Abstract
Chemistry of the actinide elements represents a challenging yet vital scientific frontier. Development of actinide chemistry requires fundamental understanding of the relative roles of actinide valence-region orbitals and the nature of their chemical bonding. We report here an experimental and theoretical investigation of the uranium methylidyne molecules X3U CH (X = F, Cl, Br), F2ClU
CH (X = F, Cl, Br), F2ClU CH, and F3U
CH, and F3U CF formed through reactions of laser-ablated uranium atoms and trihalomethanes or carbon tetrafluoride in excess argon. By using matrix infrared spectroscopy and relativistic quantum chemistry calculations, we have shown that these actinide complexes possess relatively strong U
CF formed through reactions of laser-ablated uranium atoms and trihalomethanes or carbon tetrafluoride in excess argon. By using matrix infrared spectroscopy and relativistic quantum chemistry calculations, we have shown that these actinide complexes possess relatively strong U C triple bonds between the U 6d-5f hybrid orbitals and carbon 2s-2p orbitals. Electron-withdrawing ligands are critical in stabilizing the U(VI) oxidation state and sustaining the formation of uranium multiple bonds. These unique U
C triple bonds between the U 6d-5f hybrid orbitals and carbon 2s-2p orbitals. Electron-withdrawing ligands are critical in stabilizing the U(VI) oxidation state and sustaining the formation of uranium multiple bonds. These unique U C-bearing molecules are examples of the long-sought actinide-alkylidynes. This discovery opens the door to the rational synthesis of triple-bonded actinide
C-bearing molecules are examples of the long-sought actinide-alkylidynes. This discovery opens the door to the rational synthesis of triple-bonded actinide carbon compounds.
carbon compounds.
Keywords: actinide multiple bond, heavy element, laser ablation, matrix isolation, relativistic quantum chemistry
Chemical bonding and bond order are among the most important fundamental concepts in modern chemistry since the birth of the valence theory of Lewis (1). Main-group and transition-metal compounds with multiple chemical bonds have always been fascinating to chemists because of their pivotal role in organic, inorganic, and organometallic chemistry, characteristic chemical and physical properties, and versatile applications in biological and material science (2–6). Whereas numerous organic and inorganic compounds with multiple bonds are known (7–17), f elements (lanthanides and actinides) with multiple bonds are relatively rare, except for early actinides. Such bonding has aroused great interest recently in the search for actinide complexes with multiple bonds between two actinide metals (18–21) and between actinide (An) and main-group ligands (L) (22–28).
Among the actinide complexes with An L multiple bonds, the importance of first-row elements to bond to actinide metal centers has been highlighted by Burns (22), and molecular complexes containing metal-nitride units have been prepared recently (24–26). Uranium as the leading example forms a plethora of U
L multiple bonds, the importance of first-row elements to bond to actinide metal centers has been highlighted by Burns (22), and molecular complexes containing metal-nitride units have been prepared recently (24–26). Uranium as the leading example forms a plethora of U O bonds and a handful of U
O bonds and a handful of U NR and U
NR and U CR2 (R = organic groups) bonds. Considerable interest has been developed in recent years in actinide complexes with An
CR2 (R = organic groups) bonds. Considerable interest has been developed in recent years in actinide complexes with An L double bonds, and most of these investigations have centered on organometallic systems. Examples include the compounds above with N
L double bonds, and most of these investigations have centered on organometallic systems. Examples include the compounds above with N U
U N linkages (26) and organoimido (An
N linkages (26) and organoimido (An NR) and phosphinidene (An
NR) and phosphinidene (An PR) groups (29, 30). The matrix isolation technique has revealed several inorganic uranium compounds with covalent triple bonds, including NUN, CUO, and NUO+ cation (31–33), which are isoelectronic with the ubiquitous uranyl dication. However, An
PR) groups (29, 30). The matrix isolation technique has revealed several inorganic uranium compounds with covalent triple bonds, including NUN, CUO, and NUO+ cation (31–33), which are isoelectronic with the ubiquitous uranyl dication. However, An L multiple bonds are usually formed between hard-acidic, high-valent actinides and hard Lewis bases, particularly F−, O2−, and NR2− (34, 35), and no actinide alkylidyne complexes with An
L multiple bonds are usually formed between hard-acidic, high-valent actinides and hard Lewis bases, particularly F−, O2−, and NR2− (34, 35), and no actinide alkylidyne complexes with An CR triple bonds are known so far. Because of the high orbital energies of carbon, LnAn
CR triple bonds are known so far. Because of the high orbital energies of carbon, LnAn CR type of carbyne compounds is not expected to be highly stable. Well designed ligands that can stabilize the actinide center at their stable oxidation states are needed to accommodate the actinide
CR type of carbyne compounds is not expected to be highly stable. Well designed ligands that can stabilize the actinide center at their stable oxidation states are needed to accommodate the actinide carbon multiple bonds.
carbon multiple bonds.
High-oxidation state transition metal alkylidene and alkylidyne complexes have received increasing attention over the past three decades owing to their importance as catalysts in a variety of synthetic organometallic processes (36). Recently, we have prepared simple methylidene and methylidyne molecules through the reaction of laser-ablated early transition metal atoms with methane or methyl halides (37). These studies were extended to the accessible actinide metal atoms Th and U for the preparation of the first actinide methylidene species HXAn CH2 (X = H, F, Cl, Br) (38–41). Although Mo and W reactions also formed the analogous H2XM
CH2 (X = H, F, Cl, Br) (38–41). Although Mo and W reactions also formed the analogous H2XM CH methylidynes (42–46), the H2XU
CH methylidynes (42–46), the H2XU CH counterparts were energetically too high to be produced in these experiments (40). However, very recent investigations with the heavy metals Zr, Hf, and Re in trihalomethane reactions have demonstrated that the highly exothermic driving force for halogen transfer from carbon to heavy metal fosters the formation of the low-energy, very stable trihalo metal carbynes (47, 48).
CH counterparts were energetically too high to be produced in these experiments (40). However, very recent investigations with the heavy metals Zr, Hf, and Re in trihalomethane reactions have demonstrated that the highly exothermic driving force for halogen transfer from carbon to heavy metal fosters the formation of the low-energy, very stable trihalo metal carbynes (47, 48).
We report here an integrated experimental and theoretical study of the actinide-methylidyne species, namely F3U CH, Cl3U
CH, Cl3U CH, Br3U
CH, Br3U CH, F2ClU
CH, F2ClU CH, and F3U
CH, and F3U CF, which render the long-sought U
CF, which render the long-sought U C triple bonds in methylidyne compounds. Detailed bonding analysis based on a variety of relativistic quantum chemistry calculations indicates that the U
C triple bonds in methylidyne compounds. Detailed bonding analysis based on a variety of relativistic quantum chemistry calculations indicates that the U C triple bonds are composed of one (df-sp)σ bond and two (df-p)π bonds. Interestingly the U
C triple bonds are composed of one (df-sp)σ bond and two (df-p)π bonds. Interestingly the U C bond length and bond strength are tunable by changing the electronegativity of the neighboring atoms attached to the U
C bond length and bond strength are tunable by changing the electronegativity of the neighboring atoms attached to the U C bond, making these complexes attractive reactive intermediates. This discovery of the An
C bond, making these complexes attractive reactive intermediates. This discovery of the An C triple-bonded complexes provides important insights and is expected to assist in the rational synthesis of these compounds in larger quantities, which might be stabilized by electron-withdrawing substituents and steric protection of the U
C triple-bonded complexes provides important insights and is expected to assist in the rational synthesis of these compounds in larger quantities, which might be stabilized by electron-withdrawing substituents and steric protection of the U C bonds with bulky ligands.
C bonds with bulky ligands.
Results and Discussion
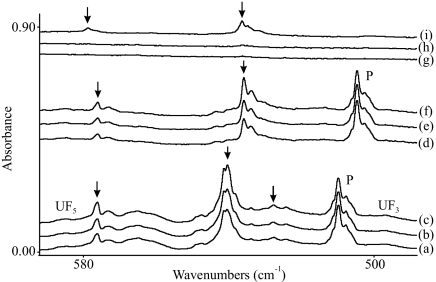
Reactions of laser-ablated U atoms were done with fluoroform, chloroform, bromoform, carbon tetrafluoride, and several isotopic modifications (CHX3, 13CHX3, CDX3 [X = F, Cl, Br], and CF4) as precursors in excess argon (0.5%, 1%, or 2% concentrations) during condensation onto an 8 K cesium iodide window, as described (49, 50). Depleted high-purity uranium metal targets (ORNL) were filed to remove surface contaminants. Common bands in uranium experiments with different precursors are limited to oxides and nitrides (38–41, 49). Infrared spectra were recorded on a Nicolet 550 spectrometer after sample deposition, after annealing, and after irradiation by using a 175 W mercury arc street lamp. Infrared spectra from reactions of fluoroform or carbon tetrafluoride reagents with uranium are shown in Fig. 1, and the results from reactions with chloroform and bromoform are detailed in Fig. 2.
Fig. 1.
Infrared spectra in the 590–490 cm−1 region for laser-ablated U atoms codeposited with fluoromethanes in excess argon at 8 K. U and 1% CHF3 in argon codeposited for 1 h (a), after >290 nm irradiation (b), and after >220 nm irradiation (c). U and 1% CDF3 in argon codeposited for 1 h (d), after >290 nm irradiation (e), and after >220 nm irradiation (f). U and 1% CF4 in argon codeposited for 1 h (g), after >290 nm irradiation (h), and after >220 nm irradiation (i). Precursor absorptions are labeled P.
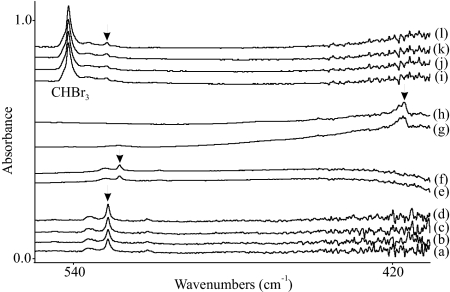
Fig. 2.
Infrared spectra in the 550–410 cm−1 region for laser-ablated U atoms codeposited with chloroform in excess argon at 8 K. U and 0.5% CHCl3 in argon codeposited for 1 h (a), after λ > 290 nm irradiation (b), after λ > 220 nm irradiation (c), and after annealing to 30 K (d). U and 0.5% 13CHCl3 in argon codeposited for 1 h (e), and after λ > 220 nm irradiation (f). U and 0.5% CDCl3 in argon codeposited for 1 h (g), and after λ > 220 nm irradiation (h). U and 2% CHBr3 in argon codeposited for 1 h (i), after λ > 290 nm irradiation (j), after λ > 220 nm irradiation (k), and after annealing to 30 K (l).
The geometries, vibrational frequencies, and electronic structures of the potential uranium product complexes were calculated by using relativistic density functional theory (DFT) with the generalized gradient PW91 approach (51) as implemented in ADF 2006.1 (52). Inasmuch as the 6s and 6p semicore orbitals are important for actinide bonding, they are included explicitly in the variational space along with the 5f, 6d, 7s, and 7p valence orbitals, whereas frozen-core approximation was applied to the U [1s2-5d10] atomic core. Slater basis sets with the quality of triple-zeta plus two polarization functions (TZ2P) were used. The zero-order regular approximation was used to account for the relativistic effects (53). We also performed ab initio calculations at the level of coupled-cluster with single, double, and perturbative triple excitations [CCSD(T)] (54) on X3UCH (X = H, F) with use of the Stuttgart quasi-relativistic pseudopotential and valence basis set for U and 6-31+G* basis sets for C, F, and H (55, 56). The optimized CCSD(T) U C distances (1.926 Å in H3UCH) lie in the same range as those from DFT calculations, indicating that the later are applicable for evaluating these close-shell triple-bond actinide systems.
C distances (1.926 Å in H3UCH) lie in the same range as those from DFT calculations, indicating that the later are applicable for evaluating these close-shell triple-bond actinide systems.
In the fluoroform spectra stable binary uranium fluorides give rise to very weak absorptions at 496 and 584 cm−1 for UF3 and UF5, respectively (57), which shows that uranium abstracts fluorine from the precursor molecule. Three new bands marked with arrows in Fig. 1a are observed at 576.2, 540.2, and 527.5 cm−1 in the infrared spectrum recorded after the initial reaction of U and CHF3. These bands increase by 30% on UV irradiation (λ > 290 nm) and another 20% on further UV irradiation (λ > 220 nm). A more dilute 13CHF3 sample gave only the most intense absorption shifted to 539.2 cm−1, which demonstrates clearly that carbon is involved in the product species. The reaction with CDF3 shown in Fig. 1d gives the same upper band, with the strong band shifting to 535.9 cm−1 and the lower band shifting to the low 400 cm−1 poor-signal region. Because CF4 is less reactive, on reagent codeposition (Fig. 1g) weaker bands are detected at 578.7 and 536.4 cm−1, which increase slightly during the first irradiation (λ > 290 nm), but undergo major growth in the second irradiation (λ >220 nm) (Fig. 1i). These bands are stable on annealing the sample to 30 K, and they increase slightly on further UV irradiation.
The new absorptions in the U F stretching region are due to a reaction product trapped in solid argon, which is a uranium species other than binary UFx (x = 1–6) (57). After our previous work on the titanium reaction with CF4, which produced the electron-deficient methylidyne complex F3TiCF (58), and analogous work with heavy metal atoms (47, 48), which produced the F3MCH molecules, we consider these absorptions for assignment to the F3UCH and F3UCF uranium-bearing methylidyne compounds. Relativistic quantum chemistry calculations have been performed on various structural isomers, isotopomers, and different electronic states of these molecules to determine the thermodynamic stability and to validate the experimental assignments. The optimized geometries of X3U
F stretching region are due to a reaction product trapped in solid argon, which is a uranium species other than binary UFx (x = 1–6) (57). After our previous work on the titanium reaction with CF4, which produced the electron-deficient methylidyne complex F3TiCF (58), and analogous work with heavy metal atoms (47, 48), which produced the F3MCH molecules, we consider these absorptions for assignment to the F3UCH and F3UCF uranium-bearing methylidyne compounds. Relativistic quantum chemistry calculations have been performed on various structural isomers, isotopomers, and different electronic states of these molecules to determine the thermodynamic stability and to validate the experimental assignments. The optimized geometries of X3U CH (X = F, Cl, Br) and F3U
CH (X = F, Cl, Br) and F3U CF by using the PW91 approach for the singlet ground-state molecules are depicted in Fig. 3, and their vibrational frequencies and IR intensities are listed in Tables 1 and 2.
CF by using the PW91 approach for the singlet ground-state molecules are depicted in Fig. 3, and their vibrational frequencies and IR intensities are listed in Tables 1 and 2.
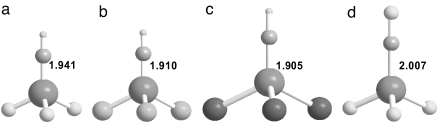
Fig. 3.
Optimized molecular structures of F3U CH (a), Cl3U
CH (a), Cl3U CH (b), Br3U
CH (b), Br3U CH (c), and F3U
CH (c), and F3U CF (d) by using PW91. The U
CF (d) by using PW91. The U C bond lengths are in angstroms.
C bond lengths are in angstroms.
Table 1.
Observed and calculated fundamental vibrational frequencies for the C3v F3U≡CX (X = H, D, F) molecules*
| Mode description | F3U≡CH |
F3U≡13CH |
F3U≡CD |
F3U≡CF |
||||
|---|---|---|---|---|---|---|---|---|
| Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | |
| C-X str, a1 | 2,979 (2) | 2,969 (2) | —‡ | 2,200 (1) | —‡ | 1,268 (312) | ||
| U≡CX str, a1 | —‡ | 747 (46) | —†‡ | 721 (42) | —‡ | 717 (41) | — § | 441 (34) |
U F sym str, a1 F sym str, a1
|
576.2 | 585 (122) | —† | 585 (123) | 576.2 | 586 (123) | 578.7 | 589 (118) |
U F antisym str, e F antisym str, e |
540.2 | 561 (284) | 539.2 | 559 (280) | 535.9 | 541 (207) | 536.4 | 544 (177) |
| U≡C-X bend, e | 527.5 | 508 (34) | —† | 506 (24) | — § | 412 (49) | — | 311 (28) |
*Vibrational frequencies (cm−1) and intensities (km/mol, in parentheses) are calculated by using PW91/TZ2P (see text). Three real lower-frequency bending modes (a1, e, e) are not listed. Absorptions are observed in argon matrix.
†Sample too dilute to observe weaker bands.
‡Absorption masked by very intense precursor band.
§Region noisy because of low detector response at the instrumental limit.
Table 2.
Observed and calculated fundamental vibrational frequencies for the C3v X3U≡CH (X = Cl, Br) molecules*
| Mode description | Cl3U≡CH |
Cl3U≡13CH |
Cl3U≡CD |
Br3U≡CH |
||||
|---|---|---|---|---|---|---|---|---|
| Obs.† | Calc. | Obs. | Calc. | Obs. | Calc. | Obs. | Calc. | |
C H str, a1 H str, a1
|
3,001 (2) | 2,991 (2) | 2,219 (7) | 3,005 (3) | ||||
| U≡CX str, a1 | —‡ | 770 (69) | —‡ | 744 (65) | —‡ | 738 (64) | —‡ | 777 (70) |
| U≡C-H bend, e | 527.2 | 522 (224) | 522.8 | 518 (218) | 415.9 | 410 (216) | 527.6 | 527 (178) |
U X sym str, a1 X sym str, a1
|
339 (29) | 339 (29) | 339 (29) | 225 (84)§ | ||||
U X antisym str, e X antisym str, e |
329 (140) | — | 329 (140) | — | 326 (100) | 216 (10)§ | ||
*Vibrational frequencies (cm−1) and intensities (km/mol, in parentheses) are calculated by using PW91/TZ2P (see text). Three real lower-frequency bending modes (a1, e, e) are not listed.
†Absorptions are observed in argon matrix.
‡Absorption masked by very intense precursor band.
§Mode symmetries reversed.
The upper bands in Fig. 1 are assigned to the symmetric U F stretching mode, which shows very little change between these two F3U
F stretching mode, which shows very little change between these two F3U CR (R = H, F) molecules. The strongest absorption is due to the very strong degenerate U
CR (R = H, F) molecules. The strongest absorption is due to the very strong degenerate U F stretching mode, which shifts slightly from F3UCH to F3U13CH, to F3UCD and to F3UCF. Finally, the weak 527.5 cm−1 band is assigned to the degenerate H
F stretching mode, which shifts slightly from F3UCH to F3U13CH, to F3UCD and to F3UCF. Finally, the weak 527.5 cm−1 band is assigned to the degenerate H C
C U bending mode, which is weak in this molecule probably, in part, because of intensity gained by the nearby degenerate U
U bending mode, which is weak in this molecule probably, in part, because of intensity gained by the nearby degenerate U F stretching mode. In the deuteriated species, this mode shifts into a region of low signal near our limit of detection.
F stretching mode. In the deuteriated species, this mode shifts into a region of low signal near our limit of detection.
This assignment is confirmed in chloroform and bromoform experiments, where the H C
C U bending mode gains substantial infrared intensity and shifts slightly to 527.2 and to 527.6 cm−1, respectively, and the U
U bending mode gains substantial infrared intensity and shifts slightly to 527.2 and to 527.6 cm−1, respectively, and the U X stretching frequencies are much lower because of the heavier halogen mass. The lack of a significant difference between the chloroform and bromoform reaction product absorptions demonstrates clearly that the halogen atoms in the product structure are farther separated from the hydrogen atom than in the precursor haloform molecules. Notice in Fig. 2 that the strong new band with chloroform at 527.2 cm−1 increases on UV irradiation, and shifts to 522.8 cm−1 with 13CHCl3 and to 415.9 cm−1 with the CDCl3 precursor. The PW91 calculation of frequencies (Table 2) for isotopic Cl3UCH molecules reveals one strong absorption at 522 cm−1, which exhibits almost exactly the same isotopic shifts as the observed new product absorption and defines the bending mode. We note that the frequency of the H
X stretching frequencies are much lower because of the heavier halogen mass. The lack of a significant difference between the chloroform and bromoform reaction product absorptions demonstrates clearly that the halogen atoms in the product structure are farther separated from the hydrogen atom than in the precursor haloform molecules. Notice in Fig. 2 that the strong new band with chloroform at 527.2 cm−1 increases on UV irradiation, and shifts to 522.8 cm−1 with 13CHCl3 and to 415.9 cm−1 with the CDCl3 precursor. The PW91 calculation of frequencies (Table 2) for isotopic Cl3UCH molecules reveals one strong absorption at 522 cm−1, which exhibits almost exactly the same isotopic shifts as the observed new product absorption and defines the bending mode. We note that the frequency of the H C
C U bending mode increases as the C
U bending mode increases as the C U bond becomes stronger and shorter in the X3U
U bond becomes stronger and shorter in the X3U CH series, and the reaction of excited U atom with chloroform and bromoform is even more favorable than the reaction with fluoroform.
CH series, and the reaction of excited U atom with chloroform and bromoform is even more favorable than the reaction with fluoroform.
The C H stretching modes are too weak to be observed here, and the C
H stretching modes are too weak to be observed here, and the C F stretching mode is masked by very intense CF4 precursor absorption. Finally, the U
F stretching mode is masked by very intense CF4 precursor absorption. Finally, the U CH stretching mode is unfortunately in the region of very strong haloform precursor bands, and the U
CH stretching mode is unfortunately in the region of very strong haloform precursor bands, and the U CF stretching mode is predicted at the low end of our region of detection where the signal-to-noise is diminished. However, in the molecule of lower symmetry, F2ClU
CF stretching mode is predicted at the low end of our region of detection where the signal-to-noise is diminished. However, in the molecule of lower symmetry, F2ClU CH, prepared from the reaction with Freon 22, the U
CH, prepared from the reaction with Freon 22, the U CH stretching mode is found as a weak band at 671.3 cm−1, which is shifted to 648.7 cm−1 by using 13CHF2Cl, confirming the vibrational assignment of U
CH stretching mode is found as a weak band at 671.3 cm−1, which is shifted to 648.7 cm−1 by using 13CHF2Cl, confirming the vibrational assignment of U CH stretching mode. Note that the large 22.6 cm−1 carbon-13 shift for the U
CH stretching mode. Note that the large 22.6 cm−1 carbon-13 shift for the U CH stretching mode agrees well with theoretical calculations; our PW91 calculation predicts the U
CH stretching mode agrees well with theoretical calculations; our PW91 calculation predicts the U CH mode in F2ClU
CH mode in F2ClU CH at 682.4 cm−1 with an isotopic shift of 23.6 cm−1.
CH at 682.4 cm−1 with an isotopic shift of 23.6 cm−1.
Our calculations also indicate that the X3U CH (X = F, Cl, Br) and F3U
CH (X = F, Cl, Br) and F3U CF molecules prefer a singlet ground state, with the triplet and quintet states being much higher in energy. The methylidynes are indeed more stable than their methylidene and methyl uranium halide counterparts, and the predicted C3v molecular symmetry, vibrational frequencies, infrared absorption intensities, and isotopic frequencies are all in excellent agreement with the experimental observations, as shown in Tables 1 and 2. This agreement confirms our vibrational assignments and the identification of uranium methylidyne molecules.
CF molecules prefer a singlet ground state, with the triplet and quintet states being much higher in energy. The methylidynes are indeed more stable than their methylidene and methyl uranium halide counterparts, and the predicted C3v molecular symmetry, vibrational frequencies, infrared absorption intensities, and isotopic frequencies are all in excellent agreement with the experimental observations, as shown in Tables 1 and 2. This agreement confirms our vibrational assignments and the identification of uranium methylidyne molecules.
Additional chemical support for this exothermic α-halogen transfer reaction of the uranium systems is found in our subsequent investigations with Re, Mo, and W atoms (48, 59). These metals react with haloforms to give analogous X3M CH molecules with C3v symmetry for M = Mo and W. In the case of Re, Jahn–Teller distortion lowers the symmetry to Cs, which increases the IR intensity of the C
CH molecules with C3v symmetry for M = Mo and W. In the case of Re, Jahn–Teller distortion lowers the symmetry to Cs, which increases the IR intensity of the C H stretching absorption due to polarization of the triple bond, and high C
H stretching absorption due to polarization of the triple bond, and high C H stretching frequencies characteristic of sp hybridization are also observed. Like U the heavy group 6 metals Mo and W also support hexavalency, leading to the formation of the symmetric X3M
H stretching frequencies characteristic of sp hybridization are also observed. Like U the heavy group 6 metals Mo and W also support hexavalency, leading to the formation of the symmetric X3M CH molecules. In addition to the MX3 stretching and H
CH molecules. In addition to the MX3 stretching and H C
C M deformation fundamentals, high C
M deformation fundamentals, high C H and M
H and M C stretching frequencies are observed to further characterize these methylidyne molecules (59).
C stretching frequencies are observed to further characterize these methylidyne molecules (59).
To understand the relative roles of the U 6p, 5f, and 6d orbitals and their chemical bonding, a series of theoretical analyses have been performed. Table 3 lists the natural charges, natural localized molecular orbitals (NLMOs), natural hybrid orbitals, and the bond orders calculated for X3U CH and F3U
CH and F3U CF molecules. The orbital contributions to the σ- and π-orbitals in X3U
CF molecules. The orbital contributions to the σ- and π-orbitals in X3U CH are listed in Table 4 for comparison with those of the formal triple bonds in uranyl. The natural population analysis (60) reveals that U carries highly positive charges in these U(VI) complexes, which helps to form stable molecules by both covalent and ionic interactions. The DFT calculations predict that the U
CH are listed in Table 4 for comparison with those of the formal triple bonds in uranyl. The natural population analysis (60) reveals that U carries highly positive charges in these U(VI) complexes, which helps to form stable molecules by both covalent and ionic interactions. The DFT calculations predict that the U C distances in H3UCH (a model compound), Br3UCH, Cl3UCH, F3UCH, and F3UCF are 1.901, 1.905, 1.910, 1.941, and 2.007 Å, respectively. These U
C distances in H3UCH (a model compound), Br3UCH, Cl3UCH, F3UCH, and F3UCF are 1.901, 1.905, 1.910, 1.941, and 2.007 Å, respectively. These U C distances are slightly longer than the sum of the U and C triple-bond radii (17), but correlate well with what is expected from the experimentally measured distances for U
C distances are slightly longer than the sum of the U and C triple-bond radii (17), but correlate well with what is expected from the experimentally measured distances for U C (2.29 Å) and U
C (2.29 Å) and U C (2.54 Å) bonds (61). The U
C (2.54 Å) bonds (61). The U C triple bonds in the X3U
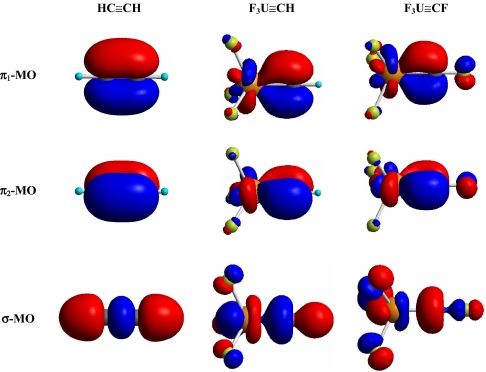
C triple bonds in the X3U CR complexes are further established by theoretical calculations from different methodologies. As shown in Fig. 4, the canonical molecular orbitals from DFT calculations also reveal one σ- and two π-orbitals with six electrons, consistent with orthodox triple-bond models (1, 2).
CR complexes are further established by theoretical calculations from different methodologies. As shown in Fig. 4, the canonical molecular orbitals from DFT calculations also reveal one σ- and two π-orbitals with six electrons, consistent with orthodox triple-bond models (1, 2).
Table 3.
Natural charges (qN), natural localized molecular orbital (NLMO) compositions, natural hybrid orbitals, and bond U-C orders
| qU | qC | NLMO | BOW | BOM | BOGJ | |
|---|---|---|---|---|---|---|
| H3U≡CH | 2.45 | −1.17 | 22% U(s0.05p0.05d1.03f) + 78% C(sp1.06) | 2.51 | 2.47 | 3.06 |
| 39% U(d0.34f) + 61% C(p) | ||||||
| F3U≡CH | 2.87 | −0.95 | 18% U(s0.10p0.05d0.96f) + 82% C(sp0.97) | 2.48 | 2.38 | 2.88 |
| 47% U(d0.19f) + 53% C(p) | ||||||
| Cl3U≡CH | 2.42 | −0.91 | 20% U(s0.09p0.03d1.05f) + 80% C(sp0.96) | 2.53 | 2.40 | 2.90 |
| 48% U(d0.19f) + 52% C(p) | ||||||
| Br3U≡CH | 2.35 | −0.93 | 21% U(s0.09p0.03d1.07f) + 79% C(sp0.97) | 2.53 | 2.39 | 2.90 |
| 47% U(d0.21f) + 53% C(p) | ||||||
| F3U≡CF | 2.78 | −0.33 | 11% U(s0.45p0.03d1.27f) + 89% C(sp0.45) | 2.14 | 2.18 | 2.57 |
| 51% U(d0.19f) + 49% C(p) |
Only one of the two π-orbitals is listed as they are equivalent. BOW, natural Wiberg bond order; BOM, Mayer bond order; BOGJ, Gophinatan–Jug bond order.
Table 4.
PW91 MO percentages (%) of U valence and semicore orbitals in uranyl and X3UCH (X = H, F) molecules
| U |
C/O |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MO | 6 s | 6p | 5f | 6d | 7 s | 7p | 2 s | 2p | |
| UO22+ | σu | 8 | 57 | 2 | 33 | ||||
| σg | 14 | 2 | 7 | 77 | |||||
| πu | 1 | 34 | 64 | ||||||
| πg | 19 | 79 | |||||||
| H3U≡CH | π | 43 | 7 | 38 | |||||
| σ | 1 | 15 | 5 | 11 | 7 | 21 | |||
| F3U≡CH | π | 39 | 9 | 46 | |||||
| σ | 6 | 12 | 13 | 2 | 12 | 26 | |||
*Orbital contribution <1% is not shown.
Fig. 4.
Comparison of the σ- and π-molecular orbitals of ethyne HC CH and the uranium-methylidyne F3U
CH and the uranium-methylidyne F3U CH and F3U
CH and F3U CF complexes. (Isosurface value = 0.05 atomic unit.)
CF complexes. (Isosurface value = 0.05 atomic unit.)
The effective bond orders calculated by using different formalisms are also listed in Table 3 which agree well with triple-bond covalency. A further support of the U C triple bonds in these complexes are obtained from calculations of the characteristic topology of the electron localization function (ELF), which measures the spatial distribution of paired electrons and exhibits maxima at the most probable positions of localized electron pairs (62, 63). Fig. 5 illustrates the ELF isosurfaces of HCCH, F3UCH, Cl3UCH, Br3UCH, and F3UCF. The polarized ring attractors between U and C indicate that all of these actinide molecules possess triple bonds as is the case for acetylene. In addition, the calculated NLMOs (60) reveal the formation of the U
C triple bonds in these complexes are obtained from calculations of the characteristic topology of the electron localization function (ELF), which measures the spatial distribution of paired electrons and exhibits maxima at the most probable positions of localized electron pairs (62, 63). Fig. 5 illustrates the ELF isosurfaces of HCCH, F3UCH, Cl3UCH, Br3UCH, and F3UCF. The polarized ring attractors between U and C indicate that all of these actinide molecules possess triple bonds as is the case for acetylene. In addition, the calculated NLMOs (60) reveal the formation of the U C triple bonds by one σ-bond between the U dfσ and C spσ hybrid orbitals and two π-bonds between the U dfπ and C pπ orbitals, thus providing unequivocal support to the formation of triple bonds in these methylidyne molecules. As shown by the NLMO results and the orbital contributions listed in Tables 3 and 4, the threefold symmetry of the X3U
C triple bonds by one σ-bond between the U dfσ and C spσ hybrid orbitals and two π-bonds between the U dfπ and C pπ orbitals, thus providing unequivocal support to the formation of triple bonds in these methylidyne molecules. As shown by the NLMO results and the orbital contributions listed in Tables 3 and 4, the threefold symmetry of the X3U CR molecules allows significant hybridization or orbital mixing between U 6d and 5f orbitals, which enhances the bonding strengths of the U
CR molecules allows significant hybridization or orbital mixing between U 6d and 5f orbitals, which enhances the bonding strengths of the U C triple bonds. Table 4 also reveals that the σ-bond of F3U
C triple bonds. Table 4 also reveals that the σ-bond of F3U CH molecule involves nonnegligible U 6p hybridization with 5f/6d orbitals, similar to the “pushing from below” 6p-5f mixing in uranyl (64).
CH molecule involves nonnegligible U 6p hybridization with 5f/6d orbitals, similar to the “pushing from below” 6p-5f mixing in uranyl (64).
Fig. 5.
Isosurfaces of the electron localization function (ELF) calculated at value 0.8 for HCCH, F3UCH, Cl3UCH, Br3UCH, and F3UCF (from left to right). The ring attractors in all of these molecules clearly show the triple bonds.
Our calculations on the series X3U CR (X, R = H, F, Cl, Br, I, CH3, SiH3, CO) also reveal that the U
CR (X, R = H, F, Cl, Br, I, CH3, SiH3, CO) also reveal that the U C bond distances and bond strengths depend on the ligands and the coordination pyramidality around the U and C atoms. In particular, the strong inductive effect of electronegative fluorine supports a large positive charge on the central U atom, which helps to retain the most stable U(VI) oxidation state and to push the U 7s orbital to high energy to facilitate strong bonding between U and CR. For the halogen substitutions on U, the less electronegative ligands decrease the U
C bond distances and bond strengths depend on the ligands and the coordination pyramidality around the U and C atoms. In particular, the strong inductive effect of electronegative fluorine supports a large positive charge on the central U atom, which helps to retain the most stable U(VI) oxidation state and to push the U 7s orbital to high energy to facilitate strong bonding between U and CR. For the halogen substitutions on U, the less electronegative ligands decrease the U C bond length by increasing the effective overlap between U 6d and C 2p orbitals. Hence, among the molecules with halogen substituents, the triple bond in I3U
C bond length by increasing the effective overlap between U 6d and C 2p orbitals. Hence, among the molecules with halogen substituents, the triple bond in I3U CH is the strongest and it has the shortest U
CH is the strongest and it has the shortest U C distance (1.895 Å), close to the calculated distance in (SiH3)3U
C distance (1.895 Å), close to the calculated distance in (SiH3)3U CH (1.893 Å). The calculations on F3U
CH (1.893 Å). The calculations on F3U CR (R = F, Cl, Br, I, H, Me) also demonstrate that the U
CR (R = F, Cl, Br, I, H, Me) also demonstrate that the U C bonds are shortest for less electronegative R such as H atom.
C bonds are shortest for less electronegative R such as H atom.
The experimental results suggest that laser-energy-excited atomic uranium is a vigorous reducing agent. The reactions occur as U cleaves the strong X C (X = F, Cl, Br) bond and then two successive α-halogen transfers to uranium occur, forming the U(VI) products identified in this work. Consistent with this notion, theoretical calculations show that halogen transfer from C to U atoms is strongly favored thermodynamically, with each step being strongly exothermic because of the difference in X
C (X = F, Cl, Br) bond and then two successive α-halogen transfers to uranium occur, forming the U(VI) products identified in this work. Consistent with this notion, theoretical calculations show that halogen transfer from C to U atoms is strongly favored thermodynamically, with each step being strongly exothermic because of the difference in X U and X
U and X C bond energies. The overall reaction (1) requires initiation by using electronically excited uranium (noted U*) from laser ablation or UV irradiation, and the reaction with fluoroform is calculated to be exothermic by 183 kcal/mol with approximate treatment of the spin-orbit-coupling effects. The solid argon matrix soaks up this excess energy and stabilizes the final methylidyne product.
C bond energies. The overall reaction (1) requires initiation by using electronically excited uranium (noted U*) from laser ablation or UV irradiation, and the reaction with fluoroform is calculated to be exothermic by 183 kcal/mol with approximate treatment of the spin-orbit-coupling effects. The solid argon matrix soaks up this excess energy and stabilizes the final methylidyne product.
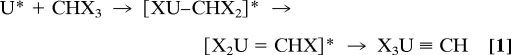 |
Through a combined experimental and theoretical effort, we have hereby shown that laser-ablated uranium atoms are quite reactive, and even the unreactive CF4 molecule falls prey and yields fluorine atoms to the active uranium metal center, leading to the formation of a relatively strong U C triple bond. Although the activation of C-halogen bonds itself is an important research field based on the need to dispose of environmentally hazardous halocarbons, the most significant aspect of this discovery is that X3U
C triple bond. Although the activation of C-halogen bonds itself is an important research field based on the need to dispose of environmentally hazardous halocarbons, the most significant aspect of this discovery is that X3U CH (X = F, Cl, Br), F2ClU
CH (X = F, Cl, Br), F2ClU CH, and F3U
CH, and F3U CF are examples of the long-sought actinide-alkylidyne molecules that have been prepared and characterized. Although the chemistry of these species prepared and isolated in a low-temperature noble-gas matrix is difficult to investigate experimentally, these unique diamagnetic X3U
CF are examples of the long-sought actinide-alkylidyne molecules that have been prepared and characterized. Although the chemistry of these species prepared and isolated in a low-temperature noble-gas matrix is difficult to investigate experimentally, these unique diamagnetic X3U CR molecules might be synthesized by coordination of U with highly electron-withdrawing ligands and steric protection of the U
CR molecules might be synthesized by coordination of U with highly electron-withdrawing ligands and steric protection of the U C bonds with bulky ligands, possibly with pseudo-threefold local symmetry. The ability of fluorine-substituted or sterically encumbering ligands (25) to generate new types of metal–ligand multiple bonds might be extended to the still-elusive actinide
C bonds with bulky ligands, possibly with pseudo-threefold local symmetry. The ability of fluorine-substituted or sterically encumbering ligands (25) to generate new types of metal–ligand multiple bonds might be extended to the still-elusive actinide carbon multiple-bond alkylidyne and arylidyne systems. The discovery of these actinide complexes with An
carbon multiple-bond alkylidyne and arylidyne systems. The discovery of these actinide complexes with An C triple bonds is potentially important for organosynthesis, catalysis, interstellar chemistry, atmospheric chemistry, and actinide chemistry under extreme conditions.
C triple bonds is potentially important for organosynthesis, catalysis, interstellar chemistry, atmospheric chemistry, and actinide chemistry under extreme conditions.
Extending the well known methylidyne chemistry of the transition metals (36) to actinide metals has proven to be a difficult challenge. Although this may simply be because actinide 6d and 5f valence orbitals do not behave like transition-metal nd orbitals (65), we indeed see glimpses of transition-metal-like behavior in the early actinides. This and our earlier research work on Th and U methylidenes (38–41) provide clues to the existence and nature of actinide carbon, multiple-bonded species.
carbon, multiple-bonded species.
Acknowledgments
The calculations were performed by using a HP Itanium2 cluster at Tsinghua National Laboratory for Information Science and Technology. We thank two anonymous reviewers for very constructive and helpful suggestions. This research was supported by National Science Foundation Grant CHE 03-52487 and by the Chinese National Key Basic Research Science Foundation (NKBRSF) Grants 2006CB932305 and 2007CB815200 and the National Natural Science Foundation of China (NNSFC) Grant 20525104.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lewis GN. J Am Chem Soc. 1916;38:762–785. [Google Scholar]
- 2.Pauling L. Nature. 1964;203:182–183. [Google Scholar]
- 3.Cotton FA, Curtis NF, Harris CB, Johnson BFG, Lippard SJ, Mague JT, Robinson WR, Wood JS. Science. 1964;145:1305–1307. doi: 10.1126/science.145.3638.1305. [DOI] [PubMed] [Google Scholar]
- 4.Fischer EO. Adv Organometallic Chem. 1976;14:1–32. [Google Scholar]
- 5.Schrock RR. Science. 1983;219:13–18. doi: 10.1126/science.219.4580.13. [DOI] [PubMed] [Google Scholar]
- 6.Lee M, Lenman M, Banas A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson PO, et al. Science. 1998;280:915–918. doi: 10.1126/science.280.5365.915. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi A, Kinjo R, Ichinohe M. Science. 2004;305:1755–1757. doi: 10.1126/science.1102209. [DOI] [PubMed] [Google Scholar]
- 8.Cotton FA, Nocera DG. Acc Chem Res. 2000;33:483–490. doi: 10.1021/ar980116o. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki N, Nishiura M, Wakatsuki Y. Science. 2002;295:660–663. doi: 10.1126/science.1067157. [DOI] [PubMed] [Google Scholar]
- 10.Frenking G. Science. 2005;310:796–797. doi: 10.1126/science.1120281. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen T, Sutton AD, Brynda M, Fettiger JC, Long GJ, Power PP. Science. 2005;310:844–847. doi: 10.1126/science.1116789. [DOI] [PubMed] [Google Scholar]
- 12.Piro NA, Figueroa JS, McKellar JT, Cummins CC. Science. 2006;313:1276–1279. doi: 10.1126/science.1129630. [DOI] [PubMed] [Google Scholar]
- 13.Radius U, Breher F. Angew Chem Int Ed. 2006;45:3006–3010. doi: 10.1002/anie.200504322. [DOI] [PubMed] [Google Scholar]
- 14.Berry JF, Bill E, Bothe E, Geoge SD, Mienert B, Neese F, Wieghardt K. Science. 2006;312:1937–1941. doi: 10.1126/science.1128506. [DOI] [PubMed] [Google Scholar]
- 15.Mindiola DJ. Acc Chem Res. 2006;39:813–821. doi: 10.1021/ar0500113. [DOI] [PubMed] [Google Scholar]
- 16.Cummins CC. Angew Chem Int Ed. 2006;45:862–870. doi: 10.1002/anie.200503327. [DOI] [PubMed] [Google Scholar]
- 17.Pyykkö P, Riedel S, Patzschke M. Chem Eur J. 2005;11:3511–3520. doi: 10.1002/chem.200401299. [DOI] [PubMed] [Google Scholar]
- 18.Gagliardi L, Roos BO. Nature. 2005;433:848–851. doi: 10.1038/nature03249. [DOI] [PubMed] [Google Scholar]
- 19.Straka M, Pyykkö P. J Am Chem Soc. 2005;127:13090–13091. doi: 10.1021/ja052723u. [DOI] [PubMed] [Google Scholar]
- 20.Cavigliasso G, Kaltsoyannis N. Inorg Chem. 2006;45:6828–6839. doi: 10.1021/ic060777e. [DOI] [PubMed] [Google Scholar]
- 21.Frenking G, Tonner R. Nature. 2007;446:276–277. doi: 10.1038/446276a. [DOI] [PubMed] [Google Scholar]
- 22.Burns CJ. Science. 2005;309:1823–1824. doi: 10.1126/science.1118701. [DOI] [PubMed] [Google Scholar]
- 23.Arney DSJ, Burns CJ, Smith DC. J Am Chem Soc. 1992;114:10068–10069. [Google Scholar]
- 24.Hayton TW, Boncella JM, Scott BL, Palmer PD, Batista ER, Hay PJ. Science. 2005;310:1941–1943. doi: 10.1126/science.1120069. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Rodriguez I, Nakai H, Zakharov LN, Rheingold AL, Meyer K. Science. 2004;305:1757–1759. doi: 10.1126/science.1102602. [DOI] [PubMed] [Google Scholar]
- 26.Evans WJ, Kozimor SA, Ziller JW. Science. 2005;309:1835–1838. doi: 10.1126/science.1116452. [DOI] [PubMed] [Google Scholar]
- 27.Gagliardi L, Pyykkö P. Angew Chem Int Ed. 2004;43:1573–1576. doi: 10.1002/anie.200353261. [DOI] [PubMed] [Google Scholar]
- 28.Ephritikhine M. Dalton Trans. 2006:2501–2516. doi: 10.1039/b603463b. [DOI] [PubMed] [Google Scholar]
- 29.Brennan JG, Andersen RA. J Am Chem Soc. 1985;107:514–516. [Google Scholar]
- 30.Arney DS, Burns CJ, Schnabel RC. J Am Chem Soc. 1996;118:6780–6781. [Google Scholar]
- 31.Green DW, Reedy GT. J Chem Phys. 1976;65:2921–2922. [Google Scholar]
- 32.Li J, Bursten BE, Liang B, Andrews L. Science. 2002;295:2242–2245. doi: 10.1126/science.1069342. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Andrews L. J Chem Phys. 1999;111:11044–11049. [Google Scholar]
- 34.Pepper M, Bursten BE. Chem Rev. 1991;91:719–741. [Google Scholar]
- 35.Clark DL. Los Alamos Sci. 2000;26:364–381. [Google Scholar]
- 36.Schrock RR. Chem Rev. 2002;102:145–179. doi: 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]
- 37.Andrews L, Cho HG. Organometallics. 2006;25:4040–4053. [Google Scholar]
- 38.Andrews L, Cho HG. J Phys Chem A. 2005;109:6796–6798. doi: 10.1021/jp052918o. [DOI] [PubMed] [Google Scholar]
- 39.Lyon JT, Andrews L. Inorg Chem. 2005;44:8610–8616. doi: 10.1021/ic051153w. [DOI] [PubMed] [Google Scholar]
- 40.Lyon JT, Andrews L. Inorg Chem. 2005;45:1847–1852. doi: 10.1021/ic051785i. [DOI] [PubMed] [Google Scholar]
- 41.Lyon JT, Andrews L, Malmqvist PÅ, Roos BO, Wang T, Bursten BE. Inorg Chem. 2006;46:4917–4925. doi: 10.1021/ic062407w. [DOI] [PubMed] [Google Scholar]
- 42.Cho HG, Andrews L. Chem Eur J. 2005;11:5017–5023. doi: 10.1002/chem.200500242. [DOI] [PubMed] [Google Scholar]
- 43.Cho HG, Andrews L. J Am Chem Soc. 2005;127:8226–8231. doi: 10.1021/ja0511568. [DOI] [PubMed] [Google Scholar]
- 44.Cho HG, Andrews L. Organometallics. 2005;24:5678–5685. [Google Scholar]
- 45.Cho HG, Andrews L, Marsden C. Inorg Chem. 2005;44:7634–7643. doi: 10.1021/ic051090h. [DOI] [PubMed] [Google Scholar]
- 46.Cho HG, Andrews L. J Phys Chem A. 2006;110:13151–13163. doi: 10.1021/jp064085n. [DOI] [PubMed] [Google Scholar]
- 47.Lyon JT, Andrews L. Organometallics. 2007;26:2519–2527. [Google Scholar]
- 48.Lyon JT, Cho HG, Andrews L, Hu HS, Li J. Inorg Chem. 2007;46:8728–8738. doi: 10.1021/ic701014u. [DOI] [PubMed] [Google Scholar]
- 49.Souter PF, Kushto GP, Andrews L, Neurock M. J Am Chem Soc. 1997;119:1682–1687. [Google Scholar]
- 50.Andrews L, Citra A. Chem Rev. 2002;102:885–911. doi: 10.1021/cr0000729. [DOI] [PubMed] [Google Scholar]
- 51.Perdew JP, Wang Y. Phys Rev B. 1992;45:13244–13249. doi: 10.1103/physrevb.45.13244. [DOI] [PubMed] [Google Scholar]
- 52.te Velde G, Bickelhaupt FM, van Gisbergen SJA, Guerra CF, Baerends EJ, Snijders JG, Ziegler T. J Comput Chem. 2001;22:931–967. [Google Scholar]
- 53.van Lenthe E, Baerends EJ, Snijders JG. J Chem Phys. 1993;99:4597–4610. [Google Scholar]
- 54.Raghavachari K, Trucks GW, Pople JA, Head-Gordon M. Chem Phys Lett. 1989;157:479–483. [Google Scholar]
- 55.Küchle W, Dolg M, Stoll H, Preuss H. J Chem Phys. 1994;100:7535–7542. [Google Scholar]
- 56.Ditchfield R, Hehre WJ, Pople JA. J Chem Phys. 1971;54:724–728. [Google Scholar]
- 57.Hunt RD, Thompson C, Hassanzadeh P, Andrews L. Inorg Chem. 1994;33:388–391. [Google Scholar]
- 58.Lyon JT, Andrews L. Inorg Chem. 2006;45:9858–9863. doi: 10.1021/ic0610379. [DOI] [PubMed] [Google Scholar]
- 59.Lyon JT, Cho HG, Andrews L. Organometallics. 2007 in press (Cr, Mo and W + CHX3, CX4) [Google Scholar]
- 60.Reed AE, Curtiss LA, Weinhold F. Chem Rev. 1988;88:899–926. [Google Scholar]
- 61.Cramer RE, Maynard RB, Paw JC, Gilje JW. J Am Chem Soc. 1981;103:3589–3590. [Google Scholar]
- 62.Becke AD, Edgecombe KE. J Chem Phys. 1990;92:5397–5403. [Google Scholar]
- 63.Silvi B, Savin A. Nature. 1994;371:683–686. [Google Scholar]
- 64.Tatsumi K, Hoffmann R. J Am Chem Soc. 1980;19:2656–2658. [Google Scholar]
- 65.Kaltsoyannis N. Chem Soc Rev. 2003;32:9–16. doi: 10.1039/b204253n. [DOI] [PubMed] [Google Scholar]