio-Tek Instruments).
Pancreatic Cancer Mouse Models.
Subconfluent MIA-ZIP4 or MIA-V cells were harvested by trypsinization and resuspended in DMEM. Only single-cell suspensions with >95% viability were used. The cells (3 × 106) were inoculated either into the right flank (s.c. tumor model) or the body of the pancreas (orthotopic tumor model) of 5- to 6-week-old male nude mice (NCI-Charles River). All mice were cared for in accordance with an animal protocol approved by Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). For the s.c. tumor model, the tumor size was measured weekly by using a digital caliper (VWR International), and the tumor volume was determined with the formula: tumor volume [mm3] = (length [mm]) × (width [mm])2 × 0.52. For intrapancreatic injections, mice were anesthetized with 2.5% avertin, and a 0.5- to 1-cm incision was made in the left subcostal region. The tumor cells (3 × 106) in a volume of 50 μl were injected into the body of the pancreas. The peritoneum and skin were closed with a 4.0 surgical suture. After 4 weeks, all surviving mice were euthanized by an overdose of CO2 exposure and evaluated macroscopically for the presence of orthotopic tumors and the metastases in the abdominal cavity. The orthotopic and metastatic tumor nodules were then explanted, counted, and measured. For both s.c. and orthotopic experiments, the animals were euthanized when their tumor size reached 2 cm in diameter or the animals became moribund during the observation period, and the time of euthanization was recorded as the time of mortality.
Zinc Transport and Concentration.
For cell lines, MIA-V and MIA-ZIP4 cells were seeded in 6-well plates and treated with TPEN at 4 μM for 1 h at 37°C. Cells were washed with PBS to remove excessive TPEN. Cells were then incubated with DMEM in the presence of 10 μM ZnCl2 for 5 min before collection. The cells were washed twice in PBS buffer. Cell pellets (≈104 cells in each sample) were digested in 70% (vol/vol) HNO3 at 70°C for 1 h. For tissues, s.c. or orthotopic primary tumors of the same size from MIA-V- or MIA-ZIP4-injected mice were collected and homogenized. Homogenized mouse tissues (≈50 mg) were placed in septum-sealed glass tubes and treated with 0.1 ml of 70% (vol/vol) HNO3 overnight. All these samples were finally diluted with HPLC-grade water to 2 ml for quantitative assay. Zinc concentrations in cell lines and tissues were determined by inductively coupled plasma mass spectrometry (ICPMS) (ELAN 9000, PerkinElmer) and normalized to the total protein content.
Statistical Analysis.
Quantitative results are shown as means ± standard deviations. The statistical analysis was performed by Student's t test for paired data between control and treated groups or one-way ANOVA for data from multiple groups. P values <0.05 were considered significant.
Acknowledgments
We thank Dr. Barry P. Rosen (Wayne State University School of Medicine, Detroit, MI) for helpful suggestions and assistance with the ICPMS. This work was supported in part by American Cancer Society Grant IRG-93-034-09, the MacDonald Research Fund 06RDM013 (M.L.), the Michael E. DeBakey Department of Surgery, Baylor College of Medicine (to M.L.), and National Institutes of Health Grants DK31127 (to R.J.C.) and R37 GM55425 (to B. P. Rosen).
Footnotes
The authors declare no conflict of interest.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Torrisani J, Buscail L. Ann Pathol. 2002;22:349–355. [PubMed] [Google Scholar]
- 3.Warshaw AL, Fernandez-del Castillo C. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 4.Fisher WE, Berger DH. Int J Gastrointest Cancer. 2003;33:79–88. doi: 10.1385/IJGC:33:1:79. [DOI] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quraishi I, Collins S, Pestaner JP, Harris T, Bagasra O. Med Hypotheses. 2005;65:887–892. doi: 10.1016/j.mehy.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King JC, Cousins RJ. In: Modern Nutrition in Health and Disease. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Baltimore: Lippincott Williams & Wilkins; 2005. pp. 271–285. [Google Scholar]
- 10.Juhasz M, Chen J, Lendeckel U, Kellner U, Kasper HU, Tulassay Z, Pastorekova S, Malfertheiner P, Ebert MP. Aliment Pharmacol Ther. 2003;18:837–846. doi: 10.1046/j.1365-2036.2003.01738.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. ANZ J Surg. 2006;76:830–842. doi: 10.1111/j.1445-2197.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 13.Liuzzi JP, Cousins RJ. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 14.Guerinot ML. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 15.Eide DJ. Pflügers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 16.Cousins RJ, Liuzzi JP, Lichten LA. J Biol Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 17.Lee R, Woo W, Wu B, Kummer A, Duminy H, Xu Z. Exp Biol Med (Maywood) 2003;228:689–696. [PubMed] [Google Scholar]
- 18.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Biochem J. 2003;375:51–59. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagara N, Tanaka N, Noguchi S, Hirano T. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Proc Natl Acad Sci USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 22.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 23.Margalioth EJ, Schenker JG, Chevion M. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarty PK, Ghosh A, Chowdhury JR. Neoplasma. 1986;33:85–90. [PubMed] [Google Scholar]
- 25.Mulay IL, Roy R, Knox BE, Suhr NH, Delaney WE. J Natl Cancer Inst. 1971;47:1–13. [PubMed] [Google Scholar]
- 26.Moore JB, Blanchard RK, McCormack WT, Cousins RJ. J Nutr. 2001;131:3189–3196. doi: 10.1093/jn/131.12.3189. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A. Bone. 2006;38:181–198. doi: 10.1016/j.bone.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Chesters JK, Boyne R. Exp Cell Res. 1991;192:631–634. doi: 10.1016/0014-4827(91)90085-9. [DOI] [PubMed] [Google Scholar]
- 29.Kim AH, Sheline CT, Tian M, Higashi T, McMahon RJ, Cousins RJ, Choi DW. Brain Res. 2000;886:99–107. doi: 10.1016/s0006-8993(00)02944-9. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Am J Pathol. 1996;148:1763–1770. [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Am J Pathol. 2000;157:1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC, Yao Q, Chen C. Cancer. 2004;101:2341–2350. doi: 10.1002/cncr.20634. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, Chen C, Yao Q. Cancer. 2006;106:2284–2294. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]