Abstract
Hydrogen gas has tremendous potential as an environmentally acceptable energy carrier for vehicles, but most hydrogen is generated from nonrenewable fossil fuels such as natural gas. Here, we show that efficient and sustainable hydrogen production is possible from any type of biodegradable organic matter by electrohydrogenesis. In this process, protons and electrons released by exoelectrogenic bacteria in specially designed reactors (based on modifying microbial fuel cells) are catalyzed to form hydrogen gas through the addition of a small voltage to the circuit. By improving the materials and reactor architecture, hydrogen gas was produced at yields of 2.01–3.95 mol/mol (50–99% of the theoretical maximum) at applied voltages of 0.2 to 0.8 V using acetic acid, a typical dead-end product of glucose or cellulose fermentation. At an applied voltage of 0.6 V, the overall energy efficiency of the process was 288% based solely on electricity applied, and 82% when the heat of combustion of acetic acid was included in the energy balance, at a gas production rate of 1.1 m3 of H2 per cubic meter of reactor per day. Direct high-yield hydrogen gas production was further demonstrated by using glucose, several volatile acids (acetic, butyric, lactic, propionic, and valeric), and cellulose at maximum stoichiometric yields of 54–91% and overall energy efficiencies of 64–82%. This electrohydrogenic process thus provides a highly efficient route for producing hydrogen gas from renewable and carbon-neutral biomass resources.
Keywords: biofilms, cellulose, electron transport, hydrogen, microbial fuel cells
Hydrogen gas produced by bacterial fermentation of glucose is limited to yields of 4 mol/mol, and typically only 2–3 mol/mol are recovered compared with a stoichiometric potential of 12 mol/mol (1, 2). Processes for hydrogen production from glucose and cellulose hydrolysis and fermentation are relatively well understood and feasible (3). However, further conversion of the remaining residual organic acids, such as acetic acid, cannot be achieved by bacteria without additional energy input. Fermentation processes for hydrogen production are therefore limited to using only sugars, and even with this substrate they are limited to a maximum yield of 33% (1, 2).
Microbial fuel cells (MFCs) provide a direct method of obtaining bioelectricity from cellulose and other biodegradable organic matter by a process called electrogenesis (4–6). Exoelectrogenic bacteria transfer electrons obtained from the oxidation of organic matter outside the cell to the MFC anode while releasing protons into solution (7). Electrons, protons, and oxygen react at the cathode, producing water. Anode potentials can approach the maximum possible based on the free energy of the substrate, which for acetic acid under neutral pH conditions is approximately −0.3 V (versus a standard hydrogen electrode).
Hydrogen gas can be produced with the same exoelectrogenic bacteria by modifying the MFC by adding a small voltage to that produced by the bacteria and omitting oxygen from the cathode (8, 9). Based on a thermodynamic analysis, the addition of >0.11 V to that generated by bacteria (−0.3 V) will generate hydrogen gas at the cathode, but voltages of >0.2 V are needed because of electrode overpotentials. This hydrogen evolution process, referred to as electrohydrogenesis, provides a route for extending biohydrogen production past the endothermic barrier imposed by the microbial formation of fermentation dead-end products, such as acetic acid. However, overall process efficiencies and hydrogen production rates have previously been low.
To substantially increase hydrogen generation rates and efficiencies, we developed a compact reactor system using chemically modified three-dimensional graphite granule anode and an anion exchange membrane. Graphite granules were treated by a high-temperature ammonia gas process to increase bacterial adhesion and overall power generation (10). Hydrogen fuel cells, and most other reactors previously examined, use a cation exchange membrane (e.g., Nafion) to keep reactor chambers separated. The membrane reduces the diffusion of hydrogen evolved at the cathode into the anode solution where it can be lost to bacterial oxidation. Cation exchange membranes preferentially transfer cations present at high concentration in solution rather than protons (e.g., Na+ and K+) (11, 12), resulting in an elevated pH at the cathode that limits the hydrogen evolution reaction and a reduced pH at the anode that limits bacterial growth. Low pH cannot be used at the anode because exoelectrogenic bacteria require near-neutral pH conditions, resulting in low proton concentrations (i.e., 10−7 M) which can limit the overall reaction rate. By using an anion exchange membrane, proton conduction was enhanced by protons being carried by negatively charged phosphate anions through the membrane.
Results and Discussion
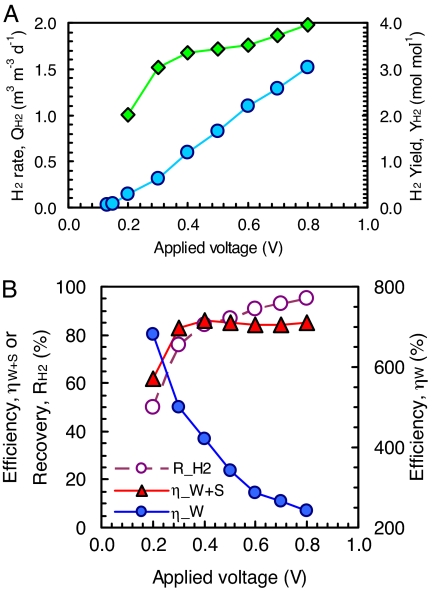
Hydrogen gas production from acetic acid, the predominant volatile acid produced from glucose or cellulose fermentation, was possible at applied voltages of >0.13 V, with the production rate increasing from 0.03 to 1.5 m3·d−1·m−3 H2 (total reactor volume; up to 10.5 m3·d−1·m−3 H2 based only on the anode liquid volume) at applied voltages of 0.2–0.8 V (Fig. 1). Increased production rates reduced the time needed for a complete batch cycle from 30 to 3 h. A reduction in cycle time accounts in part for the increased yields from 3.03 to 3.95 mol/mol H2 at applied voltages of 0.3–0.8 V. At 0.2 V, the yield decreased to 2.01 mol/mol, suggesting that hydrogen losses through the membrane and tubing became appreciable relative to low H2 production rates. The gas was nearly pure hydrogen (>99.5%) with only trace amounts of CO2 and CH4 in all experiments.
Fig. 1.
Hydrogen production rate (circles) and hydrogen yield (diamonds) (A) and energy efficiencies (filled symbols) and hydrogen recovery (open symbols) (B) from acetic acid.
The energy efficiency ranged from ηW = 681–243% when evaluated in terms of only the voltage addition (0.2–0.8 V) as a result of energy contributed by bacterial oxidation of the acetate. In comparison, hydrogen produced by water electrolysis can never exceed 100%, and it typically has efficiencies of 50–70%. The external power needed for electrohydrogenesis could be obtained in practice by using MFCs, a hydrogen fuel cell, or other renewable energy methods, such as wind and solar energy. Using gas produced by the process in a hydrogen fuel cell with an assumed energy conversion efficiency of 50% would lower overall energy efficiency at an applied voltage of 0.6 V from 288% to ηW = 144%. When evaluated on the basis of both the voltage added and the heat of combustion of the acetate added, the overall efficiencies ranged from ηW+S = 62–86% (Fig. 1B).
The electrohydrogenic process produced hydrogen at high yields from a variety of substrates (Table 1). Hydrogen was produced directly from cellulose particles with an overall hydrogen recovery of 68% and an energy recovery of 63%. The production rate of 0.11 m3·d−1·m−3 from cellulose was less than that of acetate when the two substrates were added at the same concentration (1 g/liter) likely because of the slow hydrolysis rate of the cellulose particles (13). Hydrogen production rates could be improved by increasing the rate that volatile acids are produced from cellulose through further enrichment of cellulolytic microorganisms to obtain cultures with higher intrinsic hydrolysis rates. Alternatively, adding a higher cellulose concentration would increase the overall production of volatile acids per volume of reactor and thus better match volatile acid production from cellulose with volatile acid consumption for electrohydrogenesis.
Table 1.
Hydrogen production using cellulose, glucose, or five different volatile acids at an applied voltage of 0.6 V
| Substrate | YH2, mol of H2/mol of substrate | RH2, % | Production rate, m3·d−1·m−3 | η, % | ηW+S, % |
|---|---|---|---|---|---|
| Glucose | 8.55 | 71 | 1.23 | 266 | 64 |
| Cellulose | 8.20* | 68 | 0.11 | 268 | 63 |
| Acetic acid | 3.65 | 91 | 1.10 | 261 | 82 |
| Butyric acid | 8.01 | 80 | 0.45 | 285 | 77 |
| Lactic acid | 5.45 | 91 | 1.04 | 283 | 82 |
| Propionic acid | 6.25 | 89 | 0.72 | 248 | 79 |
| Valeric acid | 8.77 | 67 | 0.14 | 263 | 66 |
*Calculated per mole of hexose equivalent.
Glucose was converted to hydrogen gas at a rate (1.23 m3·d−1·m−3) similar to that of acetate but at a lower overall recovery of 71% (8.55 mol/mol) (Table 1). This recovery is 4- to 5-times larger, however, than typically achieved through cellulose fermentation (14). The predominant acids produced by glucose fermentation include acetic, butyric, lactic, propionic, and valeric, all of which were successfully used to generate hydrogen gas in the electrohydrogenic process at energy recoveries of 66–82% (Table 1). Hydrogen production rates for lactic acid (1.04 m3·d−1·m−3) were similar to those obtained with acetic acid, whereas the lowest rate was measured using valeric acid (0.14 m3·d−1·m−3). Stoichiometric conversion efficiencies of these four volatile acids ranged from 67–90% (Table 1). The rate of mass transfer of acetic acid through the anion exchange membrane used here is low (12), suggesting that diffusive losses of volatile acids from the anode chamber through the membrane were minimal. These same volatile acids also are produced by fermentation of different types of organic matter; thus, the process developed here could be used with virtually any organic matter source as a method of high energy yield and high production rates of hydrogen.
Considerable attention is being focused on ethanol production from renewable biomass for use as a transportation fuel (15). However, the production of cellulose-derived ethanol presents substantial technical challenges because the cellulose must first be hydrolyzed and released as sugar. Ethanol production also is not practical from organic matter other than simple sugars. Moreover, fermentation-produced ethanol can only be separated from water by using highly energy demanding processes, and ethanol must be used in combustion engines that have low energy efficiencies compared with hydrogen oxidation in chemical fuel cells. Hydrogen production using electrohydrogenic reactors represents an immediate method for renewable energy production in the form of hydrogen gas for transportation. There are substantial infrastructure issues to be addressed in using hydrogen gas, but the environmental and energy-conversion efficiency benefits for hydrogen as a transportation fuel makes it worth addressing and solving these issues (16). Even if a hydrogen-based transportation system is never developed, sustainable hydrogen production from cellulose and fermentation end-products still is valuable as a sustainable method of hydrogen generation. One immediate application is for fertilizer production. Local production of fertilizers from cellulose-derived hydrogen gas could greatly reduce transportation costs for fertilizers and improve global agricultural yields and economics.
Materials and Methods
Reactor and Operation.
The reactor was constructed by clamping an anion exchange membrane (AMI-7001; Membrane International, Glen Rock, NJ) between the anode (30 mm in diameter, 20 mm long; 14 ml) and cathode (40 mm long; 28 ml) chambers (12). The anode chamber was filled with graphite granules that were 2–6 mm in diameter (El Carb 100; Graphite Sales, Birmingham, AL) at a specific surface area of As = 1,320 m2/m3, calculated as As = 6θ/d, where d = 4 mm is the average particle diameter and θ = 53% is the bed porosity. The granules were pretreated with a high-temperature ammonia gas process that increases current densities and reduces reactor acclimation times (10). A graphite rod (6.15 mm in diameter; Alfa Aesar, Ward Hill, MA) was inserted into the bed of granules, reducing the liquid volume to 6 ml. The cathode (1 cm2), made of carbon cloth and a Pt catalyst [0.5 mg/cm2 Pt; prepared as previously described (17)], was placed in the cathode chamber close to the membrane and connected to the external circuit by a titanium wire (0.68 mm in diameter; Alfa Aesar). Hydrogen gas was collected by gluing the open bottom of a glass tube (80 mm long by 16.8 mm in diameter; empty bed volume of 18 ml) containing a crimp top with a thick rubber stopper to a hole cut into the top of the cathode chamber.
Bacteria derived from a soil (cellulose-fed reactor) or wastewater (all other substrates) were inoculated and enriched on a specific substrate by using a phosphate buffer (50 mM) and nutrient medium (18) for >3 months in MFCs. A two-chamber, ferricyanide catholyte MFC was used with cellulose to minimize oxygen contamination (19). Single-chamber, air-cathode, cube-type MFCs were used for glucose, acetic, and lactic acids (4-cm electrode spacing; carbon cloth electrodes) (18), and bottle-type MFCs were used for propionic and valeric acids (graphite brush anodes) (20). Repeatable and stable cycles of power using a 1,000-Ω external resistor were obtained with each substrate, with maximum power densities of 430–450 mW/m2 for MFCs using glucose, acetic, and lactic acids; 290 mW/m2 for propionic acid; 260 mW/m2 for butyric acid; 154 mW/m2 for valeric acid; and 90 mW/m2 for cellulose. Polarization data were not obtained, however, and the use of different MFC architectures does not allow direct comparison of these different power densities (21).
The bacteria from the anode surface of an MFC were transferred into the anode chamber of the electrohydrogenic reactors. The anode chamber was filled with medium, 50 mM phosphate buffer, and one of the substrates (1 g/liter chemical oxygen demand), and the cathode chamber contained only the phosphate buffer solution. Both chambers were sparged with ultra-pure nitrogen gas (99.998%) for 30 min before the voltage was applied to the reactor. The reactor solutions were replaced when the current decreased to <50 μA. A negative voltage was applied to the circuit by connecting the working pole of a multichannel potentiostat (WMPG100; WonATech, GyeongGi-Do, Korea) to the cathode and the counter and reference poles to the anode or by connecting the positive pole of the power supply (3645A; Array Electric Co.) to the cathode and the negative pole to the anode. After five feeding cycles, the PBS concentration was increased to 200 mM. Experiments were conducted in duplicate in a constant-temperature room (30°C).
Chemicals.
Lactic acid (87.4%) and all other chemicals were obtained from Mallinckrodt (Hazelwood, MO) except that sodium acetate anhydrous (99.2%; Sigmacell Cellulose type 20) was obtained from Sigma (St. Louis, MO); glucose anhydrous was obtained from Fisher Scientific (Hanover Park, IL); and propionic (99.5%) and valeric acid (98%) were obtained from Chem Service (West Chester, PA). All solutions were prepared in deionized water.
Analytics.
Volumetric gas production in the cathode chamber was measured with an anaerobic respirometer system (AER-208; Challenge Environmental Systems, Fayetteville, AR) with the composition quantified by using a gas chromatograph (model 8610B; SRI Instruments, Torrence, CA) equipped with a thermal conductivity detector and a molecular sieve column (Carboxen 1000, 60/80 mesh, 15′ × 1/8″ SS; Supelco, Bellefonte, PA) with argon as the carrier gas. The chemical oxygen demand was measured according to standard methods (22).
Calculations.
Overall hydrogen recovery was calculated as RH2 = CERCat. The Coulombic efficiency is CE = (nCE/nth), where nth is the moles of hydrogen that could be theoretically produced and nCE = CP/(2F) is the moles of hydrogen that could be produced from the measured current, CP is the total Coulombs calculated by integrating the current over time, F is Faraday's constant, and 2 is the moles of electrons per mole of hydrogen. The cathodic hydrogen recovery was calculated as RCat = nH2/nCE, where nH2 is the total moles of hydrogen produced. Hydrogen yield (YH2) was calculated as YH2 = nH2 /ns, where ns is substrate removal calculated on the basis of chemical oxygen demand (22).
Energy recovery was calculated on the basis of electricity input as ηW = WH2/Win, where WH2 is the heat of combustion of the hydrogen produced (upper heating value of 285.83 kJ per mole of H2) and Win is the electricity input determined as Win = CPEap, where Eap is the applied voltage corrected for the power loss across a resistor used to measure current. The overall energy recovery is ηW+S = WH2/(WS + Win), where WS is the heat of combustion of the substrate (acetate; 870.28 kJ/mol). The hydrogen production rate, QH2 (measured as m3·d−1·m−3), was based on the measured daily hydrogen production normalized to the reactor volume (total reactor volume, except as indicated).
Acknowledgments
This work was supported by Air Products and Chemicals, Inc., and National Science Foundation Grant BES-0401885.
Abbreviation
- MFC
microbial fuel cell.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Logan BE. Environ Sci Technol. 2004;38:160A–167A. doi: 10.1021/es040468s. [DOI] [PubMed] [Google Scholar]
- 2.Niessen J, Schröder U, Harnish F, Scholz F. Lett Appl Microbiol. 2005;41:286–290. doi: 10.1111/j.1472-765X.2005.01742.x. [DOI] [PubMed] [Google Scholar]
- 3.Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL. Int J Hydrogen Energy. 2007;32:172–184. [Google Scholar]
- 4.Logan BE, Regan JM. Trends Microbiol. 2006;14:512–518. doi: 10.1016/j.tim.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Schubert C. Nature. 2006;441:277–279. doi: 10.1038/441277a. [DOI] [PubMed] [Google Scholar]
- 6.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, et al. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang IS, Moon H, Bretschger O, Jang JK, Park HI, Nealson KH, Kim BH. J Microbiol Biotechnol. 2007;16:163–177. [Google Scholar]
- 8.Liu H, Grot S, Logan BE. Environ Sci Technol. 2005;39:4317–4320. doi: 10.1021/es050244p. [DOI] [PubMed] [Google Scholar]
- 9.Rozendal RA, Hamelers HVM, Euverink GJW, Metz SJ, Buisman CJN. Int J Hydrogen Energy. 2006;31:1632–1640. [Google Scholar]
- 10.Cheng S, Logan BE. Electrochem Commun. 2007;9:492–496. [Google Scholar]
- 11.Rozendal RA, Hamelers HVV, Buisman CJN. Environ Sci Technol. 2006;40:5206–5211. doi: 10.1021/es060387r. [DOI] [PubMed] [Google Scholar]
- 12.Kim JR, Cheng S, Oh S-E, Logan BE. Environ Sci Technol. 2007;41:1004–1009. doi: 10.1021/es062202m. [DOI] [PubMed] [Google Scholar]
- 13.Rezaei F, Richard TL, Brennan R, Logan BE. Environ Sci Technol. 2007;41:4053–4058. doi: 10.1021/es070426e. [DOI] [PubMed] [Google Scholar]
- 14.Ren Z, Ward T, Logan BE, Regan JM. J Appl Microbiol. 2007 doi: 10.1111/j.1365–2672.2007.03477.X. [DOI] [PubMed] [Google Scholar]
- 15.Ragauskas AJ, Williams DK, Davison BH, Britovsek F, Cairney J, Eckert CA, Frederick WJJ, Hallett JP, Leak DJ, Liotta CL, et al. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 16.Schlapbach L, Züttel A. Nature. 2001;414:353–358. doi: 10.1038/35104634. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Liu H, Logan BE. Environ Sci Technol. 2006;40:364–369. [PubMed] [Google Scholar]
- 18.Liu H, Cheng S, Logan BE. Environ Sci Technol. 2005;39:658–662. doi: 10.1021/es048927c. [DOI] [PubMed] [Google Scholar]
- 19.Oh S, Logan BE. Appl Microbiol Biotechnol. 2006;70:162–169. doi: 10.1007/s00253-005-0066-y. [DOI] [PubMed] [Google Scholar]
- 20.Logan BE, Cheng S, Watson V, Estadt G. Environ Sci Technol. 2007;41:3341–3346. doi: 10.1021/es062644y. [DOI] [PubMed] [Google Scholar]
- 21.Logan BE, Aelterman P, Hamelers B, Rozendal R, Schröder U, Keller J, Freguiac S, Verstraete W, Rabaey K. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 22.Clesceri LS, Greenberg AE, Eaton AD, editors. American Public Health Association. Standard Methods for the Examination of Water and Wastewater. 20th Ed. Washington DC: Am Public Health Assoc; 1998. [Google Scholar]