Abstract
Children's intellectual development is influenced by both genetic inheritance and environmental experiences. Breastfeeding is one of the earliest such postnatal experiences. Breastfed children attain higher IQ scores than children not fed breast milk, presumably because of the fatty acids uniquely available in breast milk. Here we show that the association between breastfeeding and IQ is moderated by a genetic variant in FADS2, a gene involved in the genetic control of fatty acid pathways. We confirmed this gene–environment interaction in two birth cohorts, and we ruled out alternative explanations of the finding involving gene–exposure correlation, intrauterine growth, social class, and maternal cognitive ability, as well as maternal genotype effects on breastfeeding and breast milk. The finding shows that environmental exposures can be used to uncover novel candidate genes in complex phenotypes. It also shows that genes may work via the environment to shape the IQ, helping to close the nature versus nurture debate.
Keywords: cognitive development, gene environment interaction
For 100 years, the IQ has been at the heart of scientific and public debates about nature versus nurture (1–3). Twin studies document that differences between individuals' IQs are under strong genetic influence, but twin studies also attest to the existence of nongenetic, environmental influences on IQ, particularly for young children (4, 5). In the past 5 years, the nature versus nurture debate has shifted toward interest in how both nature and nurture work together (6). An integral part of this new focus is research that tests how genetic differences moderate the effects of environmental influences on individuals' health and behavior (7). Here we report replicated evidence that a measured genotype can moderate response to an environmental influence on children's IQ. We began our investigation of gene–environment interaction in IQ by selecting for study an environmental factor thought to influence neurodevelopment and known to predict IQ. We selected being fed breast milk (hereafter breastfeeding) as the environmental exposure because the biological processes underlying its benefits for the developing brain are increasingly well understood (8). A gene involved in these putative biological processes would be a good candidate for framing a gene–environment interaction hypothesis (9). Thus, selecting breastfeeding as the environmental exposure allowed us to nominate a novel candidate gene for this study of IQ.
Breastfeeding is thought to influence brain development through nutritional processes involving fatty acids (10). The predominant long-chain polyunsaturated fatty acids (LC-PUFAs) present in human milk, but not in cow's milk or most infant formulas, are docosahexaenoic acid (DHA; 22:6n-3) and arachidonic acid (AA or ARA; 20:4n-6) (11). Substantial amounts of DHA and AA accumulate in the human brain during the first postnatal months (12), and infants who are breastfed have higher concentrations of DHA and AA than infants fed unsupplemented formulas (13, 14). Evidence, in general, is consistent with the hypothesis that LC-PUFAs in breast milk may enhance cognitive development (15). In humans, children who are breastfed have higher IQs than children not fed breast milk (16, 17), and this advantage persists into adulthood (17). Although breastfeeding in contemporary, industrialized nations is associated with higher social class, IQ differences between breastfed children and children not fed breast milk remain significant in most observational studies even after adjustments for class-related confounding factors (16, 17). However, the essentiality of fatty acids cannot be inferred from such studies. Experimental studies, where more control can be achieved, show that animals that are fed diets deficient in n-3 fatty acids exhibit neuronal deficits, including memory, sensory, and visual abnormalities (18). DHA supplementation in rodents and nonhuman primates leads to increased brain DHA concentrations and enhanced performance on a wide variety of learning, memory, and problem-solving tasks (19–21). LC-PUFAs are thought to be important for cognitive development because they are required for efficient neurotransmission (22) and are involved in neurite outgrowth, dendritic arborization, and neuron regeneration after cell injury (23). This putative biological pathway led us to search the KEGG database (24) for genes involved in LC-PUFA metabolism, reasoning that they might moderate the effect of breastfeeding on children's IQ.
This search led us to FADS2, an attractive candidate gene because of its role in the modification of dietary fatty acids. FADS2, located on chromosome 11q12.2, encodes the delta-6 desaturase that is the rate-limiting step on the metabolic pathway leading to AA and DHA production. FADS2 gene expression is also regulated through end-product inhibition and dietary LC-PUFAs such as those available in breast milk (25). To the best of our knowledge, FADS2 polymorphisms have not been studied in relation to breast milk or to IQ. As such, we selected two SNPs (rs174575 and rs1535) as candidate biomarkers because they provided the best combination of two desirable factors: (i) they had known linkage disequilibrium (LD) throughout the region, and (ii) they had minor allele frequencies sufficiently prevalent to permit their use in tests of gene–environment interaction. Specifically, using data from CEPH (Utah residents with ancestry from northern and western Europe) HapMap trios (26), we found that these SNPs showed strong LD throughout the promoter and intragenic region of FADS2. In addition, these SNPs exhibited strong LD into the promoter and intragenic region of FADS1, a highly similar gene that borders the 5′ region of FADS2 and that is also involved in fatty acid metabolism, encoding the delta-5 desaturase (27). By genotyping these two tag SNPs (identified with the Gabriel method in Haploview v3.2 on release 20 of CEPH HapMap data), we could obtain maximum information about a candidate locus that potentially moderates breastfeeding effects on IQ [see supporting information (SI) Text and SI Figs. 3–5 for details about candidate gene and marker selection strategy]. We then tested the hypothesis that the cognitive advantage associated with breastfeeding in humans is related to genetic differences in LC-PUFA metabolism, and we replicated this test in two birth cohorts.
Results
Consistent with previous reports, the difference in IQ test scores between breastfed children and those not breastfed was 5.6 and 6.3 IQ points in the Dunedin and E-risk cohorts, respectively. Genotype was not related to IQ in either cohort. (IQ means associated with the three rs174575 genotype groups, CC, CG, and GG, were 101.1, 100.4, and 99.5 in Dunedin and 100.5, 100.7, and 100.3 in E-risk; IQ means associated with the three rs1535 groups, AA, AG, and GG, were 101.2, 100.3, and 100.9 in Dunedin and 101.0, 100.4, and 99.3 in E-risk.)
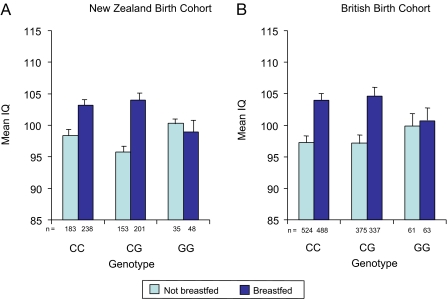
Analyses revealed that rs174575 interacted with breastfeeding to influence IQ in both the Dunedin (P = 0.035) and E-risk (P = 0.018) cohorts (Fig. 1). There was a dominant effect of the C allele in response to breastfeeding. In Dunedin, breastfed children carrying the C allele showed a 6.4-IQ-point advantage relative to children not fed breast milk (t = 6.35, P < 0.001). In contrast, GG homozygotes neither gained an advantage from breastfeeding nor suffered a disadvantage from not being fed breast milk (t = 0.50, P = 0.62) (Fig. 1A). Turning to the E-risk cohort, we found that breastfed children carrying the C allele showed a 7.0-IQ-point advantage relative to children not fed breast milk (t = 7.91, P < 0.001), whereas GG homozygotes neither gained an advantage from breastfeeding nor suffered a disadvantage from not being fed breast milk (t = 0.22, P = 0.83) (Fig. 1B).
Fig. 1.
The association between breastfeeding and IQ is moderated by a genetic polymorphism (rs174575) in the FADS2 gene. In each cohort, we estimated a hierarchical regression model (ordinary least squares) with main effects for genotype (C carriers vs. GG homozygotes) and environment (not breastfed vs. breastfed) followed by a multiplicative genotype × environment interaction term, with covariate adjustment for socioeconomic status. In the Dunedin cohort (A), the effect of breastfeeding was significant (t = 4.67, P < 0.001), the effect of rs174575 was not significant (t = 0.32, P = 0.75), and the interaction term was significant (t = 2.11, P = 0.035). In the E-risk cohort (B), the effect of breastfeeding was significant (t = 3.20, P < 0.001), the effect of rs174575 was not significant (t = 1.82, P = 0.42), and the interaction term was significant (t = 2.37, P = 0.018).
Four points are relevant for interpreting this replicated gene–environment interaction between rs174575 and breastfeeding in predicting IQ. First, it is important to rule out confounding by social class, because socioeconomic advantage is related to children's higher IQ, and in modern countries, socioeconomically advantaged women are more likely to breastfeed (Table 1). To rule out this potential confound, all significance tests reported here for the rs174575-breastfeeding interaction were conducted with covariate adjustment for social class (see SI Table 2). Second, it is important to rule out confounding by maternal IQ (28). We added statistical controls for measures of maternal cognitive ability (Table 1); the rs174575-breastfeeding interaction remained significant in both Dunedin (P = 0.03) and E-risk (P = 0.03) (see SI Table 2). Third, to interpret the interaction, it is necessary to rule out potential genotype effects on exposure to breastfeeding. Child genotype was not related to breastfeeding in either cohort; prevalence rates of breastfeeding associated with the three rs174575 genotype groups (CC, CG, and GG) were 56%, 57%, and 58% in Dunedin [χ2 (2) = 0.10, P = 0.95] and 48%, 47%, and 51% in E-risk [χ2 (2) = 0.30, P = 0.86].
Table 1.
Comparison of children in two birth cohorts, grouped according to genotype (rs174575) and breastfeeding, on tested intelligence (IQ) and covariates
| Samples and measures | rs174575 CC homozygotes |
rs174575 CG heterozygotes |
rs174575 GG homozygotes |
|||
|---|---|---|---|---|---|---|
| Not breastfed | Breastfed | Not breastfed | Breastfed | Not breastfed | Breastfed | |
| New Zealand (Dunedin) birth cohort | n = 183 | n = 238 | n = 153 | n = 201 | n = 35 | n = 48 |
| Children's IQ | 98.4 (15.2) | 103.2 (13.9) | 95.8 (12.4) | 104.0 (13.4) | 100.3 (11.2) | 98.9 (13.8) |
| Socioeconomic status (1 = low; 3 = high)* | 1.9 (0.60) | 2.0 (0.65) | 1.8 (0.55) | 2.1 (0.60) | 1.9 (0.53) | 1.9 (0.58) |
| Maternal cognitive ability† | 97.1 (15.2) | 102.4 (15.2) | 96.0 (12.9) | 103.6 (14.2) | 100.2 (13.4) | 103.3 (14.9) |
| Gestational age,‡ weeks | 40.0 (1.7) | 40.0 (1.5) | 39.7 (2.0) | 40.2 (1.5) | 39.9 (1.7) | 40.2 (1.6) |
| Birthweight,§ g | 3,399 (535) | 3,374 (491) | 3,289 (609) | 3,467 (462) | 3,431 (450) | 3,344 (347) |
| British (E-risk study) birth cohort | n = 524 | n = 488 | n = 375 | n = 337 | n = 61 | n = 63 |
| Children's IQ | 97.3 (14.1) | 104.0 (15.0) | 97.2 (13.9) | 104.6 (15.3) | 99.9 (15.3) | 100.7 (17.3) |
| Socioeconomic status (1 = low; 3 = high)* | 1.7 (0.75) | 2.3 (0.79) | 1.7 (0.76) | 2.3 (0.75) | 1.9 (0.77) | 2.4 (0.68) |
| Maternal cognitive ability† | 95.1 (14.9) | 105.0 (12.9) | 98.5 (12.7) | 105.2 (13.8) | 91.8 (14.5) | 102.6 (14.4) |
| Gestational age,‡ weeks | 36.4 (2.6) | 36.1 (2.9) | 36.4 (2.2) | 36.2 (3.1) | 36.5 (2.8) | 36.0 (3.6) |
| Birthweight,§ g | 2,452 (517) | 2,404 (572) | 2,442 (485) | 2,466 (550) | 2,490 (462) | 2,483 (690) |
Entries in the table are means and standard deviations. IQ scores were standardized to M = 100 and SD = 15 in each cohort.
*In both the Dunedin and E-risk cohorts, genotype was not associated with social class (P = 0.34 and 0.23), breastfeeding was significantly associated with social class (P < 0.001), and there was no difference in the association between breastfeeding and social class by genotype (P = 0.93 and 0.77).
†In the Dunedin cohort, maternal cognitive ability was assessed with the SRA verbal test (56). In the E-risk cohort, mothers were administered the Wide Range Achievement Test (57). Scores were standardized to M = 100 and SD = 15. In both the Dunedin and E-risk cohorts, genotype was not associated with maternal IQ (P = 0.39 and 0.84), breastfeeding was significantly associated with maternal IQ (P < 0.001), and there was no difference in the association between breastfeeding and maternal IQ by genotype (P = 0.85 and 0.34).
‡In both the Dunedin and E-risk cohorts, genotype was not associated with gestational age (P = 0.81 and 0.78), breastfeeding was associated with gestational age in Dunedin (P = 0.04) but not in E-risk (P = 0.11), and there was no difference in the association between breastfeeding and gestational age by genotype (P = 0.13 and 0.99).
§In both the Dunedin and E-risk cohorts, genotype was not associated with birth weight (P = 0.99 and 0.32), breastfeeding was not associated with birth weight (P = 0.13 and 0.61), and there was no difference in the association between breastfeeding and birth weight by genotype (P = 0.27 and 0.39).
Fourth, it is important to rule out potential genotype differences in intrauterine growth. Because small gestational age and lower birth weight have been linked to lower IQ (29, 30), a spurious rs174575-breastfeeding interaction could be produced if GG homozygotes differed from C-carriers in their intrauterine growth. However, there were no significant gestational age or birth weight differences between the genotype groups, in either cohort (Table 1).
We repeated all analyses using rs1535. We observed a significant interaction in the E-risk cohort (P = 0.01). Breastfed A-carriers had higher IQs than nonbreastfed A-carriers, whereas this advantage was not as pronounced among GG homozygotes (breastfed children with AA, AG, and GG genotypes had IQs of 104.6, 104.6, and 100.0, whereas nonbreasfted children had IQs of 97.7, 96.8, and 98.6). This interaction did not replicate in the Dunedin cohort (P = 0.55); breastfed children with AA, AG, and GG genotypes had IQs of 102.7, 103.6, and 102.7, respectively, whereas nonbreastfed children had IQs of 99.1, 96.0, and 98.4.
The interaction between children's rs174575 genotype and breastfeeding, replicated across cohorts, suggests that C-carrying children benefit from breast milk more than do GG homozygotes. An alternative hypothesis is that there are variations in human-milk composition that are related to maternal rs174575 genotype. If our findings reflected such a maternal genotype effect, we should find that, among breastfeeding mothers, rs174575 C-carrying mothers have children with higher IQs than GG mothers. To test this possibility, we collected DNA from the mothers of the E-risk children. (We were not able to collect DNA from the mothers of the Dunedin cohort.) There were no significant IQ differences among children fed breast milk as a function of maternal genotype (breast-milk-fed children of CC, CG, and GG mothers had IQs of 104.6, 103.4, and 103.9; P = 0.93). These results suggest that the rs174575 moderation of breastfeeding effects on IQ involves genetic differences in children's LC-PUFA metabolism rather than rs174575 differences among lactating women in their milk composition (see SI Table 3).
Discussion
These results suggest that genetic variation in fatty acid metabolism moderates breastfeeding effects on children's cognitive development. We confirmed the gene–environment interaction in two independent, well characterized birth cohorts who were assessed with the best available intelligence tests, and the combined interaction effect across the two studies yielded a P value of 0.005. Among GG homozygote children, the IQ advantage associated with breastfeeding was nil (−0.1 IQ points). In contrast, among children who were C-carriers, the IQ advantage was 6.8 IQ points (or 0.48 standard deviation units in the general population). This advantage corresponds to a moderate effect size (31) that is associated with many important life outcomes (32). Moreover, we were able to rule out potential confounding of the gene–environment interaction due to gene–exposure correlation, intrauterine growth differences, social class differences, and maternal cognitive ability. This study is an initial step in the process of constructive replication (33); further investigation to replicate and explain this specific gene–environment interaction is warranted.
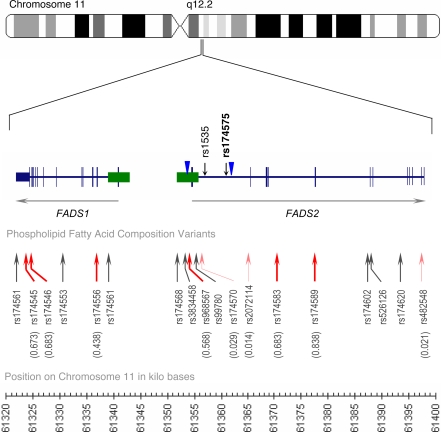
The genetic moderation of breastfeeding effects on IQ is unlikely to be directly caused by the analyzed SNP, and the molecular mechanism by which rs174575 may influence cognitive development is not known. However, rs174575 demonstrates LD with several SNPs in the FADS1 FADS2 gene cluster that have been shown to track fatty acid composition of serum phospholipids in humans (27) (Fig. 2); the rs174575 C allele is linked with the major alleles of these SNPs, which are associated with more efficient fatty acid processing, possibly due to increased transcriptional activity or to a more active protein. It may be that rs174575 influences the biosynthesis of n-6 and n-3 series LC-PUFAs from their dietary precursors. Because n-6 and n-3 fatty acids compete for the same desaturase metabolic enzymes at multiple steps, this process may operate differently in breastfed versus nonbreastfed children, whose n-6:n-3 precursor ratio is different (34). Genetic variants may also condition the feedback regulation of polyunsaturated fatty acids. The availability of dietary DHA (as in breast milk) alters gene expression in n-6 and n-3 pathways as well as expression of genes involved in synaptic plasticity (35, 36), and the genomic structure surrounding rs174575 (Fig. 2) contains sterol regulatory element transcription factor binding sites through which polyunsaturated fatty acids modify gene expression (24). In addition, through these various mechanisms, several classes of eicosanoids may also be affected and influence brain development (37).
Fig. 2.
The FADS1 and FADS2 gene structure on chromosome 11q12.2. The FADS1 and FAD2 genes are arranged in a head-to-head orientation. The figure shows the position of rs174575 in relation to the hypothetical promoter regions (green rectangles), hypothetical transcription factor binding site SRE (blue arrowheads) (54), and 18 SNPs that have been related to individual differences in fatty acid composition among humans (27). Of these 18 SNPs, 9 (shown in red) are validated in publicly available data via HapMap. r2 between rs174575 and each of the nine markers found in HapMAP CEU (26) is noted in parentheses. Further suggesting common regulatory mechanisms for these two genes, publicly available gene expression data on lymphoblastoid cell lines from 57 unrelated CEPH individuals (GEO accession no. GDS2106) show that FADS1 gene expression correlates 0.78 with FADS2 gene expression. Similarly, in mouse BXD strains, FADS1 and FADS2 gene expression correlate 0.61 (55).
Our finding has implications for neuroscience and early child development. Human milk is widely promoted as good for the brain, and DHA and AA may be needed for optimal intellectual development (23). However, although evidence for this process is mounting, it has not yet been proven (12). The replicated finding here that breastfeeding effects on IQ depend on genetic variation in fatty acid metabolism supports a likely neurobiological pathway uniting the gene, environmental exposure, and phenotype and strengthens the need for nutrigenomic studies to trace it in the laboratory (38). Randomized controlled trials comparing the neurodevelopment of infants fed DHA-supplemented versus unsupplemented formula have yielded inconsistent results (15). Our finding suggests an intriguing explanation. Unobserved genetic heterogeneity in fatty acid metabolism—and in response to other supplemented nutrients—may dilute supplementation effects. If genetically responsive subgroups can be identified for analysis, modest benefits may be revealed as stronger than previously thought for some children.
The finding also has implications for behavioral genomics. It is reasonable to ask whether FADS2 is a “gene for” IQ. There was no overall main effect of genotype on IQ. However, breastfed rs174575 C-carriers scored 4.1 IQ points higher than GG homozygotes (104.0 vs. 99.9, P = 0.02). This finding suggests that under human ancestral conditions, when all infants were breastfed, genetic variation in FADS2 could have influenced individual differences in intelligence. The interaction also offers a clue about why the FADS2 locus has not appeared in the first genome-wide scans for intelligence (39). Genome-wide scans aim to uncover genes having direct main effects (i.e., genes that show associations with phenotypes regardless of participants' environments). Such scans are inefficient for detecting genes whose effects are conditional on environmental exposures. In contemporary samples of which a nontrivial proportion of participants are not fed breast milk, any link between variation in FADS2 and IQ would be concealed. The link between genetic variation in fatty acid metabolism and IQ can be revealed only against a specific environmental background (that was universal before formula feeding). This hypothesis suggests that biological interrogation of FADS2 in relation to intelligence can be pursued further in mammalian species. Comparative genomics shows that rs174575 is in a sequence conserved between human (hg17), chimpanzee (panTro1), dog (canFAm1), rat (rn3), and mouse (mm5) having regulatory potential (40), and there are nonhuman animal models for dietary deprivation/supplementation (41) and for the study of intelligence (42).
Finally, the finding has implications for the public understanding of genetics. The pendulum of opinion surrounding nature versus nurture has swung back and forth, yielding global estimates of heritability versus “environmentality” that have overlooked the contribution of interactions between specific genes and specific experiences (6). To date, research on gene–environment interactions has been dominated by the search for genetic variants that increase disease susceptibility to environmental pathogens (43); for example, carriers of “short” 5-HTT alleles who encounter stressful life events are at risk of becoming depressed (44); carriers of “rapid” NAT2 alleles who eat red meat are at risk of developing colorectal cancer (45). However, genes are not only implicated in disease; here we have shown that a genetic variant (in FADS2) may also enhance a favorable response (increased IQ) to a salubrious exposure present throughout human ancestry (breastfeeding).
Materials and Methods
Study 1.
Participants in the first cohort were members of the Dunedin Multidisciplinary Health and Development Study, which tracks the health and behavior of a birth cohort of 1,037 children. This sample was constituted at age 3, when the investigators enrolled 91% of consecutive births between April 1972 and March 1973 in Dunedin, New Zealand. Cohort families represent the full range of socioeconomic status in the general population of New Zealand's South Island. Details about the sample are reported elsewhere (46). The sample has been followed to age 32 with 96% retention.
Breastfeeding was assessed in interviews with mothers when the children were 3 years old; 57% of the cohort children were breastfed, consistent with breastfeeding rates in New Zealand in the early 1970s (47). Those infants not breastfed generally received formula feeding prepared from dried cow's milk powder.
IQ was measured at ages 7, 9, 11, and 13 years with the Wechsler Intelligence Scale for Children–Revised (48). IQ scores from the four assessments were combined to form an overall score. The children's IQ scores ranged from 55 to 147 and were normally distributed.
DNA was obtained from 97% of study members as adults. To avoid potential problems of population stratification, DNA from cohort members of Maori origin was excluded from our analyses.
Study 2.
Participants in the second cohort were members of the Environmental Risk (E-risk) Longitudinal Twin Study, which tracks the development of a sample of 2,232 children. This E-risk sample was drawn from a 1994–1995 birth register of twins born in England and Wales (49). The E-risk sample was constructed in 1999–2000, when 1,116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments, forming the base cohort for the longitudinal E-risk study. Details about the sample are reported elsewhere (50). Because each study family contains two children, all statistical analyses reported here were corrected conservatively for the nonindependence of the twin observations by using tests based on the sandwich or Huber/White variance estimator (51).
Breastfeeding was assessed by a postal questionnaire completed by mothers when the children were 2 years old; 48% of the cohort children were breastfed. Infants not breastfed received formula feeding (before LC-PUFA supplementation became widely available in the U.K.).
IQ was measured at age 5 by using a short form of the Wechsler Preschool and Primary Scale of Intelligence–Revised (52) comprising Vocabulary and Block Design subtests, and by following procedures described by Sattler (ref. 53, table H-6). The children's IQs ranged from 52 to 145 and were normally distributed.
DNA was obtained via buccal swabs from 2,140 children (96% of the sample) and their mothers. Non-Caucasian participants (9%) were excluded from our analyses.
SNP genotyping protocols are summarized in SI Table 4. In both cohorts, genotyping was performed blind to measures of breastfeeding exposure and IQ phenotype.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (United States), the Medical Research Council (United Kingdom), and the Health Research Council (New Zealand). A.C. and T.E.M. are Royal Society–Wolfson Research Merit Award holders.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704292104/DC1.
References
- 1.Gould SJ. The Mismeasure of Man. New York: Norton; 1981. [Google Scholar]
- 2.Herrnstein RJ, Murray C. The Bell Curve. New York: Free Press; 1994. [Google Scholar]
- 3.Gray JR, Thompson PM. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard TJ, McGue M. Science. 1981;212:1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- 5.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioural Genetics. New York: Freeman; 2000. [Google Scholar]
- 6.Ridley M. Nature via Nurture. New York: Harper Collins; 2003. [Google Scholar]
- 7.Caspi A, Moffitt TE. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. Pediatrics. 2005;115:496–506. [Google Scholar]
- 9.Moffitt TE, Caspi A, Rutter M. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Infant Formula: Evaluating the Safety of New Ingredients. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 11.Sauerwald TU, Demmelmair H, Koletzko B. Lipids. 2001;36:991–996. doi: 10.1007/s11745-001-0810-9. [DOI] [PubMed] [Google Scholar]
- 12.Heird WC, Lapillonne A. Annu Rev Nutr. 2005;25:549–571. doi: 10.1146/annurev.nutr.24.012003.132254. [DOI] [PubMed] [Google Scholar]
- 13.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- 14.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- 15.McCann JC, Ames BN. Am J Clin Nutr. 2005;82:281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JW, Johnstone BM, Remley DT. Am J Clin Nutr. 1999;70:525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen EL, Michaelson KF, Sanders SA, Reinisch JM. J Am Med Assoc. 2002;287:2365–2371. doi: 10.1001/jama.287.18.2365. [DOI] [PubMed] [Google Scholar]
- 18.Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Behav Neurosci. 2002;116:1022–1031. doi: 10.1037//0735-7044.116.6.1022. [DOI] [PubMed] [Google Scholar]
- 19.Carrie I, Guesnet P, Bourre JM, Frances H. Br J Nutr. 2000;83:439–447. [PubMed] [Google Scholar]
- 20.Champoux M, Hibbeln JR, Shannon C, Majchrzak S, Soumi SJ, Salem N, Jr, Higley JD. Pediatr Res. 2002;51:273–281. doi: 10.1203/00006450-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi T, Fukumoto Y, Harada E. Behav Brain Res. 2002;131:193–203. doi: 10.1016/s0166-4328(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 22.Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. J Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- 23.Marszalek JR, Lodish HF. Ann Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 24.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura MT, Nara TY. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 26.The International HapMap Consortium. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Hienrich J. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 28.Der G, Batty GD, Deary IJ. Br Med J. 2006;333:945–948. doi: 10.1136/bmj.38978.699583.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matte TD, Bresnahan M, Begg MD, Susser E. Br Med J. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawlor DA, Clark H, Davey Smith G, Leon DA. Pediatrics. 2006;117:e894–e902. doi: 10.1542/peds.2005-2412. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 32.Gottfredson LS. Intelligence. 1997;24:79–132. [Google Scholar]
- 33.Lykken DT. Psych Bull. 1968;70:151–159. doi: 10.1037/h0026141. [DOI] [PubMed] [Google Scholar]
- 34.Yehuda S. World Rev Nutr Diet. 2003;92:37–56. doi: 10.1159/000073791. [DOI] [PubMed] [Google Scholar]
- 35.Rao JS, Ertley RN, DeMar JJC, Rapoport SI, Bazinet RP, Lee H-J. Mol Psychiatry. 2006;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 36.Kitajka K, Puskás LG, Zvara A, Kackler JL, Barceló-Coblijn G, Yeo YK, Tibor F. Proc Natl Acad Sci USA. 2002;99:2619–2624. doi: 10.1073/pnas.042698699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uauy R, Hoffman DR, Peirano R, Birch DG, Birch EE. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- 38.Wainwright PE. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 39.Posthuma D, Luciano M, Geus EJ, Wright MJ, Slagboom PE, Montgomery G, Boomsma DI, Martin NG. Am J Hum Genet. 2005;36:65–76. doi: 10.1086/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, Roskin KM, Schwartz M, Sugnet CW, Thomas DJ, et al. Nucleic Acids Res. 2003;31:51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedorova I, Salem N., Jr Prostaglandins Leukot Essen Fatty Acids. 2006;75:271–289. doi: 10.1016/j.plefa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Plomin R. Nat Rev Neurosci. 2001;2:136–141. doi: 10.1038/35053584. [DOI] [PubMed] [Google Scholar]
- 43.Hunter DJ. Nat Rev Genet. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 44.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, McClay J, Mill J, Martin J, Brathwaite A, et al. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, Hennekents CH, Kelsey KT, Hunter DJ. Cancer Res. 1998;58:3307–3311. [PubMed] [Google Scholar]
- 46.Moffitt TE, Caspi A, Rutter M, Silva P. Sex Differences in Antisocial Behaviour: Conduct Disorder, Delinquency, and Violence in the Dunedin Longitudinal Study. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 47.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JA, Herbison GP, Poulton R. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Manual for the Wechsler Intelligence Scale for Children–Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 49.Trouton A, Spinath F, Plomin R. Twin Res. 2002;5:444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 50.E-Risk Study Team. Moffitt TE. J Child Psychol Psychiatry. 2002;43:201–235. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 51.Statacorp. Stata Statistical software, Release 8.2. College Station, TX: Stata Corporation; 2005. [Google Scholar]
- 52.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence–Revised. London: Psychological Corporation; 1990. [Google Scholar]
- 53.Sattler JM. Assessment of Children: WISC-III and WPPSI-R Supplement. San Diego: Jerome M. Sattler; 1992. [Google Scholar]
- 54.Nara TY, He WS, Tang C, Clarke SD, Nakamura MT. Biochem Biophys Res Commun. 2002;296:111–117. doi: 10.1016/s0006-291x(02)00851-3. [DOI] [PubMed] [Google Scholar]
- 55.Gatti D, Maki A, Chesler EJ, Kosyk O, Kirova R, Lu L, Manly KF, Qu Y, Williams RW, Perkins A, et al. Hepatology. 2007;46:548–557. doi: 10.1002/hep.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thurstone TG, Thurstone LL. The SRA Verbal Form. Chicago: Science Research Associates; 1973. [Google Scholar]
- 57.Wilkinson GS. The Wide Range Achievement Test. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.