Abstract
Histone deacetylase (HDAC) inhibitors reactivate tumor suppressor gene transcription; induce cancer cell differentiation, growth arrest, and programmed cell death; and are among the most promising new classes of anticancer drugs. Myc oncoproteins can block cell differentiation and promote cell proliferation and malignant transformation, in some cases by modulating target gene transcription. Here, we show that tissue transglutaminase (TG2) was commonly reactivated by HDAC inhibitors in neuroblastoma and breast cancer cells but not normal cells and contributed to HDAC inhibitor-induced growth arrest. TG2 was the gene most significantly repressed by N-Myc in neuroblastoma cells in a cDNA microarray analysis and was commonly repressed by N-Myc in neuroblastoma cells and c-Myc in breast cancer cells. Repression of TG2 expression by N-Myc in neuroblastoma cells was necessary for the inhibitory effect of N-Myc on neuroblastoma cell differentiation. Dual step cross-linking chromatin immunoprecipitation and protein coimmunoprecipitation assays showed that N-Myc acted as a transrepressor by recruiting the HDAC1 protein to an Sp1-binding site in the TG2 core promoter in a manner distinct from it's action as a transactivator at E-Box binding sites. HDAC inhibitor treatment blocked the N-Myc-mediated HDAC1 recruitment and TG2 repression in vitro. In neuroblastoma-bearing N-Myc transgenic mice, HDAC inhibitor treatment induced TG2 expression and demonstrated marked antitumor activity in vivo. Taken together, our data indicate the critical roles of HDAC1 and TG2 in Myc-induced oncogenesis and have significant implications for the use of HDAC inhibitor therapy in Myc-driven oncogenesis.
Keywords: c-Myc, N-Myc
Recruitment of histone deacetylase (HDAC) proteins, such as HDAC1, to chromatin results in the removal of acetyl groups from nucleosomal histones and histone deacetylation (reviewed in refs. 1–3). Histone deacetylation, which leads to transcriptional repression of key tumor suppressor genes, is a common contributing factor to human tumorigenesis (1, 2). HDAC inhibitors induce cancer cell differentiation, growth arrest and programmed cell death, with little toxicity against normal nonmalignant cells (1, 4, 5). Early clinical trials using HDAC inhibitors in patients demonstrate that HDAC inhibitor treatment leads to tumor regression and symptomatic improvement in some heavily pretreated and multiply relapsed patients, with a surprisingly low side-effect profile (4, 5). Little is known of the cellular and molecular factors that determine resistance or sensitivity to HDAC inhibitors.
Myc family oncoproteins, such as N-Myc and c-Myc, are structurally related nuclear proteins that affect cell signaling and phenotype by binding to cognate DNA sequences and modulating target gene transcription (6, 7). Many Myc target genes have been identified, but few have been linked to specific cancer phenotypes (6).
N-Myc and c-Myc are commonly up-regulated in human cancer, leading to cell proliferation, arrested differentiation, and malignant transformation (7, 8). N-Myc amplification and consequent N-Myc overexpression are seen as a clonal feature in 20–25% of tumors, and correlates with poor prognosis in patients with neuroblastoma (9, 10). Considerable evidence suggests that neuroblastoma is, in part, caused by arrested neuroblast differentiation and resistance to spontaneous regression (9, 11). Amplification of the c-Myc oncogene was found in 15% of breast cancer patients in a metaanalysis of 3,797 patients from 26 published articles and correlated with poor prognosis (12). However, the molecular basis for the oncogenic action of Myc oncoproteins is still unknown.
We found that published cDNA microarray gene profiling studies of HDAC inhibitor-treated cell lines and tissues identified tissue transglutaminase (TG2) as one of the common transcriptional targets of HDAC inhibitors (13–16). Our own microarray data showed that TG2 was the gene most significantly repressed by N-Myc in neuroblastoma cells. TG2 is widely expressed in normal tissues and is a multifunctional enzyme that catalyzes transamidation and multimerization of proteins (17). We have, therefore, carried out experiments to examine the role of TG2 in the anti-cancer efficacy of HDAC inhibitors and in the oncogenic effects of Myc.
Results
Transcriptional Activation of TG2 Contributes to HDAC Inhibitor-Induced Growth Inhibition in Neuroblastoma and Breast Cancer Cells.
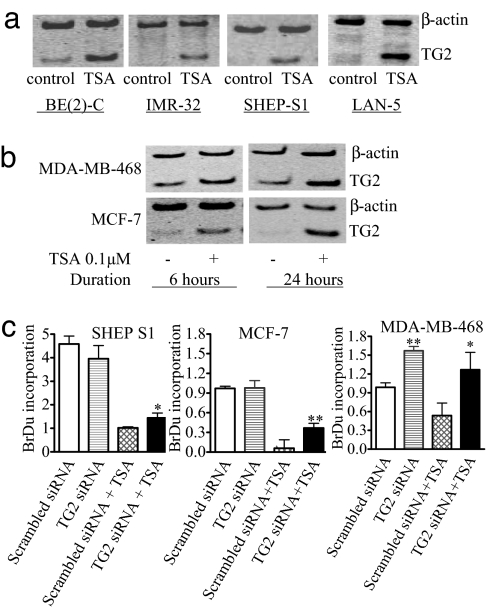
In a search for transcriptional targets critical for HDAC inhibitor-induced anti-cancer effects, we reviewed published cDNA microarray gene profiling studies and found that TG2 is commonly up-regulated by HDAC inhibitors, such as Trichostatin A (TSA), SAHA, and butyrate, in cancer cells of various organ origins, such as leukaemia and liver, renal, nasopharyngeal, and breast cancer (13–16). To validate this observation, we performed semiquantitative RT-PCR analysis of TG2 gene expression in neuroblastoma and breast cancer cells. As shown in Fig. 1a, treatment with 0.1 μM TSA for 6 h reactivated TG2 gene expression in BE(2)-C, IMR-32, SHEP S1, and LAN-5 neuroblastoma cells. Immunoblot analysis with an antibody that identified chromatin histone H4 revealed a marked increase in histone acetylation 3 h after TSA treatment (supporting information (SI) Fig. 7), confirming that TSA acetylated histones in the cells. Likewise, TSA up-regulated TG2 expression in MCF-7 and MDA-MB-468 breast cancer cells (Fig. 1b). In contrast, TSA did not have an effect on TG2 transcription in normal nonmalignant MCF-10A1 human mammary epithelial cells, MRC-5 human lung fibroblasts, human mammary epithelial cells (HMECs), human umbilical vein epithelial cells (HUVEC), primary murine ganglia or bone marrow-derived lymphocytes (SI Fig. 8). These data suggested that TG2 gene transcription is repressed by HDAC activity across cancer cells from various tissue types but not in normal nonmalignant cells.
Fig. 1.
Up-regulation of TG2 by the HDAC inhibitor TSA and its role in cell proliferation in neuroblastoma and breast cancer cells. (a) BE(2)-C, IMR-32, SHEP S1, and LAN-5 neuroblastoma cells were treated with control or 0.1 μM TSA for 6 h, followed by RNA extraction and semiquantitative competitive RT-PCR. (b) MCF-7 and MDA-MB-468 breast cancer cells were treated with control or TSA for 6 or 24 h, followed by RNA extraction and RT-PCR. (c) SHEP S1, MCF-7, and MDA-MB-468 cells were transfected with control or TG2 siRNA for 8 h, followed by treatment with control or TSA for 48 h and incubation with BrDu for the last 6 h. BrDu incorporation was measured as OD units of absorbance. *, P < 0.05 and **, P < 0.01 indicate a statistically significant increase in BrDu incorporation. Error bars indicate standard error.
To assess the role of TG2 gene up-regulation in HDAC inhibitor-induced cell growth arrest, we used neuroblastoma SHEP S1, breast cancer MCF-7, and MDA-MB-468 cells. SHEP S1 and MCF-7 cells expressed a very low basal level of TG2, whereas MDA-MD-468 cells showed higher TG2 expression (SI Fig. 9). Treatment of the SHEP S1, MCF-7, and MDA-MB-468 cells with 0.1 μM TSA did not induce cell death but significantly activated TG2 gene expression. Both basal and HDAC inhibitor-induced TG2 gene expression was markedly reduced by TG2-specific siRNA (SI Fig. 9). In the absence of TSA, TG2 siRNA transfection had no effect on cell proliferation in SHEP S1 and MCF-7 cells but increased cell proliferation in MDA-MB-468 cells (Fig. 1c). Although TSA suppressed cell proliferation, TG2 siRNA partly blocked this effect in all three cell lines tested, indicating that TG2 reactivation is partly required for the growth arrest induced by HDAC inhibitors in the cancer cells.
TG2 has been reported to induce apoptosis by activating a BAX conformational change leading to BAX-mediated mitochondrial apoptosis (18), one of the main pathways through which HDAC inhibitors induce apoptosis (19). We therefore tested whether up-regulation of TG2 could be responsible for HDAC inhibitor-induced apoptosis. Treatment with TSA at the dosage of 0.5 μM induced significant cell death in MCF-7 cells but not in SHEP S1 and MDA-MB-468 cells (<10% as quantified by trypan blue exclusion assay), whereas 0.1 μM TSA induced significant cell death in BE(2)-C cells. As shown in SI Fig. 10a, compared with scrambled siRNA, TG2 siRNA did not affect the proportion of MCF-7 or BE(2)-C cells stained with TUNEL 48 h after TSA treatment (P > 0.05). Treatment with TSA for 48 h induced BAX protein expression in both BE(2)-C (data not shown) and MCF-7 cells (SI Fig. 10b). However, knocking-down TG2 with siRNA did not have an effect on the proportion of cells positive for total BAX, or conformationally changed active BAX, as analyzed by immunocytochemistry staining with an anti-BAX N-20 antibody for total BAX and anti-BAX 6A7 antibody for active BAX (SI Fig. 10b). These results did not support a role for TG2 in HDAC inhibitor-induced apoptosis.
TG2 Transcription is Repressed by N-Myc in Neuroblastoma Cells and by c-Myc in Breast Cancer Cells.
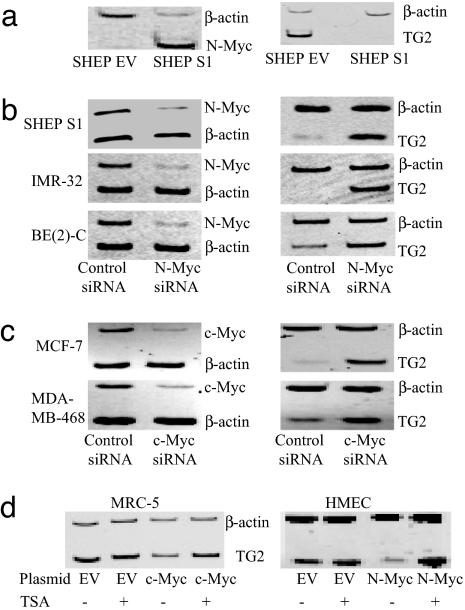
To identify transcriptional targets of the N-Myc oncoprotein, we performed microarray experiments comparing mRNA expression in SHEP S1 neuroblastoma cells stably transfected with an N-Myc-expressing construct and control SHEP EV cells stably transfected with an empty vector. In total, 110 genes were down-regulated, and 28 were up-regulated, by >3-fold (P < 0.05). Those genes with a >4.5-fold difference have been listed in SI Table 1. Among these N-Myc transcriptional target gene candidates, the most significantly modulated was TG2, which was down-regulated by 50-fold. Competitive RT-PCR analysis of three representative N-Myc target gene candidates, including TG2, fibronectin (FN1), and procollagen-lysine oxoglutarate dioxygenase2, in the two cell lines confirmed the validity of the microarray data (Fig. 2a and SI Fig. 11).
Fig. 2.
TG2 is transcriptionally repressed by N-Myc and c-Myc in neuroblastoma and breast cancer cells and activated by TSA in nonmalignant cells overexpressing N-Myc or c-Myc. (a) The effect of N-Myc on TG2 gene expression was examined by semiquantitative competitive RT-PCR of mRNA from N-Myc overexpressing SHEP S1 or empty vector control SHEP EV cells. (b) SHEP S1, BE(2)-C, and IMR-32 neuroblastoma cells were transfected with scrambled siRNA or siRNA specifically targeting N-Myc, followed by RNA extraction, and RT-PCR. (c) MCF-7 and MDA-MB-468 breast cancer cells were transfected with scrambled or c-Myc siRNA, followed by RNA extraction and RT-PCR. (d) Nonmalignant MRC-5 and HMEC cells were transfected with a control empty vector or a construct overexpressing c-Myc or N-Myc, respectively, and treated with control or TSA for 24 h. c-Myc, N-Myc, and TG2 expression was examined by RT-PCR.
To confirm that the transcriptional suppression of TG2 by N-Myc was not due to subclone selection of stable transfectant cells, TG2 expression was analyzed in SHEP TET-OFF cells, which were stably transfected with a tetracycline withdrawal-inducible, N-Myc-expressing construct. As shown in SI Fig. 12a, withdrawal of tetracycline for 48 h induced N-Myc gene expression by 50% and reduced TG2 gene expression by 50% in the SHEP TET-OFF cells (P < 0.05). To determine whether TG2 was an N-Myc target gene across neuroblastoma cell lines, we transiently transfected siRNAs targeting N-Myc or scrambled control siRNA into S-type (substrate-adherent type) N-Myc stable transfectant SHEP S1, N-type (neuronal type) N-Myc-amplified IMR-32, and I-type (intermediate type) N-Myc-amplified BE(2)-C cells (20, 21). The N-Myc siRNAs knocked down N-Myc expression by >70% in all of the three cell lines, and up-regulated TG2 expression by 90% in SHEP S1, 120% in BE(2)-C cells, and >1,100% in IMR-32 cells (Fig. 2b).
c-Myc and N-Myc are known to activate gene transcription through common mechanisms (6, 7). We therefore tested the hypothesis that c-Myc might also suppress TG2 transcription in breast cancer cell lines. As shown in Fig. 2c, repression of c-Myc expression in MCF-7 and MDA-MB-468 mammary cancer cell lines by a specific siRNA significantly up-regulated TG2 gene transcription (P < 0.01). These findings indicated that Myc oncoproteins share a common mechanism in suppressing TG2 gene transcription.
We next transiently transfected nonmalignant MRC5 fibroblasts with c-Myc and HMECs with N-Myc, then treated the cells with 0.1 or 0.05 μM TSA for 24 h and assessed the effect on TG2 transcription. Although Myc overexpression alone slightly (statistically nonsignificantly) decreased basal TG2 expression in these two nonmalignant cell types, we observed a significant increase in TG2 expression after TSA treatment (P < 0.001) (Fig. 2d and SI Fig. 12b).
Repression of TG2 Expression by N-Myc Is Required for Neuritic Differentiation Arrest in Neuroblastoma Cells.
N-Myc-induced malignant transformation has been associated with arrest of differentiation and subsequent indefinite cell proliferation (22). To test whether suppression of TG2 gene expression is responsible for N-Myc-induced neuroblastoma cell differentiation arrest, we transfected scrambled siRNA, TG2 siRNA, N-Myc siRNA, or TG2 siRNA plus N-Myc siRNA into N-Myc-amplified I-type BE(2)-C cells and N-type IMR-32 neuroblastoma cells. As shown in SI Fig. 13, N-Myc siRNA knocked down N-Myc and up-regulated TG2, TG2 siRNA knocked down TG2, and N-Myc siRNA in combination with TG2 siRNA knocked down both N-Myc and TG2 gene expression in both cell lines. Although scrambled siRNA and TG2 siRNA alone did not show a significant effect on cell morphology, N-Myc siRNA alone induced neurite outgrowth within 72 h of transfection, and neurite formation was more dramatic 48 h later (Fig. 3). In contrast, cotransfection of TG2 siRNA blocked N-Myc siRNA-induced neuritic differentiation.
Fig. 3.
N-Myc blocks neuroblastoma cell differentiation by suppressing TG2 gene transcription. BE(2)-C (a) and IMR-32 (b) cells were transfected with scrambled control siRNA (A), TG2 siRNA (B), N-Myc siRNA (C), or N-Myc siRNA plus TG2 siRNA (D). Five days after transfection, cell differentiation was assessed by analyzing neurite outgrowth under phase contrast microscopy. Cell images were captured and stored, and neurite outgrowth was quantified. Error bars indicate standard error.
N-Myc Represses TG2 Transcription by Directly Recruiting the HDAC1 Protein to the Sp1-Binding Site of the TG2 Gene Core Promoter.
Recent studies have shown that c-Myc can repress transcription of the p21WAF1 gene by directly interacting with the p21WAF1 core promoter containing binding sites for Sp1, the transcription factor required for active transcription of p21WAF1 (23, 24). Based on these findings, we tested the hypothesis that N-Myc might repress TG2 transcription through a direct mechanism.
Dual cross-linking ChIP was applied to N-Myc-amplified neuroblastoma cells (IMR-32 and LAN-1), using specific antibodies against N-Myc, Sp1, and HDAC1 proteins (25). A preimmune serum was used as a negative control to determine the baseline of the nonspecific background. As shown in Fig. 4a and SI Fig. 14, all tested antibodies could efficiently immunoprecipitate the TG2 core promoter that contained the Sp1-binding sites (Amplicon B). A DNA region (Amplicon A) located ≈1.6 kb from the TG2 core promoter, was tested in ChIP as a negative control. In addition, to exclude possible artifacts that could be generated by using the dual cross-linking procedure, we verified that binding of N-Myc to canonical E-Box sequences was not compromised. Specifically, we tested N-Myc binding to the E-box sequences present within the promoter of the APEX-1 (Amplicon D) and nucleolin (Amplicon F) genes, direct targets of c-Myc and N-Myc (26–28). Dual cross-linking did not affect the binding of N-Myc to its cognate sites (Fig. 4a). We obtained similar results with a second neuroblastoma cell line, IMR-32 (SI Fig. 15). The N-Myc transrepressor protein complex appeared to have distinct protein components from the N-Myc transactivator complex, because specific antibodies did not identify Max, Tip-60, or TRRAP in the repressor complex, but, in contrast, all three proteins were present in the known N-Myc transactivation complex (Fig. 4a) (6). Conversely, HDAC1 was not present in the activator complexes.
Fig. 4.
N-Myc represses TG2 gene transcription by recruiting HDAC1 to the TG2 gene core promoter. (a) Dual cross-linking ChIP and quantitative PCR were applied to LAN-1 cells. Quantitative PCR with primers targeting the Sp1-binding site (Amplicon B) or Amplicon A, 1.6 kb up-stream of TG2 gene transcription start site, was performed in triplicate. (Upper Left) Fold enrichment of a given DNA region immunoprecipitated with anti-N-Myc, Max, Sp1, HDAC1, Tip-60, and TRRAP antibodies was calculated as the ratio between the enrichment obtained with a specific antibody compared with preimmune serum. Results were the average of three independent dual cross-linking ChIP experiments. (Upper Right) Dual cross-linking ChIP was performed on LAN-1 cells treated with control or TSA for 24 h, when a maximal transcriptional reactivation of TG2 was observed. Error bars indicate standard error. Dual ChIP analysis of APEX-1 and nucleolin genes was performed as a control. Amplicons C and E correspond to regions far from the transcription start site. Amplicons D and F, near the transcription start site, carry E-box sequences. (b) Luciferase activity of the two reporters TG2 and ΔTG2, transfected into SHEP TET-OFF cells, was determined in the presence (-TET) or absence (+TET) of N-Myc expression and normalized to that of renilla.
To further confirm that the TG2 core promoter region carrying the Sp1 binding sites was required for N-myc mediated repression, the wild-type TG2 promoter and one derivative lacking the core region were cloned into a luciferase reporter and separately transfected into SHEP TET-OFF cells. Luciferase activity of the reporters was monitored as a function of N-Myc expression. Results displayed in Fig. 4b showed that the Sp1 core promoter region was required for N-Myc to repress transcription of the TG2 gene.
To understand the dynamics of how N-Myc, Sp1, and HDAC1 contributed to TG2 repression, dual cross-linking ChIP was performed on LAN-1 neuroblastoma cells treated with TSA for 24 h. Our results showed that TSA dramatically reduced the association of HDAC1 with the TG2 promoter (P < 0.01), but not that of Sp1 and N-Myc (Fig. 4a and SI Fig. 16). Because TSA induced reactivation of TG2 transcription, this result suggested that N-Myc required HDAC1 to repress TG2 transcription, possibly by direct interaction. To prove this point, we tested the possibility that N-Myc and HDAC1 can interact in situ. Nuclear extracts obtained from LAN-1 cells were incubated with specific anti-N-Myc antibodies or with preimmune IgG used as a negative control. The IP-complexes were subsequently separated in an SDS/PAGE and analyzed by Western blot, using antibodies that recognized Sp1, HDAC1, Max, and Tip-60. Results shown in Fig. 5a showed that the anti-N-Myc antibodies coimmunoprecipitated Sp1, HDAC1, Max, and Tip-60, thus confirming that N-Myc was directly complexed in both the activator and repressor complexes identified by our dual cross-linking ChIP experiments. However, when the same nuclear extracts were incubated first with an anti-HDAC1 antibody, only Sp1 and N-Myc were identified in the repressor complex.
Fig. 5.
N-Myc directly interacts with Sp1 and HDAC1 through its carboxyl-terminal domain. (a) Protein coimmunoprecipitation (IP) of N-Myc or HDAC1. One milligram of nuclear protein extract from LAN-1 cells was incubated with either a preimmune serum, or an anti-N-Myc antibody (Left) or an anti-HDAC1 antibody (Right). The purified IP-complex was analyzed by Western blot, using antibodies for the following proteins: Sp1, HDAC1, Max, and Tip-60. Lane 1, input; lane 2, preimmune serum IgG IP; lane 3, anti-N-Myc or anti-HDAC1 antibody IP. (b) GST-N-Myc fusion proteins carrying different N-Myc domains were incubated with nuclear extracts expressing HA-HDAC1 or HA-Tip60. GST pull down complexes were analyzed by Western blot analysis, using an anti-HA monoclonal antibody.
We next sought to determine whether the N-Myc protein domain directly interacting with the repressor complex was distinct from the domain responsible for the interaction with the activator complex. We generated four different GST-N-Myc deletion mutant expression constructs (Fig. 5b). GST-N-Myc proteins were expressed in bacteria and then immobilized on glutathione-agarose beads. The derived beads were incubated with nuclear extracts from cells transfected with HA-HDAC1 or HA-Tip-60. We found that the two complexes bound distinct N-Myc domains. HDAC1 bound only the C-terminal N-Myc DNA binding domain, whereas Tip-60 bound the N-terminal Myc Box I and II domains, consistent with the literature for c-Myc (Fig. 5b) (6, 29).
To assess the necessity of the Sp1 protein for the TG2 transrepressor complex, we transiently transfected LAN-1 cells with an Sp1 siRNA and assessed the TG2 expression. We found that inhibition of Sp1 expression was associated with a marked increase in TG2 transcription, confirming that Sp1 was also necessary for the repressor complex (SI Fig. 17).
TG2 Is Only Expressed in N-Myc-Negative Cells in Neuroblastoma Tumor Tissue and Is Up-Regulated by HDAC Inhibitor Treatment in Vivo.
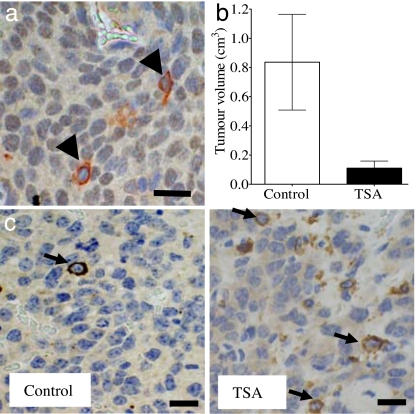
Last, we examined the relationship between TG2 and N-Myc expression and the effects of HDAC inhibitor treatment on TG2 expression in vivo. Four-week-old homozygote N-Myc transgenic mice develop palpable neuroblastoma in the abdomen at an incidence of 100% (11). Cohorts of four homozygous N-Myc transgenic mice at 4 weeks of age were treated with control or TSA for 1 week before being euthanized. As shown in Fig. 6a, without drug intervention, the majority of neuroblastoma tumor cells were positively stained with N-Myc in the nucleus. We found that each TG2-positive tumor cell was negative for N-Myc expression and that every N-Myc positive cell was negative for TG2 expression. This indicated that N-Myc suppressed TG2 gene expression in the neuroblastoma tumor tissues in vivo. Treatment with TSA for 1 week significantly reduced both tumor volume (Fig. 6b) and weight (data not shown) by 8-fold (P < 0.01) in the N-Myc transgenic mice and dramatically increased the percentage of TG2-positive tumor cells in the residual neuroblastoma tumors (Fig. 6c) (P < 0.01).
Fig. 6.
TG2 is expressed exclusively in N-Myc-negative neuroblastoma cells and is up-regulated by an HDAC inhibitor in vivo. (a) Neuroblastoma tissue sections from N-Myc transgenic mice were double-labeled with an anti-N-Myc antibody, which was visualized by using DAB (brown, in the nucleus), and an anti-TG2 antibody, which was visualized by using AEC (red in the cytoplasm, indicated with arrowheads). The nucleus was counterstained with haematoxylin (blue). (b) Neuroblastoma-bearing N-Myc transgenic mice were treated with control or 20 mg per kilogram of body weight of TSA for 1 week before euthanizing. Tumor volume was measured. Error bars indicate standard error. (c) Formalin-fixed tissue sections from the mice were subjected to immunohistochemical assessment of TG2 protein expression, which was visualized by using DAB. The nucleus was counterstained with haematoxylin. Arrows indicate positive cytoplasmic TG2 staining in tumor cells. (Scale bar, 10 μm.)
Discussion
More than a dozen HDAC inhibitors are currently in clinical trials for the treatment of malignancies of almost all organ origins, and the HDAC inhibitor SAHA is already in clinical use for the treatment of cutaneous lymphoma. In this study, we have demonstrated that TG2 is a common transcriptional target of a HDAC inhibitor in both neuroblastoma and breast cancer but not in normal nonmalignant cells and that transcriptional activation of TG2 contributes to HDAC inhibitor-induced cell growth inhibition.
TG2 is a multifunctional protein that has structural and functional homology to both transglutaminases and BH3-only protein families (17, 18). TG2 promotes programmed cell death by inducing a proapoptotic conformational change in the BAX protein and activation of the mitochondrial apoptosis pathway (18, 30), which has been defined as one of the main pathways through which HDAC inhibitors exert their cytotoxic effects (19). However, our results show that up-regulation of TG2 does not contribute to HDAC inhibitor-induced apoptosis.
This study has demonstrated that TG2 is commonly repressed by the N-Myc oncoprotein in neuroblastoma cells and by c-Myc in breast cancer cells and that the neuritic differentiation of neuroblastoma cells induced by N-Myc siRNA depends on transcriptional activation of TG2. The transamidation activity of TG2 has been confirmed to be essential for the neuroblastoma and leukemia cell differentiation response to retinoid therapy (31–33), and TG2 overexpression alone induces neuritic differentiation in neuroblastoma cells (31). Therefore,we conclude that suppression of TG2 is essential for the differentiation block in N-Myc overexpressing neuroblastoma cells. Moreover, HDAC inhibitor therapy alone reverses the action of N-Myc and c-Myc on the transcriptional suppression of TG2, which coincides with marked anti-tumor effects in N-Myc transgenic mice. Our data suggest a general mechanism by which Myc oncoproteins affect the malignant phenotype and highlight the importance of HDAC inhibitors for the treatment of cancer types overexpressing Myc oncoproteins.
Myc family proteins are well known to activate gene transcription by binding to a Myc-responsive E-box and to inhibit gene transcription by binding to a repressive initiator element at the target gene promoter (34). c-Myc has more recently been reported to suppress p21WAF1 transcription through binding to the p21WAF1 core promoter at an Sp1-binding site (24); however, the mechanism of transcriptional repression is unknown. Our study has shown that N-Myc can recruit the HDAC1 protein via its DNA binding domain to the TG2 gene core promoter at the Sp1-binding site and that HDAC inhibitor treatment reactivates TG2 gene transcription without affecting N-Myc and Sp1 binding to the Sp1-binding site. This suggests that N-Myc and HDAC1 are contemporaneously bound to Sp1, which is bound to DNA at its consensus binding site, and that recruitment of HDAC1 is essential for N-Myc-induced transcriptional suppression of TG2. Although HDAC1 has been shown to bind Sp1 (35), our data provide evidence that a Myc oncoprotein can suppress transcription through recruiting HDAC1 to a target gene promoter and that Myc-mediated target gene suppression could be reversed by HDAC inhibitor treatment in cell lines and in tumor tissues in vivo. More importantly, our data indicates that Myc oncoproteins may possess a more widespread capacity for transcriptional suppression of tumor suppressor genes by recruiting HDAC1 proteins to target gene promoters at Sp1-binding sites.
Because TG2 has been demonstrated to suppress tumor growth, metastasis, and angiogenesis in animal models of colon cancer and melanoma (36, 37), our findings highlight TG2 as a potential drug development target for the treatment of cancers overexpressing Myc oncoproteins. Furthermore, our findings provide support for the use of HDAC inhibitor therapy in cancer types overexpressing Myc oncoproteins and suggest that therapies that augment the expression or function of TG2 may have a synergistic therapeutic effect on cancer when combined with HDAC inhibitors.
Materials and Methods
cDNA Microarray.
Direct-labeling cDNA microarray experiments were carried out in triplicate with cDNA microarray slides printed in the Peter MacCallum Cancer Centre (Melbourne, Australia) and analyzed with GeneSpring.
siRNA Transfection.
Cells were transfected with scrambled or target gene-specific siRNA, using Lipofectamine 2000. siRNAs specifically targeting c-Myc, TG2, or N-Myc were purchased from Dharmacon and/or Ambion.
Cell Proliferation and Differentiation Assays.
The cell proliferation assay was carried out with the BrdU Colorimetric Cell Proliferation Kit (Roche). Cell differentiation assay was carried out as described in ref. 31.
Dual Step Cross-Linking Chromatin Immunoprecipitation.
Dual-step ChIP was performed essentially as described by Nowak et al. (25), with 5 μg of IgG as a control, anti-N-Myc (BD Biosciences), anti-Sp1 (Upstate Biotechnology), anti-HDAC1 (Upstate Biotechnology) anti-Max (Santa Cruz Biotechnology), anti-TRAPP and anti-TIP60 [kindly provided by B. Amati (European Institute of Oncology, Milian, Italy)] antibodies. Promoter regions were detected with quantitative PCR with specific primer pairs listed in SI Methods.
GST Pull-Down Assay.
GST-N-myc proteins were expressed in E.coli, purified, and immobilized onto glutathione agarose beads (Sigma). GST beads were then incubated with nuclear extracts expressing either HA-Tip60 or HA-Hdac1 proteins. Purified complexes were separated on SDS/PAGE and analyzed by Western Blot analysis, using an anti HA monoclonal antibody (Sigma).
Protein Coimmunoprecipitation Assay.
Nuclear protein lysates (1 mg) extracted from LAN-1 cells were immunoprecipitated with either mouse preimmune serum or an anti-N-Myc monoclonal antibody. Eluted proteins were finally immunoblotted with anti-HDAC1 antibody.
Luciferase Assay.
The TG2 promoter construct was generated by inserting the DNA region between −1,603 bp and +378 bp of the TG2 promoter into the pGL2-basic reporter (Promega) upstream of the TK-TATA box. The ΔTG2 vector was obtained by deleting the −77/+378-bp region from the TG2 vector. Firefly or renilla luciferase activity was measured with a dual luciferase assay kit (Promega)
Animal Studies.
Four-week-old homozygous N-Myc transgenic mice with spontaneous abdominal neuroblastoma (11) were injected intraperitoneally with control or TSA at 20 mg/kg of body weight daily for 7 days (38). After mice were killed, tumor tissues were paraffin-embedded. All studies involving animals were approved by the Animal Care and Ethics Committee of the University of New South Wales, Sydney, Australia. For single immunostaining, mouse tissue sections were probed with anti-TG2 antibody and developed with diaminobenzidine (DAB). For double-labeling immunohistochemistry, tissue sections were first incubated with mouse anti-N-Myc antibody. After peroxidase inactivation, the sections were reprobed with anti-TG2 antibody and developed by 3-Amino-9-ethylcarbazole (AEC).
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council Australia, Cancer Council New South Wales, and Cancer Institute New South Wales, Australia (M.H. M.D.N., and G.M.M.); the United States National Cancer Institute and a University of New South Wales Faculty Grant (to T.L.); and the Italian Association for Research on Cancer, the Italian Ministry of University and Research, University of Bologna, and Regional Program for Industrial Research-ITT Emilia Romagna Region, Italy (G.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705524104/DC1.
References
- 1.Liu T, Kuljaca S, Tee A, Marshall GM. Cancer Treat Rev. 2006;32:157–165. doi: 10.1016/j.ctrv.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Bolden JE, Peart MJ, Johnstone RW. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 3.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 4.Kelly WK, Marks PA. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone RW. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 6.Patel JH, Loboda AP, Showe MK, Showe LC, McMahon SB. Nat Rev Cancer. 2004;4:562–568. doi: 10.1038/nrc1393. [DOI] [PubMed] [Google Scholar]
- 7.Pelengaris S, Khan M, Evan G. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 8.Nesbit CE, Tersak JM, Prochownik EV. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 9.Brodeur GM. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 10.Maris JM, Matthay KK. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 11.Hansford LM, Thomas WD, Keating JM, Burkhart CA, Peaston AE, Norris MD, Haber M, Armati PJ, Weiss WA, Marshall GM. Proc Natl Acad Sci USA. 2004;101:12664–12669. doi: 10.1073/pnas.0401083101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deming SL, Nass SJ, Dickson RB, Trock BJ. Br J Cancer. 2000;83:1688–1695. doi: 10.1054/bjoc.2000.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba T, Yokosuka O, Arai M, Tada M, Fukai K, Imazeki F, Kato M, Seki N, Saisho H. J Hepatol. 2004;41:436–445. doi: 10.1016/j.jhep.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 14.He LZ, Tolentino T, Grayson P, Zhong S, Warrell RP, Jr, Rifkind RA, Marks PA, Richon VM, Pandolfi PP. J Clin Invest. 2001;108:1321–1330. doi: 10.1172/JCI11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray SG, Qian CN, Furge K, Guo X, Teh BT. Int J Oncol. 2004;24:773–795. doi: 10.3892/ijo.24.4.773. [DOI] [PubMed] [Google Scholar]
- 16.Keen JC, Garrett-Mayer E, Pettit C, Mack KM, Manning J, Herman JG, Davidson NE. Cancer Biol Ther. 2004;3:1304–1312. doi: 10.4161/cbt.3.12.1458. [DOI] [PubMed] [Google Scholar]
- 17.Lorand L, Graham RM. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 18.Rodolfo C, Mormone E, Matarrese P, Ciccosanti F, Farrace MG, Garofano E, Piredda L, Fimia GM, Malorni W, Piacentini M. J Biol Chem. 2004;279:54783–54792. doi: 10.1074/jbc.M410938200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang XD, Gillespie SK, Borrow JM, Hersey P. Mol Cancer Ther. 2004;3:425–435. [PubMed] [Google Scholar]
- 20.Ross RA, Spengler BA. J Natl Cancer Inst. 2004;96:1192–1193. doi: 10.1093/jnci/djh262. [DOI] [PubMed] [Google Scholar]
- 21.Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, Cheung NK, Ross RA. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimmer MR, Weiss WA. Curr Opin Pediatr. 2006;18:634–638. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]
- 23.Gartel AL, Shchors K. Exp Cell Res. 2003;283:17–21. doi: 10.1016/s0014-4827(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 24.Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, Tyner AL. Proc Natl Acad Sci USA. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak DE, Tian B, Brasier AR. Biotechniques. 2005;39:715–725. doi: 10.2144/000112014. [DOI] [PubMed] [Google Scholar]
- 26.Menssen A, Hermeking H. Proc Natl Acad Sci USA. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perini G, Diolaiti D, Porro A, Della Valle G. Proc Natl Acad Sci USA. 2005;102:12117–12122. doi: 10.1073/pnas.0409097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN, Golub TR. Proc Natl Acad Sci USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. EMBO Rep. 2003;4:575–580. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piacentini M, Farrace MG, Piredda L, Matarrese P, Ciccosanti F, Falasca L, Rodolfo C, Giammarioli AM, Verderio E, Griffin M, Malorni W. J Neurochem. 2002;81:1061–1072. doi: 10.1046/j.1471-4159.2002.00898.x. [DOI] [PubMed] [Google Scholar]
- 31.Tucholski J, Lesort M, Johnson GV. Neuroscience. 2001;102:481–491. doi: 10.1016/s0306-4522(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 32.Balajthy Z, Csomos K, Vamosi G, Szanto A, Lanotte M, Fesus L. Blood. 2006;108:2045–2054. doi: 10.1182/blood-2004-02-007948. [DOI] [PubMed] [Google Scholar]
- 33.Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- 34.Li LH, Nerlov C, Prendergast G, MacGregor D, Ziff EB. EMBO J. 1994;13:4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camarero N, Nadal A, Barrero MJ, Haro D, Marrero PF. Nucleic Acids Res. 2003;31:1693–1703. doi: 10.1093/nar/gkg262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, Melino G, Griffin M. Cell Death Differ. 2006;13:1442–1453. doi: 10.1038/sj.cdd.4401816. [DOI] [PubMed] [Google Scholar]
- 37.Lentini A, Forni C, Provenzano B, Beninati S. Amino Acids. 2007;32:95–100. doi: 10.1007/s00726-006-0304-3. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, et al. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.