Abstract
After encoding, memory traces are initially fragile and have to be reinforced to become permanent. The initial steps of this process occur at a cellular level within minutes or hours. Besides this rapid synaptic consolidation, systems consolidation occurs within a time frame of days to years. For declarative memory, the latter is presumed to rely on an interaction between different brain regions, in particular the hippocampus and the medial prefrontal cortex (mPFC). Specifically, sleep has been proposed to provide a setting that supports such systems consolidation processes, leading to a transfer and perhaps transformation of memories. Using functional MRI, we show that postlearning sleep enhances hippocampal responses during recall of word pairs 48 h after learning, indicating intrahippocampal memory processing during sleep. At the same time, sleep induces a memory-related functional connectivity between the hippocampus and the mPFC. Six months after learning, memories activated the mPFC more strongly when they were encoded before sleep, showing that sleep leads to long-lasting changes in the representation of memories on a systems level.
Keywords: fMRI, hippocampus, medial prefrontal cortex, memory
New memories must undergo a period of consolidation to become stable and immune to interference (1). Consolidation occurs in the form of molecular processes at individual synapses (2) but also in the form of systems consolidation, which is a reorganization of the memory trace within different brain systems (3–5). This is most obvious for declarative memory, where recall initially depends on the hippocampus, but after some time becomes hippocampus-independent (6–8). Instead, neocortical areas, especially the medial prefrontal cortex (mPFC), are assumed to take over its function (9, 10). In a recent functional imaging study, Takashima et al. (11) showed that both regions display opposite activity over the course of 3 months; whereas the hippocampal contribution to memory recall decreases with time, the prefrontal one rises.
During the last few years, an important contribution of sleep to memory consolidation has been revealed (12, 13). Sleep prevents forgetting and makes memories resistant to interference, especially when it closely follows learning (14, 15). In particular, animal research has shown that sleep provides the conditions for a hippocampal–neocortical dialogue and information transfer (16, 17). Low levels of cholinergic neuromodulation disinhibit hippocampal–neocortical feedback synapses (18), and hippocampus and neocortex show synchronous activity during sleep (19). Together, these findings support the idea that sleep modifies the trace of a recently stored memory. In the present experiment, we tested this hypothesis using functional MRI (fMRI) to characterize brain activity related to free recall immediately, 48 h, and 6 months after learning a declarative memory task. The contribution of sleep to systems memory consolidation was tested by allowing subjects to sleep or by sleep depriving them during the first night after learning.
Results
Subjects were tested on a word-pair memory task in two conditions, following a within-subject cross-over design. On the first evening of each condition, subjects learned a new randomized list of word pairs containing 90 pairs of semantically related concrete nouns with the instruction to imagine a picture containing both objects of a pair. In one condition (sleep, S), cued recall was tested on the first evening immediately after learning (PRE). Then subjects were allowed to sleep during two nights before being retested (POST). In the other condition (sleep deprivation, SD), subjects were sleep-deprived for 24 h after learning and immediate testing. Then they slept on the second postencoding night, which preceded the retest session. Sleep and sleep-deprivation conditions were arranged in random order. Additionally, subjects came back for an unannounced followup retest 6 months after the initial sessions, during which recall of words from both conditions was tested. Brain activity during all learning and recall sessions was recorded with fMRI.
The brain activity measured during learning and recall sessions, tested against the corresponding baseline activity during the Korean letter task, showed that similar brain regions were activated during learning and retrieval. Activity was centered bilaterally in the occipital extrastriate cortices, extending anteriorly up to the fusiform gyri (Fig. 1; learning: [−34 −90 22], Z = 5.46, PFWE < 0.001; [38 −84 10], Z = 5.29, PFWE < 0.001; recall: [−36 −88 0], Z = 6.59, PFWE < 0.001; [36 −88 4], Z = 6.31, PFWE < 0.001). During learning, additional activity was found in the right supramarginal gyrus ([62 −40 32], Z = 5.09, PFWE = 0.004), an area also active during working memory tasks (20). Thus, during learning and recall of pairs of concrete nouns, mainly extrastriate sites of primary object representation are strongly activated (21).
Fig. 1.
Brain activity during learning and recall tasks, respectively, for words correctly recalled 2 days after learning as compared with the Korean control task. Activity during learning (A) and cued recall of word pairs (B) is centered mainly in the extrastriate visual system. Note that words represented concrete objects, and subjects were instructed to imagine a picture containing both items of a pair, which explains the strong implication of this pathway known to be crucial for object representation (21). No significant activity was found in area 17 of the visual cortex, perhaps reflecting the imaginary nature of the task. Maps displayed at PFWE < 0.05.
The effect of sleep on systems memory consolidation becomes apparent when comparing brain activity elicited by correct recall of word pairs from the sleep and sleep deprivation conditions. Both conditions differ in the way hippocampal activity changes over the initial 2-day retention interval. This activity is centered in the subiculum of the right hippocampus and closely follows the outline of the hippocampus, bordering on the amygdala (POST-PRE × S-SD, [26 −16 −22], Z = 3.92, PSVC = 0.003, Fig. 2A). Although there were no significant differences in brain responses between conditions during immediate recall, activity in the right hippocampus was significantly stronger during POST recall after sleep than after sleep deprivation (Fig. 2B; [26 −16 −24], Z = 3.33, PSVC = 0.017). No other brain area showed significant effects in these contrasts, and none was significantly more active after SD compared with the S condition.
Fig. 2.
Changes in hippocampal activity during correct word recall over sleep and sleep deprivation. (A) Between the immediate (PRE) and 2-day delayed (POST) recall sessions, the hippocampal involvement in correct word recall increases significantly, but only when subjects slept during the first night after learning (S-SD × POST-PRE). (B) Hippocampal activity ([26 −16 −22]) across the whole 6-month retention interval. In the sleep condition (open circles), activity increases from 30 min to 2 days (Z = 3.00, PSVC = 0.04), whereas no significant changes can be found when subjects were sleep-deprived the night after learning (filled circles). S and SD conditions differ only at the 2-day interval (t15 = 3.4, P = 0.003).
Behavioral data supplement this conclusion and confirm the beneficial effect of sleep on declarative memory consolidation (22). Immediately after learning, the number of correctly recalled pairs in the cued recall procedure was 55.7 ± 3.4 words before SD and 51.2 ± 3.5 words before S (t17 = 1.4, P = 0.17), each out of a new random list of 90 related word pairs. Between PRE and POST recall sessions, subjects forgot on average 3.1 ± 1.1 words after SD (5.6 ± 2.0% of words initially remembered) and only 0.1 ± 0.8 words (0.2 ± 1.6%) after S (t17 = 2.6, P = 0.02). Results could not be accounted for by persisting effects of sleep deprivation during retest. Reaction times in a psychomotor vigilance task did not differ between sessions or conditions (sleep deprivation, PRE: 279 ± 5.5 ms, POST: 279 ± 5.8 ms; sleep, PRE: 276 ± 5.1 ms, POST: 279 ± 6.6 ms; all comparisons P > 0.4). Subjective ratings of fatigue at the time of testing also did not differ between conditions (sleep deprivation, PRE: 2.0 ± 0.2, POST: 2.1 ± 0.3; sleep, PRE: 2.3 ± 0.3, POST: 2.2 ± 0.3; all comparisons, P > 0.3). In addition, during learning and recall sessions, learning blocks were interleaved with blocks during which subjects had to perform an attention task, which required them to detect either one or two slightly darker characters in a string of Korean letters. The number of correct answers (87.7 ± 1.9 of 90 trials) was comparable in all sessions (all comparisons P > 0.25), indicating a high compliance of subjects in both conditions.
To address the issue of hippocampal–neocortical interactions, we assessed the functional connectivity of the above-described hippocampal location by means of psychophysiological regression. During correct word recall in the POST session after SD, hippocampal activity was preferentially coupled with that of an area with a maximum in the left precuneus ([−18 −58 22], Z = 5.33, PFWE < 0.001). After S, however, in addition to the precuneus ([10 −52 26], Z = 4.22, PSVC < 0.001), a strong functional relation was observed between the hippocampus and the ventral mPFC [−6 36 −18], Z = 4.11, PSVC < 0.001, Fig. 3; for a complete list of activations, see supporting information (SI) Table 1]. This relation was significantly stronger than in the SD condition (Z = 4.05, PSVC = 0.002). This important result shows that 48 h after encoding, retrieval engages the mPFC only in proportion to hippocampal activity. This finding is compatible with an early consolidation stage, during which the hippocampus still plays a central role in memory retrieval but interacts with the mPFC, suggesting the emergence of a hippocampal–neocortical information transfer.
Fig. 3.
Areas functionally related to the hippocampus during correct word recall on day two after sleep or sleep deprivation. Two days after learning, if subjects were allowed to sleep, the hippocampus was functionally connected to the precuneus and the mPFC during correct word recall (blue). However, if subjects were sleep-deprived the night after learning, the prefrontal sites did not relate to hippocampal activation (red).
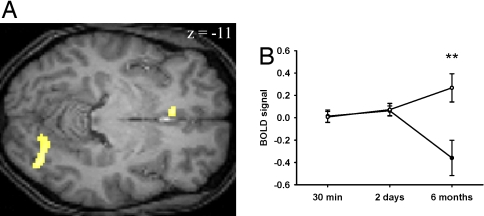
Six months later, as expected, subjects had forgotten most word pairs from both their S and SD conditions. They remembered on average more word pairs learned during the S (7.3 ± 1.0%) than the SD condition (6.5 ± 1.3%), although the difference was not significant (t17 = 0.5, P = 0.65). Nevertheless, brain responses recorded during correct recall still differed significantly between conditions at the random effects level, showing these differences were strong and very stable across subjects. Correct recall of word pairs learned before sleep, as compared with pairs learned before sleep deprivation, evoked a larger response in the left ventral mPFC ([−8 26 −8], Z = 3.25, PSVC = 0.023; Fig. 4). In addition, activation was found in the right inferior occipital gyrus, an area initially active during learning ([36 −84 −12], Z = 3.48, PSVC = 0.012; for complete results see SI Table 2). Thus, after 6 months, retrieval of words learned before sleep no longer preferentially involves the hippocampus but directly engages the mPFC. At the same time, occipital areas initially recruited during encoding are active. On the other hand, correct recall of words learned before SD activated the left hippocampus more strongly than recall of words learned before sleep, although this result did not survive correction for multiple comparisons ([−36 −22 −22], Z = 3.85, Puncorr < 0.001; for complete results, see SI Table 2). This finding might indicate that sleep deprivation on the first postencoding night hinders the plastic changes that initiate memory consolidation. It is important to note that our results represent relative differences in brain activity between sleep and sleep deprivation conditions. Hippocampus and mPFC are probably both involved to some degree in short- and long-delayed recall. Their relative contribution, however, varies depending on whether subjects slept or were sleep-deprived after learning.
Fig. 4.
Differences in brain activity during the 6-month retest session for correctly recalled words learned before sleep vs. before sleep deprivation (S-SD). (A) Correct word recall after 6 months activates the mPFC and the occipital cortex more strongly for words from the sleep condition than for words from the sleep-deprivation condition. Note that at the 2-day interval, no activity per se was found, but only a strong functional relation to hippocampal activity. Now, at the 6-month interval, independent mPFC activity is found, but no more significant hippocampal activity. (B) The difference in brain activity in the mPFC developed mainly during the interval between the 2-day and 6-month recall sessions. It is supported by a steady increase in mPFC activity for words from the S condition over the 6-month period (open circles) and a marked drop in mPFC activity for words from the SD condition (filled circles) during the 6-month session ([−6 26 −10], Z = −3.87, PSVC = 0.004).
Discussion
In summary, our findings show that sleep during the first night after learning profoundly influences the trace of declarative memories at a systems level. Initially, during retrieval 2 days after learning, the hippocampus is more active when subjects are allowed to sleep after learning. Simultaneously, hippocampal activity modulates activity in the mPFC. These findings are an indication of sleep-dependent intrahippocampal memory processing during sleep and suggest a hippocampal–mPFC interplay at an early stage of memory consolidation. In the long term, these processes initiated during this first night transform the memory trace in such a way that the cortical correlates of retrieval 6 months after encoding still reflect whether subjects slept during the first postlearning night. These results confirm models based on animal data, which predict a sleep-dependent shift of retrieval function from the hippocampus toward the neocortex during systems memory consolidation (10, 16, 17).
Our results are also in good agreement with the findings of Takashima et al. (11), who observe in fMRI a decrease in hippocampal and a concurrent increase in mPFC activity over 3 months. The present data further show that this shift in function depends on sleep. Additionally, we show that both sites are functionally connected at an early stage of memory consolidation. The study by Takashima et al. (11) additionally reports a correlation between the amount of slow-wave sleep (SWS) in a short nap and left hippocampal activity decrease during an immediately following picture recognition task (11). It is more difficult to reconcile that result with the data presented here. It should be noted, however, that it pertains to much shorter retention intervals (2–3 h) and very short periods of sleep (<14 min of SWS on average).
Although the hippocampus has long been known as an area closely related to memory storage, the role of the mPFC has only more recently received increasing attention as a neocortical site that participates in memory storage (10, 23–25). It has been proposed to play an important role for remote memories, as does the hippocampus for recent ones. For instance, in rodents, mPFC lesions produce stronger amnesia for remote than for new memories (5). Moreover, recent functional imaging studies in humans have shown participation of the hippocampus in memory retrieval to increase with recency of the memory trace, whereas that of the mPFC increased with the age of the memory (11, 26). Important functional relations between the hippocampal formation and the mPFC are also supported by direct anatomical connections and the fact that the mPFC and hippocampus activity are coordinated by the theta rhythm (27, 28) and, during sleep, by sleep spindles (29). Our finding of a functional connectivity during word recall expands these findings and shows a temporary interaction of both sites 48 h after learning. Results indicate a comparatively stable effect and large effect size in the mPFC at 6-month recall. The effect was consistent at the group level over all 18 subjects and remained significant, despite larger error variances at the individual level because of the relatively small number of events available.
Together, our findings are in good agreement with the current standard model of memory consolidation. Similar temporal gradients for the involvement of the medial temporal lobe have been found in a wide range of studies (30). After lesions to the hippocampus, animal studies often find impairment for memories <1 month old (5). If memories can be integrated into previously acquired schemas, memories become independent of the hippocampus even after 48 h (8). On the other hand, although hippocampal involvement decreases over time, involvement of cortical areas, e.g., in the frontal cortex and anterior cingulate, increases over a 25-day interval, again similar to the findings in the present study (31). In humans, studies in patients with hippocampal lesions usually show retrograde amnesia with a much longer duration, impairing memory across periods of several years (30). The differences in the time course between those patient studies and the data presented here might be explained because in lesion studies, a participation of the hippocampus is completely excluded, whereas we observe only relative changes between conditions. Interestingly, when studying a recognition task with fMRI, Stark and Squire (32) found a course of hippocampal activity similar to the one described here. In healthy subjects, activity peaked after 24 h and declined after 1 week in both hippocampi, however without reaching statistical significance.
An interesting and somewhat surprising finding was that the hippocampus was not the main site of activity during either learning or recall, even if significance thresholds were substantially lowered. This lack of hippocampal activity seems to be at odds with many previous studies showing a robust hippocampal contribution to memory (30, 33). However, it might be explained by the design of the task and the stimulus material used. Words are already well represented in memory, whereas pictures, which are used by many other studies, always represent novel aspects. In addition, pairs were already related, which might have further reduced the strength of hippocampal activity (34). Thus, hippocampal linking, relative to semantic processing of the words, seems to be of only secondary importance for task-related brain activity. This, however, in no way means that hippocampal activation during encoding or retrieval can be excluded. The contrast between S and SD conditions, which has a much higher sensitivity because semantic processing-related activity is held constant, shows that the hippocampus is involved to a varying degree at different time points.
The principal activity during task performance was found in extrastriate sensory areas, which are involved in object representation (21). In this respect, our results correspond to the findings by Wheeler et al. (35) that remembering sounds and pictures activates respective sensory-related brain areas. These areas might therefore be the actual storage sites of the encoded items (i.e., the concept corresponding to the learned words), although the hippocampus and later the mPFC are in charge of linking the individual objects to form the new memory (10, 36). Our finding that both the mPFC and areas of the primary sensory cortex were active during the 6-month recall session supports this notion of remote declarative memories being eventually encoded in distributed neocortical networks.
In the present study, behavioral effects, especially at 6-month recall, although present, are less obvious than the underlying differences in brain activity. This finding can be explained with the dual nature of declarative memory storage. Both systems, the hippocampal and the neocortical, store memories in a redundant way. Their main difference lies in their temporal properties and susceptibility to interference (6, 37). According to a recent study using similar word-pair lists, it seems likely that a much greater influence on the behavioral aspects of the task, especially after longer retention intervals, would be seen when presenting interfering material before recall (15). The findings reported here are in agreement with the consolidation model of memory, that new memories are initially stored in the hippocampus, where they are susceptible to new interfering stimuli. During sleep, they are transferred and integrated into existing memories residing in other cortical areas and therefore resistant to interference.
According to a current hypothesis, the role of sleep is to homeostatically downscale synaptic connectivity to compensate increases because of plastic processes occurring during wakefulness (38, 39). This implies that sleep is regulated locally in those neuronal populations initially involved in learning. Going beyond this possibility, our contention is that sleep actively promotes systems consolidation. Our results entail that sleep-dependent changes in activity can be detected in brain regions not originally recruited during learning, and that activity of brain areas can both increase and decrease depending on sleep.
Materials and Methods
Subjects and General Procedure.
The aim of the experiments was to compare brain activity during recall of previously learned verbal material when subjects slept or were sleep-deprived on the first night after learning. The experiments followed a randomized within-subject cross-over design. Subjects participated in both a sleep and sleep-deprivation condition. Each condition began in the evening between 18:30 and 20:30 with a learning session and an immediate recall task (PRE). After two nights of normal sleep (S) or one night of sleep deprivation and one night of recovery sleep (SD), a second recall session followed (POST). A third, unannounced, recall test was performed after ≈6 months (average, 163 ± 4 days), testing words from both the S and SD condition. During all tasks, brain activity was measured by using fMRI.
Eighteen paid volunteers (nine male) participated in this study. They were 18–30 years of age, nonsmokers, and right-handed. They reported to be in good health, with no sleep disorders and no disturbances of the sleep–wake cycle during the last 6 weeks. Experiments were approved by the Ethics Committee of the Medical Faculty of the University of Liège, and subjects gave written informed consent.
During sleep deprivation, subjects were under constant surveillance, playing games and watching films. Physical activity was kept to a minimum, and intake of caffeine and food was prohibited during the night. At 08:00, subjects were allowed to leave the laboratory and follow their usual daytime activities. Sleep duration and compliance with the sleep-deprivation regime during daytime were verified by actimetry starting 48 h before the experiments. Subjects reported an average sleep duration of 7:59 ± 0:16 h during the two nights before the experiments. In the S condition, they slept 8:20 ± 0:20 h and 7:57 ± 0:14 h, respectively, during the two nights after learning. In the SD condition, they slept 13:01 ± 1:42 h during the recovery night. Sleep durations were calculated from sleep logs.
Behavioral Tasks.
Learning and recall sessions took place inside the MRI scanner, where subjects were lying in a supine position. Stimuli were presented by using Cogent 2000 (http://www.vislab.ucl.ac.uk/Cogent) on a back-projection screen visible to the subject through a mirror attached to the head coil. Eye position was continuously monitored and recorded during scanning by using an ASL Model 504 eye-tracking system (Applied Science Laboratories). Each session consisted of 90 pairs of words, randomly intermixed with 90 displays of Korean letters and 180 fixation crosses.
Subjects were asked to learn 90 visually presented pairs of French nouns by forming a mental image of both objects. During the PRE and POST recall sessions, all previously learned words were tested. During the 6-month recall session, 45 randomly selected pairs learned during the SD and 45 pairs learned during the S condition were presented. The total duration of a learning or recall session was ≈23 min. Word pairs for learning were randomly selected from a list of 360 pairs. Words were of high concreteness and low emotional valence (40). Words consisted of 4–10 letters. Pairs were of medium to high semantic relatedness, but difficulty was such that guessing of the second words of the pairs was not a successful strategy. Each pair was presented once for 3.5 s. During the cued recall procedure, subjects were presented for 3.5 s with the first word of each pair. They were instructed to remember the second word with the help of the mental picture they imagined previously. After each word, a fixation cross was presented, and subjects had to indicate whether they remembered the second word by pressing one of two keys. It is important to note that after scanning had ended, responses were verified by explicit verbal recall during another presentation of the words. To exclude possible confounds of performance and recall confidence, only those words were entered into fMRI analysis that were indicated as remembered during scanning and correctly named during subsequent oral recall. Statistical comparisons were made by using two-sided t tests for paired samples.
Strings of Korean letters were used as explicit baseline stimuli to control attentional load and visual stimulation and to prevent memory-related brain activity during the baseline task (20, 41). Two strings of six letters separated by a ‘-’ were displayed in the center of the screen at random intervals interspersed between learning and recall trials. Letters were presented for 1.5–7 s. One or two of these letters were slightly darker than the others. Because the difference in brightness was small, these could not be detected as popouts. Subjects had to scan the letter strings and find the darker ones. During the learning sessions, they were instructed to rest their gaze on the darker letters for a short moment before searching the next one. Eye movements were tracked online and recorded to confirm subjects' compliance. During the recall task, subjects had to indicate the number of darker letters by means of a keyboard during the following presentation of the fixation cross. A fixation cross, which was used as an implicit baseline, was always shown in the center of the screen between the other types of stimuli and presented for a random interval of 1–12 s. To maintain attention during the learning task, on 50% of trials, the cross became slightly darker after some time. Subjects had to respond to these changes by pressing a key. During the recall task, subjects indicated their answers to preceding stimuli by pressing a key during presentation of the fixation cross.
Right-handedness was confirmed with the Edinburgh Inventory (42). Before PRE and POST sessions, reaction time was measured in a shortened version of the psychomotor vigilance task (PVT), which repeatedly measured simple reaction time over a period of 5 min (43). Reaction times were summarized by using the median, because their distribution is skewed to the left. Medians, which are approximately normally distributed, were analyzed by using a two-factor within-subject General Linear Model (GLM). Subjective fatigue was tested with a five-point rating scale.
fMRI Data Acquisition and Analysis.
Whole-brain functional T2*-weighted MRI data were acquired by using a 3-T Allegra scanner (Siemens Medical Solutions) by using a single-shot echo planar imaging (EPI) sequence (voxel size: 3.44 × 3.44 × 3.9 mm3; matrix size: 64 × 64 × 32; repetition time = 2,130 ms; echo time = 40 ms; flip angle = 90°). Data were analyzed in SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5) with a mixed-effects model. The hemodynamic responses to correctly recalled words, incorrectly recalled words, and Korean letter strings were compared in a full factorial random effects model with the factors condition (S vs. SD) and time (PRE vs. POST). For data from the 6-month recall, words from the S and SD conditions were compared in within-session contrasts. For analysis of the time course, data from PRE, POST, and 6-month sessions were combined into one model. Functional connectivity of the hippocampus to other brain areas during correct word recall was assessed with a physiological regression analysis (44). The activity of the hippocampal voxel maximally activated after sleep during the POST test was used as the seed voxel for this voxel-by-voxel regression analysis ([26 −16 −22]), which allowed to find the brain areas covarying in time with the responses of the hippocampus to correct word recall.
Preprocessing included realignment to the first volume, spatial normalization to a standard EPI template, and smoothing with a 8 mm FWHM Gaussian kernel. Data were analyzed by using a mixed-effects model (45). At the first level, brain responses to stimulus events convolved with a standard hemodynamic response function and the signal related to head movements were modeled by using a GLM for fixed effects. The first six scans were discarded to allow for magnetic saturation effects. High-pass filtering was implemented in the matrix design by using a cutoff period of 128 s to remove low-frequency drifts from the time series. Serial correlations in the fMRI signal were estimated by using a first-order autoregressive plus white noise model and a restricted maximum likelihood (ReML) algorithm. The effects of interest consisted in the main effects of presentation of correctly recalled words, incorrectly recalled words, and Korean letter strings. They were tested by linear contrasts, generating statistical parametric maps. These summary statistic images were further smoothed (6-mm FWHM Gaussian Kernel) and entered in a second-level analysis. Data of all subjects were combined in a full factorial random effects model. Main effects and interactions were then calculated and tested by using one-sided t contrasts. ReML estimates of variance components were used to allow for unequal variance and possible deviations from sphericity introduced by dependencies in the repeated measures design. β values were tested for normality with a Kolmogorov–Smirnov test, and no departure from the normality assumption was detected (P > 0.2).
The common effects of learning or recall sessions were calculated from respective PRE and POST sessions. Here, correctly recalled words were compared with the Korean letter task, which was used as an explicit baseline. The forgotten or incorrectly recalled words were not chosen as baseline, because these had a high variability, thus lowering statistical power. An exploratory analysis with a lowered threshold (Puncorr < 0.01) showed activity mainly in various mainly frontal regions related to memory-unspecific processes like attention and response processing. For comparisons between S and SD conditions, the baseline was not relevant, because words from both conditions could be compared directly. PRE-POST and 6-month sessions were analyzed in separate models. Responses to words correctly recalled at 2 days (PRE-POST) or 6 months, respectively, were compared between the S and SD condition. For analysis of the time course and the extraction of β values in volumes of interest, all sessions were included in one model. Again, responses to correctly recalled words were compared between the S and SD conditions. Only correctly recalled words were included in the analyses to study brain activity independent of the degree of memory recall.
Corrections for multiple testing were applied where mentioned by using either the family-wise error correction for the whole brain (FWE) or in a small volume of interest (SVC). SVC for the right hippocampus was done in a 10-mm sphere centered on the coordinate reported by Bosshardt et al. (26) ([26 −18 −22]), who used a comparable word-pair association task. SVC for the mPFC was done in a 10-mm sphere centered on the coordinate reported by Takashima et al. ([−2 32 −10]), where subjects had to memorize photographs (11).
All coordinates are given as standard Montreal Neurological Institute coordinates and correspond to the maxima of the reported cluster of activation. Coordinates were labeled by using the Anatomy 1.3 toolbox available at www.fz-juelich.de/ime/spm_anatomy_toolbox. All significant clusters with a size >10 voxels are reported. All subjects were included in the analyses; only in the analysis of the 6-month recall session did two subjects have to be excluded, because they did not remember any words from the SD condition. All data are given as mean ± standard error of mean. Maps are displayed at Puncorr < 0.001 if not otherwise indicated. Functional images are displayed on the structural image of one typical subject, normalized to the same stereotactic space.
Supplementary Material
Acknowledgments
This study was supported by the Belgian Fonds National de la Recherche Scientifique (FNRS), the Fondation Médicale Reine Elisabeth, and the University of Liège. M.B., T.T.D.-V., A.D., M.D., V.S., G.V., G.R., and P.M. are supported by the FNRS. M.S. is supported by an Erwin Schrödinger fellowship of the Austrian Science Fund (FWF; J2470-B02). S.G. was supported by an Emmy Noether fellowship from the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705454104/DC1.
References
- 1.McGaugh JL. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Malenka RC, Nicoll RA. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 3.Dudai Y. Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Hu Y, Tsien JZ. Prog Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Takehara K, Kawahara S, Kirino Y. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClelland JL, McNaughton BL, O'Reilly RC. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 7.Squire LR, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 8.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 9.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Frankland PW, Bontempi B. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 11.Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernandez G. Proc Natl Acad Sci USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maquet P. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 13.Stickgold R. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 14.Gais S, Lucas B, Born J. Learn Mem. 2006;13:259–262. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Buzsáki G. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 17.Hasselmo ME. Trends Cognit Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 18.Hasselmo ME, McGaughy J. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- 19.Sirota A, Csicsvari J, Buhl D, Buzsáki G. Proc Natl Acad Sci USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulesu E, Frith CD, Frackowiak RS. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 21.Grill-Spector K. Curr Opin Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 22.Born J, Rasch B, Gais S. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 23.Simons JS, Spiers HJ. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 24.Destrebecqz A, Peigneux P, Laureys S, Degueldre C, Del FG, Aerts J, Luxen A, van der LM, Cleeremans A, Maquet P. Brain Res Cogn Brain Res. 2003;16:391–398. doi: 10.1016/s0926-6410(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 25.Takehara-Nishiuchi K, Nakao K, Kawahara S, Matsuki N, Kirino Y. J Neurosci. 2006;26:5049–5058. doi: 10.1523/JNEUROSCI.4381-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosshardt S, Degonda N, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Henke K. Hippocampus. 2005;15:1026–1040. doi: 10.1002/hipo.20105. [DOI] [PubMed] [Google Scholar]
- 27.Irle E, Markowitsch HJ. Neuroscience. 1982;7:2637–2647. doi: 10.1016/0306-4522(82)90088-4. [DOI] [PubMed] [Google Scholar]
- 28.Jones MW, Wilson MA. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siapas AG, Wilson MA. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 30.Squire LR, Stark CE, Clark RE. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 31.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 32.Stark CE, Squire LR. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H, Yonelinas AP, Ranganath C. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davachi L, Wagner AD. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler ME, Petersen SE, Buckner RL. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess N, Becker S, King JA, O'Keefe J. Philos Trans R Soc London Ser B. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gluck MA, Myers CE. Annu Rev Psychol. 1997;48:481–514. doi: 10.1146/annurev.psych.48.1.481. [DOI] [PubMed] [Google Scholar]
- 38.Huber R, Ghilardi MF, Massimini M, Tononi G. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 39.Tononi G, Cirelli C. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Content A, Mousty P, Radeau M. L'Année Psychologique. 1990;90:551–566. [Google Scholar]
- 41.Stark CE, Squire LR. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oldfield RC. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 43.Dinges DF, Powell JW. Behav Res Methods Instrum Comput. 1985;17:652–655. [Google Scholar]
- 44.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 45.Penny WP, Holmes AP. In: Human Brain Function. Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny WD, editors. London: Elsevier; 2004. pp. 843–850. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.