Abstract
Hepatitis C virus (HCV) is a single-stranded positive-sense RNA virus of the Flaviviridae family. HCV-infected hepatocytes are known to produce reactive oxygen species (ROS), which initiate lipid peroxidation, a reaction that converts polyunsaturated fatty acids, such as arachidonate, into reactive carbonyls that inactivate proteins. To study the effect of lipid peroxidation on HCV replication, we administered arachidonate to Huh7 cells that harbor an HCV replicon (Huh7-K2040 cells). After incubation in medium supplemented with arachidonate but deprived of lipid-soluble antioxidants, the cellular amount of malondialdehyde (MDA), a product of lipid peroxidation, increased markedly in Huh7-K2040 cells but not in parental Huh7 cells that do not harbor an HCV replicon. This increase was followed by a sharp reduction (>95%) in HCV RNA. Both of these events were prevented when cells were treated with vitamin E, a lipid-soluble antioxidant. After prolonged incubation of Huh7-K2040 cells with arachidonate in the absence of lipid-soluble antioxidants, the amount of MDA decreased after the reduction in the amount of HCV RNA. Thus, in the presence of arachidonate and in the absence of lipid-soluble antioxidants, HCV replication induces lipid peroxidation that reduces the amount of HCV RNA. Our results provide a mechanism for the previous observation that polyunsaturated fatty acids inhibit HCV replication [Kapadia SB, Chisari FV (2005) Proc Natl Acad Sci USA 102:2561–2566], and they suggest that these agents may be effective in inhibiting HCV replication in vivo.
Keywords: lipid peroxidation, polyunsaturated fatty acids
Hepatitis C virus (HCV) exacts a heavy toll on public health. Approximately 170 million people worldwide are infected persistently with HCV, and these individuals account for most cases of chronic liver disease and hepatic failure (1). Current IFN-based therapies are ineffective in many patients infected by HCV, and they are plagued with adverse effects (2), underscoring the need for new therapeutic strategies.
HCV is a single-stranded positive-sense RNA virus of the Flaviviridae family (3). The 9.6-kb HCV genome encodes a single polyprotein that is posttranslationally processed into at least 10 structural and nonstructural (NS) proteins. Among the NS proteins, NS3, NS4A, NS4B, NS5A, and NS5B are sufficient to support replication of the HCV RNA, as illustrated by the replication capacity of HCV subgenomic replicons (4). An HCV subgenomic replicon is an engineered HCV RNA expressing a selectable marker gene, neo, in place of the structural coding region. A heterologous viral internal ribosomal entry site is inserted after the neomycin resistance cassette to direct the translation of the viral nonstructural proteins NS3–5B. When human hepatoma Huh7 cells are transfected with HCV replicons and selected with G418, a cell line can be established in which HCV RNA is constantly replicated (4).
A hallmark of HCV infection is the generation of reactive oxygen species (ROS) and oxidative stress (5). ROS generation has been reported in HCV infected patients (6–8), Huh7 cells infected by an infectious clone of HCV (9), and Huh7 cells that contain HCV replicons (10, 11). ROS that are located in membranes are eradicated by lipid-soluble antioxidants, such as vitamin E (12). When production of ROS exceeds the scavenger activity of these lipid-soluble antioxidants, ROS in membranes trigger lipid peroxidation, a nonenzymatic chain of reactions that converts polyunsaturated fatty acids into toxic reactive carbonyls, which inactivate proteins by formation of covalent protein conjugates (13). Interestingly, Kapadia and Chisari (14) recently reported that polyunsaturated fatty acids inhibit HCV replication, but the mechanism of this inhibition is not known.
In the current studies, we used the HCV replicon system to examine the impact of lipid peroxidation on HCV replication. Using Huh7 cells harboring an HCV replicon, we observed that exposure to polyunsaturated fatty acids in the absence of lipid-soluble antioxidants resulted in an acute increase in the amount of malondialdehyde (MDA), a product of lipid peroxidation. This increase was followed by a reduction in HCV RNA. Inasmuch as the generation of MDA relies on ROS produced by HCV replication, prolonged incubation of cells with polyunsaturated fatty acids in the absence of lipid-soluble antioxidants led to a decline in the amount of MDA after the reduction in HCV RNA. This decline in lipid peroxidation allows inhibition of HCV replication without generation of cellular toxicity.
Results
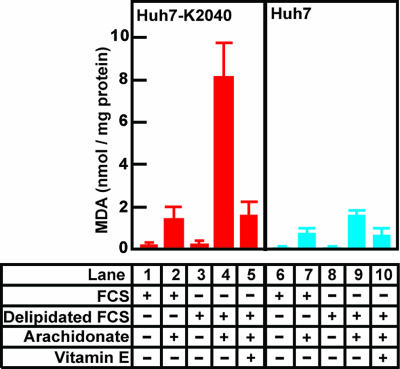
HCV replication generates ROS (10, 11), which have the potential to trigger lipid peroxidation. To monitor the production of reactive carbonyls generated by lipid peroxidation, we measured the amount of MDA produced by Huh7-K2040 cells, a line of Huh7 cells that harbor an HCV replicon (15). MDA is a product of lipid peroxidation and is commonly used as an index of this process (16). When incubated in normal culture medium supplemented with FCS, the Huh7-K2040 cells produced very little MDA (Fig. 1, lane 1). Addition of arachidonate, a substrate for lipid peroxidation, raised the amount of MDA by ≈8-fold in these cells (Fig. 1, lane 2). Inasmuch as lipid peroxidation is likely to be inhibited by lipid-soluble antioxidants present in FCS, we next measured the amount of MDA produced in cells cultured in medium containing FCS that had been depleted of all lipid-soluble materials by extraction with organic solvents (delipidated FCS) (17). This treatment elicited only a slight increase in the mount of MDA (Fig. 1, lane 3). However, under these conditions the addition of arachidonate led to a 48-fold increase in the amount of MDA (Fig. 1, lane 4). This massive increase was reversed by addition of vitamin E, a lipid-soluble antioxidant (Fig. 1, lane 5). Parental Huh7 cells that do not harbor an HCV replicon produced only modest amounts of MDA when incubated with arachidonate in delipidated FCS (Fig. 1, lane 9). This result suggests that ROS generated by HCV replication lead to the generation of MDA (and possibly other reactive carbonyls) in the presence of a polyunsaturated fatty acid, such as arachidonate, when lipid-soluble antioxidants are absent.
Fig. 1.
Increased production of MDA in Huh7-K2040 cells incubated in medium containing delipidated FCS and arachidonate. On day 0, Huh7 (blue) or Huh7-K2040 (red) cells were set up at 7 × 105 cells per 60-mm dish. On day 1, cells were switched to medium supplemented with 10% of FCS or delipidated FCS in the presence or absence 0.3 μM vitamin E or 0.1 mM arachidonate that is conjugated with BSA as indicated. Sixteen hours later, on day 2, cells were harvested, and the amount of MDA in cells was determined as described in Materials and Methods. Values (mean ± SD) from three independent experiments are presented.
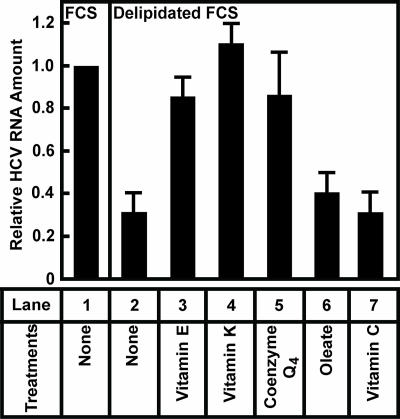
The results from Fig. 1, together with an observation that HCV replication is inhibited by polyunsaturated fatty acids (14), raise the possibility that products of lipid peroxidation repress HCV replication. If this is the case, then removal of lipid-soluble antioxidants from the culture medium should inhibit HCV replication. To test this hypothesis, we incubated Huh7-K2040 cells in medium supplemented with delipidated FCS that is deprived of lipid-soluble antioxidants. Incubation in this medium resulted in a 70% decrease in the amount of HCV RNA (Fig. 2, lane 2). Restoration of vitamin E to the culture medium raised the amount of HCV RNA to the level that was observed in control cells incubated in medium supplemented with FCS (Fig. 2, lane 3). Addition of vitamin K or coenzyme Q4, two other lipid-soluble antioxidants (18, 19), also restored replication of HCV (Fig. 2, lanes 4 and 5). Addition of oleate, a fatty acid that is abundant in FCS, did not rescue the replication of HCV (Fig. 2, lane 6). Interestingly, addition of a water-soluble antioxidant vitamin C also did not restore HCV replication (Fig. 2, lane 7), suggesting a specific requirement of lipid-soluble antioxidants for HCV replication. Although HCV replication was reduced in delipidated FCS, the Huh7-K2040 cells did not appear to be harmed by this treatment, because they continued to grow at the same rate as observed in the cells cultured in FCS (data not shown).
Fig. 2.
Lipid-soluble antioxidants are required for efficient HCV replication in Huh7-K2040 cells. On day 0, Huh7-K2040 cells were set up at 4 × 105 cells per 60-mm dish. On day 2, cells were switched to medium supplemented with 10% of indicated serum and treated with 0.3 μM indicated lipids or vitamins. Seventy-two hours later, on day 5, cells were harvested, and the amount of HCV RNA was determined by quantitative real-time PCR analysis. Values (mean ± SD of three independent experiments) are presented relative to that in control cells cultured in medium supplemented with FCS, which is set at 1.
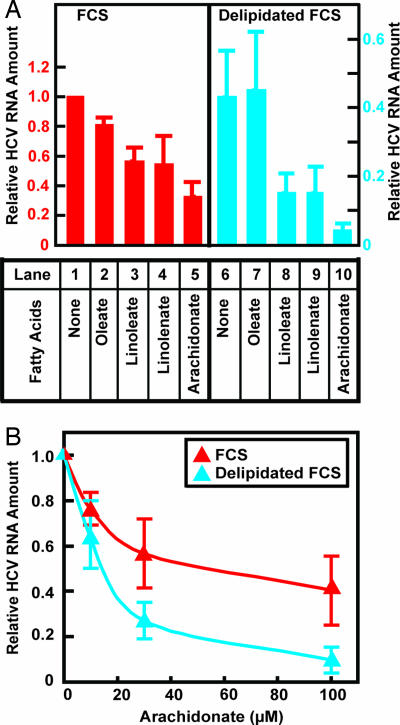
We next sought to determine whether addition of polyunsaturated fatty acids would further reduce HCV RNA in cells grown in delipidated FCS. In medium containing FCS, the addition of polyunsaturated fatty acids such as linoleate, linolenate, and arachidonate led to a 50–70% decrease in the amount of HCV RNA (Fig. 3A, lanes 3–5). The inhibitory effect was enhanced when the cells were cultured in medium containing delipidated FCS (Fig. 3A, lanes 8–10). The combination of delipidated FCS and arachidonate reduced the amount of HCV RNA by 95% compared with control cells cultured in FCS without added fatty acids (Fig. 3A, lane 10). Arachidonate was more potent than linoleate and linolenate in inhibiting HCV replication, probably because it contains more double bonds, thus allowing it to produce more reactive carbonyls after lipid peroxidation (13). Unlike the results with polyunsaturated fatty acids, treatment of Huh7-K2040 cells with oleate, a monounsaturated fatty acid that is not subject to lipid peroxidation (13), did not significantly affect replication of HCV in cells that were cultured in medium supplemented with either FCS (Fig. 3A, lane 2) or delipidated FCS (Fig. 3A, lane 7).
Fig. 3.
Polyunsaturated fatty acids inhibit HCV replication more potently in Huh7-K2040 cells cultured in delipidated FCS than in FCS. On day 0, Huh7-K2040 cells were set up at 4 × 105 cells per 60-mm dish. (A) On day 2, cells were switched to medium supplemented with 10% of FCS (red) or delipidated FCS (blue) and treated with 0.1 mM indicated BSA-conjugated fatty acids. Seventy-two hours later, on day 5, cells were harvested, and the amount of HCV RNA was determined by quantitative real-time PCR analysis. Values (mean ± SD of three independent experiments) are presented relative to the control cells that were cultured in medium supplemented with FCS without any treatment, which is set at 1. The scale for relative HCV RNA in cells cultured in FCS is different from that in cells cultured in delipidated FCS because incubation in delipidated FCS alone without any treatment of fatty acids resulted in a 60% reduction in the amount of HCV RNA. (B) On day 2, cells were switched to medium supplemented with 10% of FCS (red) or delipidated FCS (blue) and treated with indicated amount of BSA-conjugated arachidonate. Seventy-two hours later, on day 5, cells were harvested, and the amount of HCV RNA was determined by quantitative real-time PCR analysis. For cells incubated with either FCS or delipidated FCS, values (mean ± SD of three independent experiments) are plotted relative to that in control cells that were not treated with arachidonate, which is set at 1.
Fig. 3B shows an experiment in which we analyzed the amount of arachidonate required to inhibit HCV replication. When Huh7-K2040 cells were cultured in medium supplemented with FCS, the IC50 for arachidonate was ≈60 μM (Fig. 3B). The IC50 was decreased to 10 μM when these cells were incubated in medium supplemented with delipidated FCS (Fig. 3B).
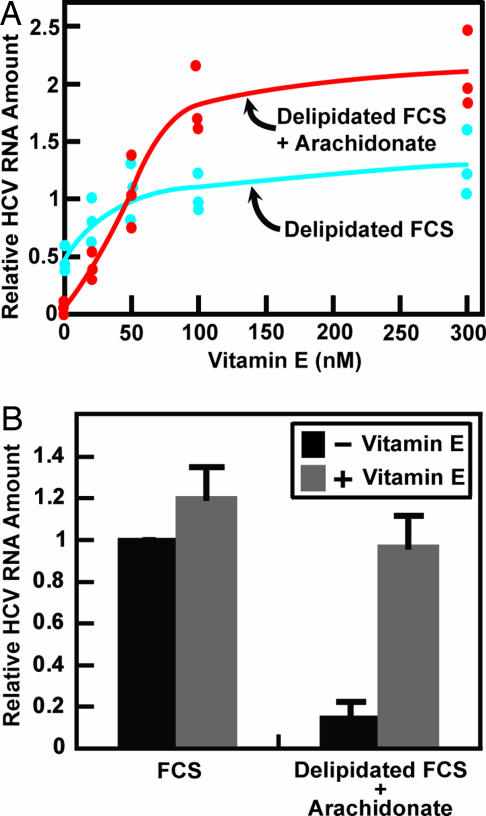
If polyunsaturated fatty acids inhibit HCV replication through lipid peroxidation, then this effect should be countered by lipid-soluble antioxidants. To test this hypothesis, Huh7-K2040 cells cultured in medium containing delipidated FCS with or without arachidonate supplementation were treated with various amounts of vitamin E. As expected, when cells were cultured in the absence of arachidonate, vitamin E restored the amount of HCV RNA to the level observed in cells cultured in FCS, which is set at 1 in Fig. 4A. In the presence of arachidonate, vitamin E increased the amount of HCV RNA from 5% to 190% of that in control cells cultured in FCS (Fig. 4A). The concentration of vitamin E that produced a half-maximal effect in both culture conditions was 40 nM (Fig. 4A). Fig. 4B shows that addition of vitamin E to cells cultured in FCS did not further increase the amount of HCV RNA, indicating that vitamin E became limiting only in delipidated FCS but not in FCS.
Fig. 4.
Vitamin E restores HCV replication in Huh7-K2040 cells treated with arachidonate. On day 0, Huh7-K2040 cells were set up at 4 × 105 cells per 60-mm dish. (A) On day 2, except for control cells that were continued to be maintained in medium containing 10% FCS, cells were switched to medium supplemented with 10% delipidated FCS in the absence (blue) or presence (red) of 0.1 mM BSA-conjugated arachidonate and treated with indicated amount of vitamin E. Seventy-two hours later, on day 5, cells were harvested, and the amount of HCV RNA was determined by quantitative real-time PCR analysis. Values are plotted relative to the control cells cultured in medium supplemented with FCS without any treatment, which is set at 1. Results from three independent experiments are presented, each denoted by an individual dot. (B) On day 2, cells were switched to medium supplemented with 10% of FCS or 10% delipidated FCS with addition of 0.1 mM BSA-conjugated arachidonate. These cells were then treated with or without 0.3 μM vitamin E. Seventy-two hours later, on day 5, cells were harvested, and the amount of HCV RNA was determined and presented as described in A. Results (mean ± SD) from three independent experiments are shown.
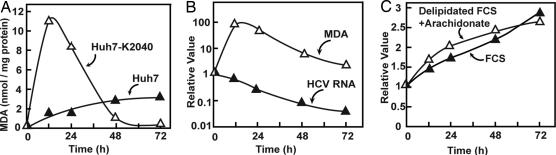
The inhibition of HCV replication by replication-dependent lipid peroxidation creates a paradox whereby HCV should inhibit its own replication, which in turn should cause a drop in reactive carbonyls produced by lipid peroxidation. Eventually, a new steady state should be reached in which replication of HCV continues at a low level that does not produce enough ROS to inhibit HCV replication. To test this hypothesis, we performed chronic experiments in which we measured the amount of MDA in Huh7 and in replicon-bearing Huh7-K2040 cells that were cultured in medium containing delipidated FCS supplemented with arachidonate (Fig. 5). In Huh7 cells, small amounts of MDA accumulated during 72 h of treatment (Fig. 5A, filled triangles). In Huh7-K2040 cells, MDA increased markedly in the first 12–24 h and then decreased to a level that was even lower than that in Huh7 cells after 48 h of treatment (Fig. 5A, open triangles). To determine whether the decrease in the amount of MDA correlates with the reduction in HCV RNA, we measured the amounts of MDA and HCV RNA in Huh7-K2040 cells that were cultured in medium containing delipidated FCS supplemented with arachidonate for various amounts of time. As shown in Fig. 5B, MDA production reached a maximum after Huh7-K2040 cells were switched into this medium for 12 h, at which time HCV RNA was decreased by only 40%. After that, the decrease in the amount of MDA paralleled the reduction in HCV RNA (Fig. 5B). After 72 h, the amount of MDA and HCV RNA were both reduced by >90% (Fig. 5B). Incubation of Huh7-K2040 cells in delipidated FCS supplemented with arachidonate did not generate cellular toxicity, because the rate of cell growth measured by the increase in total cellular protein in this culture condition was not different from that in cells cultured under normal condition in medium supplemented with FCS (Fig. 5C). The lack of cellular toxicity is most likely attributable to the transient rather than persistent activation of lipid peroxidation (Fig. 5 A and B). Long-term treatment with reactive carbonyls generated by lipid peroxidation is known to be toxic to cells (13). Similar results shown in Fig. 5 were observed in one other independent experiment and were shown in supporting information (SI) Fig. 6.
Fig. 5.
Prolonged incubation of Huh7-K2040 cells with arachidonate in the absence of lipid-soluble antioxidants. On day 0, Huh7 or Huh7-K2040 cells were set up at 7 × 105 cells per 60-mm dish. On day 1, cells were switched to medium supplemented with 10% of FCS or 10% delipidated FCS containing 0.1 mM BSA-conjugated arachidonate. After incubation in these medium for the indicated amount of time, cells were harvested. (A) The amount of MDA in Huh7 and Huh7-K2040 cells cultured in medium containing delipidated FCS and arachidonate was quantified as described in Fig. 1. (B) The amount of MDA and HCV RNA was quantified in Huh7-K2040 cells incubated in medium containing delipidated FCS and arachidonate. (C) The amount of protein in Huh7-K2040 cells that were cultured in either medium was determined. (B and C) Values shown are relative to the amount in cells harvested on day 1 immediately before medium were changed (time 0), which is set at 1. (A–C) Data from a representative experiment are reported. Similar results were observed in one other independent experiment and were shown in SI Fig. 6.
Discussion
Studies have reported that HCV replication produces ROS (10, 11), which are normally detoxified by lipid-soluble antioxidants, such as vitamin E (12). In the current study, we show that HCV replication is inhibited by lipid peroxidation that can be blocked by lipid-soluble antioxidants such as vitamin E (Figs. 2–4). When cultured in medium deprived of lipid-soluble antioxidants and containing arachidonate, Huh7-K2040 cells exhibited markedly elevated lipid peroxidation as measured by the production of MDA (Fig. 1). The high rate of lipid peroxidation led to reduced HCV replication (Figs. 2–4). Consequently, the rate of MDA production declined (Fig. 5 A and B). Eventually, a new steady state was reached in which HCV replicated at a low level that did not produce sufficient ROS to further inhibit HCV replication. Consistent with this notion, addition of arachidonate in the absence of lipid-soluble antioxidants to Huh7-K2040 cells inhibited HCV replication by >95% during the first three days of the treatment (Fig. 5B), but longer treatment (up to 6 days) did not further change the amount of HCV RNA (data not shown).
We also examined whether polyunsaturated fatty acids in the absence of lipid-soluble antioxidants also inhibited HCV replication in Huh7-derived cells infected by the JFH1 strain of HCV (data not shown). Unfortunately, such treatment was toxic to these cells, which made the results difficult to be interpreted.
Exactly how lipid peroxidation inhibits HCV replication remains unclear. Several products of lipid peroxidation such as MDA and 4-hydroxy-2-nonenal are known to inactivate proteins by formation of covalent protein conjugates (13). Thus, these products of lipid peroxidation may inactivate HCV NS proteins or host proteins required for HCV replication. Moreover, MDA is able to bind RNA covalently (20), a reaction that might allow MDA to inactive HCV RNA directly.
The current study explains the previous observation that polyunsaturated fatty acids inhibit HCV replication in Huh7 cells that harbor HCV replicons (14). If such inhibition occurs in livers of infected patients, the possibility exists that the balance between lipid peroxidation and HCV replication may help to determine the level of HCV RNA. It will be interesting to examine whether HCV viral load is inversely correlated with the amount of serum MDA in HCV-infected patients. This data also raise the possibility that dietary supplement or pharmacological preparation of polyunsaturated fatty acids (21) may help to suppress HCV replication in patients. Based on our in vitro data, peroxidation of polyunsaturated fatty acids only occurs in cells in which HCV is actively replicating (Figs. 1 and 5B). If this selectivity also occurs in vivo, then such treatment may be able to inhibit HCV replication without intolerable toxicity.
Materials and Methods
Materials.
We obtained vitamin E (α-tocopherol), coenzyme Q4, vitamin K, oleate, linoleate, linolenate, and arachidonate from Sigma and defatted BSA from Roche Molecular Biochemicals. Delipidated FCS was prepared exactly as described in ref. 17. Briefly, 500 ml of FCS was mixed with 400 ml of 1-butanol and 600 ml of isopropyl ether at room temperature for 20 min, followed by a 20-min incubation on ice. After centrifugation, the aqueous phase was reextracted with 200 ml of isopropyl ether, recentrifuged, subjected to evaporation under a stream of nitrogen gas, lyophilized, reconstituted with 200 ml of distilled water, and dialyzed against PBS. Multiple aliquots were stored at −20°C.
Cell Culture.
Huh7 cells were maintained in medium A (Dulbecco's modified Eagle medium with 4.5 g/liter glucose, 100 units/ml penicillin, 100 μg/ml streptomycin sulfate, and 10% FCS). Huh7-K2040 cells are Huh7 cells that harbor a genotype 1b HCV subgenomic replicon (15). They were maintained in medium A supplemented with 200 μg/ml G418. Both cells were maintained in monolayer culture at 37°C in 5% CO2. Huh7-K2040 cells were a gift from M. Gale (University of Washington, Seattle, WA).
Quantitative Real-Time PCR.
The measurement of HCV or cellular RNA was performed by real-time PCR analysis with a protocol described in ref. 22. Briefly, RNA was extracted from cells pooled from two 60-mm dishes, using the RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from the DNA-free RNA, using TaqMan reverse-transcription reagents (Applied Biosystems). Triplicate samples of first strand cDNA were subjected to real-time PCR quantification, using forward and reverse primers for the indicated RNA with human 36B4 as an invariant control. Relative amounts of mRNA were calculated by using the comparative CT method.
Lipid Peroxidation Analysis.
The extent of lipid peroxidation was measured by the amount of MDA, which was assayed by the thiobarbituric acid reactive substances (TBARS) kit (ZeptoMetrix). Cells pooled from two 60-mm dishes were resuspended in 300 μl of buffer A (1.5 mM KH2PO4, 8.1 mM Na2HPO4, 2.7 mM KCl, and 137 mM NaCl, pH 7.4) and homogenized by passing through 22-gauge needles 10 times followed by sonication at 100% amplitude for 5 min in a microprocessor-controlled ultrasonic water bath (Lab-Line Instruments). The amount of MDA in 40 μl of cell homogenates was measured by a spectroscopic method described in manufacturer's protocol. The absorbance was read by a SAFIRE plate reader, using Xfluor4 software (Tecan) at 532 nm. The activity of lipid peroxidation was expressed as the amount of MDA normalized by the amount of cellular protein.
Measurement of Protein Concentration.
Protein concentration was measured by a BCA kit (Pierce) following the manufacturer's protocol.
Preparation of BSA-Conjugated Fatty Acids.
A 10 mM stock solution of each fatty acid was prepared by diluting the free fatty acid in ethanol and precipitating it with 0.25 M NaOH. The precipitated sodium salt was then evaporated under nitrogen gas, reconstituted with 0.15 M NaCl, and stirred at room temperature for 10 min with defatted BSA [final concentration at 10% (wt/vol) in 0.15 M NaCl]. Each solution was stored in multiple aliquots at −20°C and protected from light in tubes evacuated under nitrogen gas.
Supplementary Material
Acknowledgments
We thank Michael S. Brown and Joseph L. Goldstein for their constant support and helpful comments; Michael Gale for his generous gift of Huh7-K2040 cells; Saada Abdalla for excellent technical assistance; Lisa Beatty, Marissa Hodgin, and Ijeoma Onwuneme for invaluable assistance in tissue culture; and Jeff Cormier for real-time PCR analysis. This work was supported by National Institutes of Health Grant HL-20948 and the Perot Family Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708423104/DC1.
References
- 1.Chisari FV. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 2.Feld JJ, Hoofnagle JH. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 3.Appel N, Schaller T, Penin F, Bartenschlager R. J Biol Chem. 2006;281:9833–9836. doi: 10.1074/jbc.R500026200. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann V, Körner F, Koch JO, Herian U, Theilmann L, Bartenschlager R. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 5.Seronello S, Sheikh MY, Choi J. Free Rad Biol Med. 2007;43:869–882. doi: 10.1016/j.freeradbiomed.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Kageyama F, Kobayashi Y, Kawasaki T, Toyokuni S, Uchida K, Nakamura H. Am J Gastroenterol. 2000;95:1041–1050. doi: 10.1111/j.1572-0241.2000.01979.x. [DOI] [PubMed] [Google Scholar]
- 7.Sumida Y, Nakashima T, Yoh T, Nakajima Y, Ishikawa H, Mitsuyoshi H, Sakamoto Y, Okanoue T, Kashima K, Nakamura H, et al. J Hepatol. 2000;33:616–622. doi: 10.1034/j.1600-0641.2000.033004616.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda R, Nagashima M, Sakamoto M, Yamaguchi N, Hirohashi S, Yokota J, Kasai H. Cancer Res. 1994;54:3171–3172. [PubMed] [Google Scholar]
- 9.Machida K, Cheng KTH, Lai CK, Jeng KS, Sung VMH, Lai MMC. J Virol. 2006;80:7199–7207. doi: 10.1128/JVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong G, Waris G, Tanveer R, Siddiqui A. Proc Natl Acad Sci USA. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Quinn PJ. Prog Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 13.Dalle - Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. J Cell Mol Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapadia SB, Chisari FV. Proc Natl Acad Sci USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J, Wang C, Sumpter R, Jr, Brown MS, Goldstein JL, Gale M., Jr Proc Natl Acad Sci USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janero DR. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 17.Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. J Biol Chem. 2001;276:4365–4372. doi: 10.1074/jbc.M007273200. [DOI] [PubMed] [Google Scholar]
- 18.Canfield LM, Davy LA, Thomas GL. Biochem Biophys Res Commun. 1985;128:211–219. doi: 10.1016/0006-291x(85)91666-3. [DOI] [PubMed] [Google Scholar]
- 19.Genova ML, Pich MM, Biondi A, Bernacchia A, Falasca A, Bovina C, Formiggini G, Castelli GP, Lenaz G. Exp Biol Med. 2003;228:506–513. doi: 10.1177/15353702-0322805-14. [DOI] [PubMed] [Google Scholar]
- 20.Sevilla CL, Mahle NH, Eliezer N, Uzieblo A, O'Hara SM, Nokubo M, Miller R, Rouzer CA, Marnett LJ. Chem Res Toxicol. 1997;10:172–180. doi: 10.1021/tx960120d. [DOI] [PubMed] [Google Scholar]
- 21.Bays H. Am J Cardiol. 2006;98:71–76. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.