Abstract
To investigate the consequences of macromolecular crowding on the behavior of a globular protein, we performed a combined experimental and computational study on the 148-residue single-domain α/β protein, Desulfovibrio desulfuricans apoflavodoxin. In vitro thermal unfolding experiments, as well as assessment of native and denatured structures, were probed by using far-UV CD in the presence of various amounts of Ficoll 70, an inert spherical crowding agent. Ficoll 70 has a concentration-dependent effect on the thermal stability of apoflavodoxin (ΔTm of 20°C at 400 mg/ml; pH 7). As judged by CD, addition of Ficoll 70 causes an increase in the amount of secondary structure in the native-state ensemble (pH 7, 20°C) but only minor effects on the denatured state. Theoretical calculations, based on an off-lattice model and hard-sphere particles, are in good agreement with the in vitro data. The simulations demonstrate that, in the presence of 25% volume occupancy of spheres, native flavodoxin is thermally stabilized, and the free energy landscape shifts to favor more compact structures in both native and denatured states. The difference contact map reveals that the native-state compaction originates in stronger interactions between the helices and the central β-sheet, as well as by less fraying in the terminal helices. This study demonstrates that macromolecular crowding has structural effects on the folded ensemble of polypeptides.
Keywords: energy landscape theory, excluded volume effect, molecular simulations, protein folding
Protein folding in vitro has been extensively characterized in dilute conditions. However, the intracellular environment is highly crowded because of the presence of large amounts of soluble and insoluble macromolecules, including proteins, nucleic acids, ribosomes, and carbohydrates. This means that a significant fraction of the intracellular space is not available to other macromolecular species. It has been estimated that the concentration of macromolecules in the cytoplasm is in the range of 80–400 mg/ml (1, 2). All macromolecules in physiological fluids collectively occupy between 10% and 40% of the total fluid volume (3, 4). The term “macromolecular crowding” implies the nonspecific influence of steric repulsions on specific reactions that occur in highly volume-occupied media. Because of excluded volume effects, any reaction that amplifies the available volume will be stimulated by macromolecular crowding (5–8). It is proposed that crowding provides a stabilizing effect to the folded protein indirectly because of compaction of the denatured states. Crowding can be mimicked experimentally by adding high concentrations of inert synthetic or natural macromolecules, termed crowding agents, to the systems in vitro. Experimental and theoretical work has demonstrated large effects of crowding on the thermodynamics and kinetics of many biological processes, including protein binding, folding, and aggregation (1, 9–12).
Whereas theoretical simulations have focused on very small proteins or peptides (11), experimental crowding studies have mostly involved large complex proteins (i.e., multidomain and/or disulfide containing) and often extreme solvent conditions (such as acidic pH). For example, the effects of crowding agents on the refolding of reduced denatured lysozyme (1, 9), oligomerization of GroEL subunits (13), self-assembly of the cell division protein FtsZ (14) and the capsid protein of HIV (15), and amyloid formation of the human apolipoprotein C-II (16) have been reported. A few studies have focused on the ability of crowding agents to induce conformational changes in unfolded states of proteins (17, 18). For example, it was shown that unfolded cytochrome c adopts a molten globule state in the presence of crowding agents at low pH (19). Notably, there are no detailed studies of the effects of macromolecular crowding on the behavior of small single-domain proteins that fold with simple mechanisms in dilute solutions.
To fill this gap, we have combined experimental and theoretical approaches to carefully assess the native and denatured structural ensembles, as well as the thermal stability, of a well behaved α/β model protein (i.e., apoflavodoxin) as a function of concentration of an inert spherical macromolecular crowding agent (i.e., Ficoll 70). Our in vitro and in silico results are in excellent agreement and demonstrate that crowding increases protein-thermal stability via structural enhancement in both the folded and denatured ensembles of molecules.
Results
Choice of Protein and Crowding Agent.
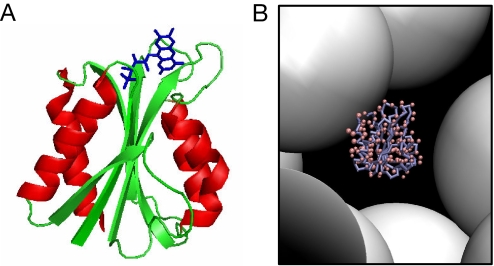
The model system, Desulfovibrio desulfuricans flavodoxin (Fig. 1A), was selected because it is a small α/β protein (148 residues) with a common fold (i.e., the flavodoxin-like fold) in which a central β-sheet is surrounded by four helices (20–22). This protein has been extensively characterized previously in our laboratory in terms of its chemical and thermal unfolding behaviors in dilute solutions (23–27). It unfolds in an apparent two-state reaction upon chemical and thermal perturbations when monitored by far-UV CD. To be able to match with simulations, flavodoxin devoid of the FMN cofactor, i.e., apoflavodoxin, is used in all work herein. Apoflavodoxin has the same folded structure as with the cofactor; it exhibits a rather modest stability (≈15 kJ/mol, pH 7) at room temperature, indicating there is span for possible improvement because of crowding effects. The crowding agent selected for this work is Ficoll 70. This agent has many advantages; it is a polysaccharide (i.e., sucrose epichlorohydrin copolymer; average molecular mass of 74 kDa) that is inert, polar, and does not interact with proteins. It behaves like a semirigid sphere (radius of ≈55 Å) (28, 29); thus it is an attractive mimic of globular macromolecules that may be present in the biological setting where proteins normally fold. In addition, it can readily be represented in computer simulations as repulsive hard spheres of the appropriate size (Fig. 1B; for volume occupancy, φc, of 25%).
Fig. 1.
Protein system used in this study. (A) Model of D. vulgaris flavodoxin (2fx2). The sequence of D. desulfuricans flavodoxin, used in our in vitro experiments, is 46% identical to that of D. vulgaris. Green, β-sheets and loops; red, α-helices; blue, FMN cofactor (removed in our experiments). (B) Snapshot of apoflavodoxin and hard spheres of the size of Ficoll 70 (volume fraction = 25%) as used in the simulations.
Ficoll 70 Increases Protein Thermal Stability in Vitro.
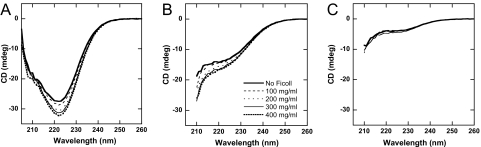
Far-UV CD at 222 nm was used to probe thermal unfolding of apoflavodoxin as a function of increasing Ficoll 70 concentrations (0–400 mg/ml, pH 7). All reactions appear as single cooperative transitions and are 70–80% reversible; in addition, there is no scan-rate dependence in accord with equilibrated reactions. As demonstrated in Fig. 2A, the presence of Ficoll 70 has a dramatic effect on apoflavodoxin thermal stability. The more Ficoll 70 is present in the samples, the higher the thermal midpoint (Tm) for apoflavodoxin. In fact, Tm increases from 45°C to 65°C, going from 0 to 400 mg/ml Ficoll 70 in Hepes buffer, pH 7 (Table 1).
Fig. 2.
In vitro thermal stability as a function of crowding agent. (A) Thermal unfolding curves for apoflavodoxin probed by far-UV CD in the presence of various amounts of Ficoll 70. (B) Tm vs. Ficoll 70 concentrations for apoflavodoxin in three different buffer conditions (squares, 10 mM Hepes; circles, 20 mM phosphate; diamonds, 40 mM phosphate plus 250 mM NaCl, all at pH 7).
Table 1.
Tm values (in kelvin) for apo-flavodoxin unfolding in different buffers (pH 7) combined with different amounts of Ficoll 70, as indicated
| Buffer | Bulk | 100 mg/ml | 200 mg/ml | 300 mg/ml | 400 mg/ml |
|---|---|---|---|---|---|
| Hepes | 317 ± 1 | 321 ± 1 | 325 ± 1 | 331 ± 1 | 337 ± 1 |
| Phosphate | 328 ± 1 | 330 ± 1 | — | 338 ± 1 | 341 ± 1 |
| Phosphate + NaCl | 342 ± 1 | 343 ± 1 | — | 346 ± 1 | — |
| Simulation | 365 | 372 (volume occupancy of 25%) | |||
The experimental midpoints were derived from CD-detected unfolding curves. All transitions were >80% reversible.
Apoflavodoxin is sensitive to buffer composition. For example, the protein is thermodynamically more stable in phosphate than in Hepes buffer (23). We found that by changing only the buffer conditions, the Tm value for apoflavodoxin can shift as much as 25°C (i.e., comparing phosphate plus salt vs. Hepes buffers at pH 7). This implies that population shifts within the native-state ensemble are possible and, moreover, that this ensemble contains a distribution of molecules with slightly different thermodynamic properties (30, 31). Nonetheless, the addition of Ficoll 70 always has a stabilizing effect on apoflavodoxin thermal stability, albeit the magnitude depends on the buffer choice (Table 1). The lower the protein stability without crowding agent in a particular buffer, the larger the stabilizing effect due to Ficoll 70 (Fig. 2B).
Structural Effects on Native State by Ficoll 70.
In Fig. 3A, we show the far-UV CD spectra of native apoflavodoxin in the presence of increasing amounts of Ficoll 70 (0–400 mg/ml; pH 7.0, 20°C). Unexpectedly, we find that the negative far-UV CD signal grows larger, suggesting gain of secondary structure, as a function of added crowding agent. The negative signal at 222 nm is raised by ≈10% in 200 mg/ml and by ≈15% at 400 mg/ml Ficoll 70. Thermally denatured apoflavodoxin (at 95°C, pH 7) also displays changes in the CD signal as a function of Ficoll 70 concentration, but the spectral shape remains characteristic of that of unfolded polypeptides (Fig. 2B). For comparison, we also collected CD spectra of apoflavodoxin unfolded in 3 M guanidine hydrochloride (GuHCl) as a function of Ficoll 70 additions (at pH 7, 20°C). As seen in Fig. 3C, the addition of Ficoll (up to 250 mg/ml tested) to the chemically denatured protein has no major effect on secondary structure content.
Fig. 3.
In vitro structural effects due to macromolecular crowding. Far-UV CD of (A) folded (pH 7, 20°C), (B) thermally unfolded (pH 7, 95°C), and (C) chemically unfolded (3 M GuHCl, pH 7, 20°C) flavodoxin in the presence of various amounts of Ficoll 70 (key for all in B).
Secondary structure estimations based on the far-UV CD spectra of folded flavodoxin (using the SOMCD neural network algorithm, http://geneura.ugr.es/cgi-bin/somcd/index.cgi) reveal that the helical content rises up to 20%, whereas the random coil contribution shrinks >10% when going from 0 to 400 mg/ml Ficoll 70 conditions (in buffer pH 7, 20°C). In the crystal structure of Desulfovibrio vulgaris flavodoxin (i.e., 2fx2, the closest structural homolog to D. desulfuricans flavodoxin in the Protein Data Bank), the protein has ≈37% helix, ≈29% β-sheet, and ≈34% random coil; interestingly, this composition is very similar to that estimated for our apoflavodoxin in 400 mg/ml Ficoll 70. Thus, with Ficoll 70, the mean of the native-state ensemble distribution in solution shifts toward a structure that is similar to that in the crystals. We note that when the same Ficoll 70 titrations were repeated with folded and unfolded forms of holoflavodoxin (i.e., with FMN bound), similar trends as for the apoform were observed (data not shown). In addition, we found that another crowding agent, Dextran 70 (a glucose polymer that adopts a rod-shape structure), also induces additional structure in folded flavodoxin (data not shown).
Simulated Effects of Crowding Matches in Vitro Data.
To assess the effects of macromolecular crowding on apoflavodoxin from a theoretical view, we computed thermodynamic properties and simulated the free-energy landscape for apoflavodoxin at different temperatures, with and without hard-sphere Ficoll 70 particles at a volume occupancy of φc = 25% (scenario depicted in Fig. 1B). The folding transition temperature was calculated from the temperature dependence of the Q parameter to be 365 K without crowder. As in the in vitro experiments, apoflavodoxin unfolds in a single thermal transition in silico that is shifted to higher temperatures (i.e., Tm of 372 K) at φc = 25% (data not shown). The stabilizing effect and the relative change in Tm value due to the presence of crowders agree well between in silico and in vitro data (Table 1).
The trend of enhanced native-like structure in the presence of crowders is seen from 1D energy profiles, F as a function of Q, at a temperature close to the folding transition [supporting information (SI) Fig. 7]. The shifts in the minimum of the folded state, from Q = 0.76 (for bulk) to Q = 0.80 (φc = 25%) and Q = 0.82 (φc = 40%), indicate augmentation of native-like structures in the folded ensemble in the presence of crowders. This type of native-state change was not observed in the previous investigation of the WW domain (11). This difference may be due to the fact that flavodoxin is longer (148 residues) and contains more complex secondary and tertiary structures than the WW domain (34 residues).
In Fig. 4, we show the resulting 2D energy landscapes for apoflavodoxin as a function of radius of gyration, Rg, and fraction of native contacts, Q, at T = 360 K for bulk (Fig. 4A) and φc = 25% (Fig. 4B) crowding conditions. Comparing Fig. 4 A with B, it is clear that the shape of the energy landscape shifts toward the low Rg region in the presence of crowder. The ensemble structures of apoflavodoxin become more compact (i.e., Rg decreases) when crowders are added. Notably, this is apparent for both the unfolded and the native-state ensembles. For the native-state ensemble, the average Rg is 4.052 ± 0.001σ (σ = 3.8 Å) for bulk conditions and 4.039 ± 0.001σ for φc = 25% conditions. Interestingly, despite the reduction in size of the native-state ensemble in the presence of crowders, the shape parameters remain the same in both cases (for bulk, Δ = 0.035 ± 0.001 and S = −0.011 ± 0.001; for φc = 25%, Δ = 0.036 ± 0.001 and S = −0.011 ± 0.001). This observation demonstrates that the effects on protein compaction due to crowding are isotropic.
Fig. 4.
In silico energy landscapes for apoflavodoxin with and without crowding. The 2D free-energy landscape as a function of Rg and Q at T = 360 K at volume fractions of crowder of zero (i.e., bulk) (A) and 25% (B). Rg is the radius of gyration in unit of σ (σ = 3.8 Å). Q is the fraction of native contacts. The color is scaled by kBT.
To reveal the molecular origin of the crowding-induced protein compaction and increased structural content, we derived difference contact maps of the folded states of apoflavodoxin between the φc = 25% and the bulk conditions (Fig. 5A). The same difference contact map was created between the two denatured states (Fig. 5B). Inspection of these maps reveal that the compaction of folded flavodoxin stems from improved interactions between the surrounding helices and the core β-sheet, as well as from less helix fraying in the terminal helices. Because helical contacts are stable in both bulk and crowded cases, these contacts do not show much of a color change in the difference map, although it is possible that the helices become more rigid in the presence of crowding agents. The extension of helices agrees well with the far-UV CD data that implied more α-helical content in folded apoflavodoxin at crowded conditions. In contrast, in the denatured state, crowding appears only to promote some native contacts between residue regions 70–90 (helix 2/loop region in folded protein) and 40–60 (loop/β-strand region in folded protein) (Fig. 5B). From an overall perspective, structural fluctuations in the native state, as measured by the rmsd for each residue, are diminished by a factor of 2 in the presence of crowders at φc = 25% (SI Fig. 8).
Fig. 5.
Mapping of in silico structural changes due to crowding against residue numbers. Difference contact map of the folded (A) and unfolded states (B) between 25% and 0% volume occupancy of crowders at T = 360 K. Secondary structure elements are indicated.
Discussion
This is a combined experimental/computational study elucidating the effects of molecular crowding on thermal and structural properties of a small single-domain globular α/β protein (i.e., 148-residue apoflavodoxin). Earlier experimental work has focused on the effects of crowding on kinetic refolding of complex proteins and on protein nonnative states at extreme conditions, whereas theoretical approaches have instead involved simple lattice models or small peptide systems (1, 9–12).
An equilibrium statistical-thermodynamic model, developed primarily by A. P. Minton (32), predicts that macromolecular crowding should increase protein thermal stability (Tm) by a magnitude of ≈5–20°C at physiological solute conditions. However, there have been few experimental verifications of this prediction. The reason is likely that many proteins unfold irreversibly and aggregate in the presence of high concentrations of crowding agents. The (irreversible) thermal midpoint for G-actin was found to increase by 5°C in the presence of 100 mg/ml PEG (33); the presence of 300 mg/ml dextran had a 3°C favorable effect on lysozyme thermal stability at pH 2 (19), and the presence of 370 mg/ml dextran raised Tm by 3.5°C for cytochrome c (34). In contrast to these modest effects, but in agreement with the prediction, we discovered that Ficoll 70 dramatically enhances apoflavodoxin (reversible) thermal stability: stabilizing effects of up to 20°C were observed in vitro. Notably, the stabilizing effect on apoflavodoxin due to crowding is similar to that observed for α-lactalbumin when placed in pores of silica glass (35, 36).
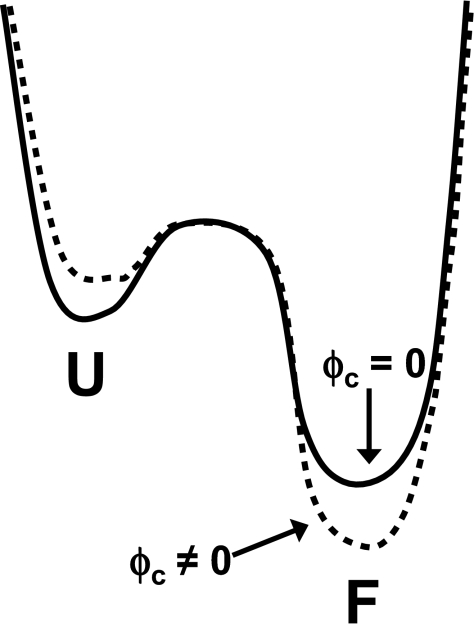
The excluded-volume theory implies that macromolecular crowding acts on unfolded-state conformations. By making the denatured state more compact and thereby less energetically favorable, the native state is indirectly stabilized (5–8). However, our experimental and computational observations of structural changes in both the native and denatured states of apoflavodoxin indicate that direct crowding effects on the native protein molecules are feasible (Fig. 6). We have observed both a compaction of the overall size of the native protein (i.e., effects on Rg) and a more native-like structure (i.e., more negative far-UV CD signal and Q value closer to 1) for apoflavodoxin in the presence of crowder. The structural effect appears larger in the folded than in the denatured ensemble although the compaction effect is more pronounced in the denatured ensemble.
Fig. 6.
Crowding affects both folded and unfolded ensembles. Schematic free-energy profile for flavodoxin folding. The enhanced stability at φc ≠ 0 (dashed curve) is due to a combination of unfolded-state destabilization and stabilizing of the folded state.
We also analyzed the changes in the folded and unfolded states due to crowding from a thermodynamic point of view. For this, the simulation data were used to compute the energy (which roughly corresponds to enthalpy), E, and the entropy, S (37), for folded and unfolded states in buffer and in 40% of crowders at 350, 360, and 370 K (SI Fig. 9 A and B). From this analysis, it emerges that there are energetic and entropic effects due to crowding in both unfolded (i.e., QU = 0.25) and folded states (i.e., QF = 0.8) of apoflavodoxin: for the ϕc = 40% condition, ΔEFU ≈ −19 kcal/mol, ΔSFU/kB ≈ −24 (S/kB is unitless) (kB, Boltzmann constant), and ΔGFU ≈ −1.72 kcal/mol at 360 K. For apoflavodoxin in buffer (i.e., bulk), ΔEFU ≈ −47.4 kcal/mol, ΔSFU/kB ≈ −66, and ΔGFU ≈ 0.12 kcal/mol at 360 K (SI Fig. 10). Interestingly, the crowding effects on folded- and unfolded-state entropy values are favorable. This may be explained by the existence of a large number of substates within the folded and unfolded ensembles as a result of crowder–protein interactions at high volume fraction of crowders. In contrast, the effect on the enthalpy/energy change due to crowding appears unfavorable, which may be explained by crowding-induced compaction having a negative effect on internal bonds (compression of structural bonds; these are modeled as harmonic springs).
Crowded conditions may cause enrichment of a large number of slightly different protein structures that are all within the native ensemble of molecules but have the highest degree of order. This can be achieved by a shift (and broadening in terms of number of substates) of the average distribution of species within the native-state ensemble toward Q = 1. In agreement, the difference contact map reveals that the secondary and tertiary structures are not altered per se, but that they become better packed and exhibit less breathing/terminal fraying in the presence of crowders. Thus, crowding forces the native ensemble of the protein molecules in solution to be on average more like they are in the crystalline state. We propose that native-state structural effects caused by macromolecular crowding may be common in vivo for globular proteins that exhibit marginal stability. The volume fraction of macromolecules may be as high as 40% in living cells. For proteins with inherent plasticity in their native states, protein–crowder interactions may play a role in the modulation of local conformations at active sites. In addition, macromolecular-crowding effects may be of high significance for intrinsically unstructured proteins (for example, FlgM), which may be unfolded in dilute solutions in vitro but adopt folded structures in the crowded in vivo environment (38).
Materials and Methods
Protein Preparation.
Flavodoxin from D. desulfuricans (American Type Culture Collection strain 29577) was expressed in Escherichia coli and purified as described (39, 40). In short, the apo-form of wild-type flavodoxin was isolated on a Q-Sepharose column and further purified by gel permeation on a Superdex-75 by using an FPLC system (Amersham–Amersham Pharmacia) (25).
In Vitro Measurements.
Apoflavodoxin native- and unfolded-state far-UV CD spectra (1-mm cell, 200–300 nm) were monitored at 20°C and 95°C, respectively, on a Jasco-810 spectrometer. Thermal unfolding experiments were carried out by following CD at 222 nm as a function of temperature. All experiments were performed in different buffers and in the presence and absence of various amounts of Ficoll 70 (Sigma). Three solvent conditions (10 mM Hepes, 20 mM phosphate, and 40 mM phosphate plus 250 mM NaCl, all at pH 7) were tested in separate experiments. Ficoll 70 was included in 100 mg/ml steps, from 0 to 400 mg/ml; higher concentrations of Ficoll 70 were not accessible due to limited solubility. Care was taken to let all buffer/Ficoll/protein mixtures equilibrate before measurements. Both unfolding (from 20°C to 95°C) and refolding (from 95°C to 20°C data points were collected every degree with a scan rate of 2.5°C per minute scan rate. Scan rates were varied between 0.1°C per minute to 2.5°C per minute; there was no scan-rate dependence observed in the process. All unfolding transitions were >80% reversible; aggregation was never observed. Differential scanning calorimetry tests were performed on Ficoll 70 solutions to assure that Ficoll itself did not exhibit any phase transitions within the temperature range of flavodoxin unfolding. These experiments revealed no detectable transitions for Ficoll (not shown). Assuming a spherical shape (28, 29), 50 mg/ml Ficoll 70 corresponds to a volume occupancy, φc, of ≈25%; 100 mg/ml to φc ≈ 50%; at Ficoll 70 concentrations >200 mg/ml; however, the spherical shape may be distorted (28, 29).
Model of Flavodoxin and Crowders.
We used a coarse-grained Cα side-chain model (Cα-SCM) (11, 41, 42) to represent apoflavodoxin [Protein Data Bank (PDB) ID no. 2FX2; this structure is for D. vulgaris flavodoxin, which is the closest structural analog to D. desulfuricans flavodoxin for which there is a PDB structure). Each amino acid (excluding glycine) is represented by using two interaction sites, one corresponding to the Cα atom and the other the center of mass of the side chain. A modified Go-like (43) interaction is used for mimicking protein-like behavior in which the principle of minimal frustration is implemented to construct a funnel-like folding energy landscape (44–46). The structural Hamiltonian that includes bond-length potential, side-chain backbone connectivity potential, bond-angle potential, and dihedral potential follows Go-like behavior in which equilibrium values are justified from the native structure.
The interaction energy for nonbonded native side-chain interactions is:
where a native contact between side chain i and side chain j, i−j >1, is determined from the native structure by using the CSU program (47). Moreover, σij = 0.9(σi + σj). We take σi to be the distance between the center of mass of side-chain atoms and the Cα atom, which is the effective van der Waals radius of a side chain. For the native contact energies εij, we use the Betancourt–Thirumalai (48) statistical potential. For backbone hydrogen-bond interactions, we consider an angular-dependent function to capture directional property of hydrogen bonds.
where A(ρ) is
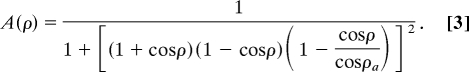 |
In this equation, ρ is the angular alignment between two interacting strands of backbones and defined in ref. 41. ρa is the pseudo dihedral angle of a canonical helical turn, 0.466 (rad). A native pair of hydrogen bonding between Cαi and Cαj is determined by using the DSSP program (49). εij for backbone hydrogen bonding between Cαi and Cαj is 2.5 kJ/mol. σij is the hydrogen bond length, 4.6 Å. The crowding particle, Ficoll 70, is modeled as an inert sphere with a radius of 55.0 Å, which is 3.8 times the radius of gyration (Rg) of flavodoxin in the native state (14.6 Å). Interactions between Ficoll 70 spheres and flavodoxin are repulsive. We choose to study volume occupancy of crowders, φc, of 25% and 40%. In most simulations, φc = 25% was used because of the limitation of computing resources.
Simulation Details and Calculations.
Thermodynamics properties are simulated in a periodic cubic box in which each length (L) is 300σ, and σ is 3.8 Å, the average distance between two adjacent Cαs in the Cα-side-chain model. L is chosen to be at least twice as long as the extended flavodoxin polypeptide. In addition, pair correlation functions between the Ficoll 70 particles in the box are computed, and L is large enough to ensure that the finite size effects are avoided in the calculation. Simulation procedures follow those in the previous study by Cheung et al. (11). The Replica Exchange Method (REM) (50) is used to enhance the sampling efficiency in the molecular simulations (51). Thermodynamic properties such as the radius of gyration (Rg), shape parameters‖ (Δ and S) (11), and the fraction of native contacts (Q) with respect to the crystal structure are computed to characterize the free-energy landscape of flavodoxin folding. The use of REM and Langevin equations of motion in the low friction limit (52) ensures that these thermodynamic properties, calculated with the weighted histogram analysis method (WHAM) (53) are converged.
Supplementary Material
Acknowledgments
Support for this project was provided by the Robert A. Welch Foundation (Grant C-1588, to P.W.-S.) and the USAMRAA (Grant W81XWH-06-1-0572, to P.W.-S.). M.S.C. is supported by the University of Houston. Computations were performed on the National Science Foundation Terascale Computing System (Teragrid, TG-MCB060051T) and at the Texas Learning Computation Center at the University of Houston.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705127104/DC1.
For a sphere, Δ is 0; S > 0 (< 0) corresponds to prolate (oblate) ellipsoid.
References
- 1.van den Berg B, Ellis RJ, Dobson CM. EMBO J. 1999;18:6927–6933. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivas G, Ferrone F, Herzfeld J. EMBO Rep. 2004;5:23–27. doi: 10.1038/sj.embor.7400056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Record MT, Jr, Courtenay ES, Cayley S, Guttman HJ. Trends Biochem Sci. 1998;23:190–194. doi: 10.1016/s0968-0004(98)01207-9. [DOI] [PubMed] [Google Scholar]
- 4.Ellis RJ, Minton AP. Nature. 2003;425:27–28. doi: 10.1038/425027a. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman SB, Minton AP. Annu Rev Biophys Biomol Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 6.Minton AP. Biophys J. 2005;88:971–985. doi: 10.1529/biophysj.104.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minton AP. J Pharmacol Sci. 2005;94:1668–1675. doi: 10.1002/jps.20417. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HX. J Mol Recognit. 2004;17:368–375. doi: 10.1002/jmr.711. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg B, Wain R, Dobson CM, Ellis RJ. EMBO J. 2000;19:3870–3875. doi: 10.1093/emboj/19.15.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uversky VN EMC. Bower KS, Li J, Fink AL. FEBS Lett. 2002;515:99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 11.Cheung MS, Klimov D, Thirumalai D. Proc Natl Acad Sci USA. 2005;102:4753–4758. doi: 10.1073/pnas.0409630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai X, Zhou Z, Bai Y, Choy WY. J Am Chem Soc. 2006;128:3916–3917. doi: 10.1021/ja057832n. [DOI] [PubMed] [Google Scholar]
- 13.Galan A, Sot B, Llorca O, Carrascosa JL, Valpuesta JM, Muga A. J Biol Chem. 2001;276:957–964. doi: 10.1074/jbc.M006861200. [DOI] [PubMed] [Google Scholar]
- 14.Rivas G, Fernandez JA, Minton AP. Proc Natl Acad Sci USA. 2001;98:3150–3155. doi: 10.1073/pnas.051634398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Alamo M, Rivas G, Mateu MG. J Virol. 2005;79:14271–14281. doi: 10.1128/JVI.79.22.14271-14281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatters DM, Minton AP, Howlett GJ. J Biol Chem. 2002;277:7824–7830. doi: 10.1074/jbc.M110429200. [DOI] [PubMed] [Google Scholar]
- 17.Tokuriki N, Kinjo M, Negi S, Hoshino M, Goto Y, Urabe I, Yomo T. Protein Sci. 2004;13:125–133. doi: 10.1110/ps.03288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu Y, Bolen DW. Biophys Chem. 2002;101–102:155–165. doi: 10.1016/s0301-4622(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 19.Sasahara K, McPhie P, Minton AP. J Mol Biol. 2003;326:1227–1237. doi: 10.1016/s0022-2836(02)01443-2. [DOI] [PubMed] [Google Scholar]
- 20.Muller F. Chemistry and Biochemistry of Flavoenzymes. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- 21.Steensma E, van Mierlo CP. J Mol Biol. 1998;282:653–666. doi: 10.1006/jmbi.1998.2045. [DOI] [PubMed] [Google Scholar]
- 22.Genzor CG, Perales-Alcon A, Sancho J, Romero A. Nat Struct Biol. 1996;3:329–332. doi: 10.1038/nsb0496-329. [DOI] [PubMed] [Google Scholar]
- 23.Muralidhara BK, Chen M, Ma J, Wittung-Stafshede P. J Mol Biol. 2005;349:87–97. doi: 10.1016/j.jmb.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 24.Muralidhara BK, Rathinakumar R, Wittung-Stafshede P. Arch Biochem Biophys. 2006;451:51–58. doi: 10.1016/j.abb.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Muralidhara BK, Wittung-Stafshede P. Biochemistry. 2003;42:13074–13080. doi: 10.1021/bi035073k. [DOI] [PubMed] [Google Scholar]
- 26.Muralidhara BK, Wittung-Stafshede P. Biochim Biophys Acta. 2005;1747:239–250. doi: 10.1016/j.bbapap.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Muralidhara BK, Wittung-Stafshede P. Biochemistry. 2004;43:12855–12864. doi: 10.1021/bi048944e. [DOI] [PubMed] [Google Scholar]
- 28.Luby-Phelps K, Castle PE, Taylor DL, Lanni F. Proc Natl Acad Sci USA. 1987;84:4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venturoli D, Rippe B. Am J Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 30.Vertrees J, Barritt P, Whitten S, Hilser VJ. Bioinformatics. 2005;21:3318–3319. doi: 10.1093/bioinformatics/bti520. [DOI] [PubMed] [Google Scholar]
- 31.Wrabl JO, Larson SA, Hilser VJ. Protein Sci. 2001;10:1032–1045. doi: 10.1110/ps.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minton AP. Biophys J. 2000;78:101–109. doi: 10.1016/S0006-3495(00)76576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tellam RL, Sculley MJ, Nichol LW, Wills PR. Biochem J. 1983;213:651–659. doi: 10.1042/bj2130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall D, Dobson CM. FEBS Lett. 2006;580:2584–2590. doi: 10.1016/j.febslet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Eggers DK, Valentine JS. J Mol Biol. 2001;314:911–922. doi: 10.1006/jmbi.2001.5166. [DOI] [PubMed] [Google Scholar]
- 36.Eggers DK, Valentine JS. Protein Sci. 2001;10:250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nymeyer H, Garcia AE, Onuchic JN. Proc Natl Acad Sci USA. 1998;95:5921–5928. doi: 10.1073/pnas.95.11.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dedmon MM, Patel CN, Young GB, Pielak GJ. Proc Natl Acad Sci USA. 2002;99:12681–12684. doi: 10.1073/pnas.202331299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helms LR, Swenson RP. Biochim Biophys Acta. 1991;1089:417–419. doi: 10.1016/0167-4781(91)90190-w. [DOI] [PubMed] [Google Scholar]
- 40.Apiyo D, Wittung-Stafshede P. Protein Sci. 2002;11:1129–1135. doi: 10.1110/ps.3840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung MS, Finke JM, Callahan B, Onuchic JN. J Phys Chem B. 2003;107:11193–11200. [Google Scholar]
- 42.Klimov DK, Thirumalai D. Proc Natl Acad Sci USA. 2000;97:2544–2549. doi: 10.1073/pnas.97.6.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udea Y, Taketomi H, Go N. Int J Peptide Res. 1975;7:445–459. [PubMed] [Google Scholar]
- 44.Onuchic JN, Luthey-Schulten Z, Wolynes PG. Annu Rev Phys Chem. 1997;48:545–600. doi: 10.1146/annurev.physchem.48.1.545. [DOI] [PubMed] [Google Scholar]
- 45.Shea JE, Brooks CL., 3rd Annu Rev Phys Chem. 2001;52:499–535. doi: 10.1146/annurev.physchem.52.1.499. [DOI] [PubMed] [Google Scholar]
- 46.Clementi C, Nymeyer H, Onuchic JN. J Mol Biol. 2000;298:937–953. doi: 10.1006/jmbi.2000.3693. [DOI] [PubMed] [Google Scholar]
- 47.Sololev V, Wade R, Vriend G, Edelman M. Proteins. 1996;25:120–129. doi: 10.1002/(SICI)1097-0134(199605)25:1<120::AID-PROT10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Betancourt MR, Thirumalai D. J Mol Biol. 1999;287:627–644. doi: 10.1006/jmbi.1999.2591. [DOI] [PubMed] [Google Scholar]
- 49.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 50.Sugita Y, Okamoto Y. Chem Phys Lett. 1999;314:141–151. [Google Scholar]
- 51.Sanbonmatsu KY, Garcia AE. Proteins. 2002;46:225–234. doi: 10.1002/prot.1167. [DOI] [PubMed] [Google Scholar]
- 52.Veitshans T, Klimov D, Thirumalai D. Fold Des. 1997;2:1–22. doi: 10.1016/S1359-0278(97)00002-3. [DOI] [PubMed] [Google Scholar]
- 53.Chodera JD, Swope WC, Pitera JW, Seok C, Dill KA. J Chem Theor Comput. 2007;3:26–41. doi: 10.1021/ct0502864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.