Abstract
Dendritic cells (DCs) represent key professional antigen-presenting cells capable of initiating primary immune responses. A specialized subset of DCs, the Langerhans cells (LCs), are located in the stratified squamous epithelial layer of the skin and within the mucosal epithelial lining of the vaginal and oral cavities. The vaginal mucosa undergoes cyclic changes under the control of sex hormones, and the renewal characteristics of the vaginal epithelial DCs (VEDCs) remain unknown. Here, we examined the origin of VEDCs. In contrast to the skin epidermal LCs, the DCs in the epithelium of the vagina were found to be repopulated mainly by nonmonocyte bone-marrow-derived precursors, with a half-life of 13 days under steady-state conditions. Upon infection with HSV-2, the Gr-1hi monocytes were found to give rise to VEDCs. Furthermore, flow cytometric analysis of the VEDCs revealed the presence of at least three distinct populations, namely, CD11b+F4/80hi, CD11b+F4/80int, and CD11b−F4/80−. Importantly, these VEDC populations expressed CD207 at low levels and had a constitutively more activated phenotype compared with the skin LCs. Collectively, our results revealed mucosa-specific features of the VEDCs with respect to their phenotype, activation status, and homeostatic renewal potential.
Keywords: Langerhans cell, monocyte, mucosa, viral infection
Dendritic cells (DCs) are the most potent initiators of adaptive immune responses (1). Several DC subsets have been defined based on their surface markers, anatomical localization, morphology, and lineage. The Langerhans cells (LCs) reside within the stratified squamous epithelial layer of the skin and of the mucosal epithelial layer lining the ocular, vaginal, cervical, and oral surfaces (2–4). LCs are well situated and equipped to ingest foreign antigens that breach these protective layers. Upon activation, LCs increase their expression of MHC class II and costimulatory molecules and migrate to the T cell areas of regional lymph nodes (LNs), where they initiate adaptive immune responses by presenting antigenic peptides to naive lymphocytes (5). However, in the mouse model of HSV infection, LCs have been found to be dispensable for generation of effector T cell responses (6, 7). Additionally, conflicting results have been found as to whether LCs are involved in the priming of T cells during contact hypersensitivity (8–10). Thus, the importance of LCs in the generation of immunity vs. tolerance remains quite controversial.
The discoveries that skin LCs are renewed by local noncirculating precursors under steady-state conditions (11) and that only under inflammatory conditions do Gr-1hi monocytes migrate into the skin and give rise to LCs (12) reveal the unique biology of the skin LCs compared with dermal DCs or blood-derived DCs. In murine blood, at least two distinct types of monocytes are present, characterized by low (Gr-1lo) or high (Gr-1hi) expression of Gr-1. Gr-1lo monocytes express high levels of CX3CR1, whereas Gr-1hi monocytes express CC chemokine receptor (CCR)2 and moderate levels of CX3CR1 (13, 14). Moreover, CCR2 expression on Gr-1hi monocytes is indispensable for their recruitment into peripheral tissues under inflammatory conditions (15). Whether monocytes give rise to DCs in the vagina during the normal female sexual cycle or under inflammatory conditions is unknown.
Sexually transmitted infections (STIs) with HIV-1, gonorrhea, chlamydia, trichomoniasis, candidiasis, and HSV-2 are transmitted via the genital mucosa and represent a major threat to human health worldwide. Despite being the major transmission route for these STIs and, therefore, of tremendous importance in prevention and treatment, the immunology of the reproductive tract remains severely understudied. Unlike the external skin, the female reproductive tissues undergo cyclical changes that are governed by the sex hormones, including changes in both the epithelial layer and the accompanying LC populations. In the murine vaginal epithelium, four subgroups of the vaginal epithelial dendritic cells (VEDCs) have been described based on immunohistochemical analysis of the MHC class II molecules and other DC markers (16). The four types of VEDCs are defined as I-A+/F4/80+, I-A+/F4/80−, I-A+/CD205+, I-A+/CD205−, and none of these populations expresses macrophage markers MOMA-1 or MOMA-2. The VEDCs have been found to contain Birbeck granule-like structures that fail to form the typical tennis-racket shape (17). Similar to the skin LCs, VEDCs have been shown to take up transluminally applied FITC-labeled tracers (18). Collectively, these studies underscore the complexities of the VEDCs in the murine vaginal epithelium that occupy their niche under hormonal influences. Although DCs in the vaginal epithelium have been assumed to be similar to the LCs present in the skin epidermis, their homeostasis and lifecycle remain unclear. Here, by employing a variety of in vivo approaches, we examined the origin and the phenotype of DCs that repopulate the vaginal epithelia of mice.
Results
Origin of the VEDC.
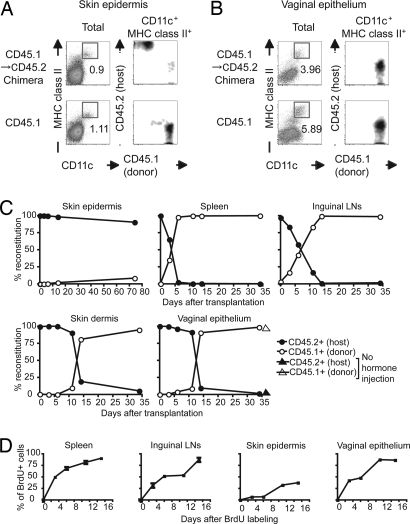
In lethally irradiated mice reconstituted with bone marrow (BM) transplants, LCs in the skin are repopulated by the host-derived precursors under steady-state conditions (11). To examine the homeostasis of the DC in the epithelia of the vagina, we used three distinct approaches. First, we examined VEDC turnover by using whole body irradiation, a procedure used by many to determine the turnover rate of various cell types in vivo. To this end, we reconstituted lethally irradiated CD45.2+ mice with BM cells isolated from the congenic CD45.1+ donor mice. Eight weeks after transplantation, chimeric mice were treated with progesterone (Depo-Provera) to synchronize them into diestrus. As reported in refs. 6, 11, 19, and 20, LCs in the skin were found to remain of the host (CD45.2) origin (Fig. 1A). In contrast, VEDCs were found to be repopulated by the progenitors of the BM origin (CD45.1) (Fig. 1B). To determine the turnover rate of VEDCs, we examined the percentages of donor vs. host by flow cytometry. After irradiation and transplantation with donor BM cells, VEDCs were found to be repopulated by donor-derived precursors with a half-life of 13 days (Fig. 1C). The rapid repopulation of the VEDCs was not attributable to the influence of the injected hormones, because mice that had not been injected with Depo-Provera also reconstituted VEDCs with similar kinetics (Fig. 1C). Indeed, a large number of CD45.1+ cells (donor) were rapidly recruited into the vaginal epithelium, but not the skin, after BM transfer [supporting information (SI) Fig. 6]. Thus, these data indicate that VEDCs repopulate from circulating BM precursors.
Fig. 1.
VEDCs repopulate from BM-derived precursors at steady state. CD45.2+C57BL6 mice were lethally irradiated and injected with BM cells isolated from CD45.1+ congenic mice. (A and B) Eight weeks after BM transfer, these BM chimeras were treated with Depo-Provera. Five days later, single-cell suspensions of the epidermis of the skin (A) or the epithelium of the vagina (B) were prepared and analyzed by flow cytometry. The DCs (CD11chi MHC class IIhi cells) were gated, and their expression of CD45.1 and CD45.2 was analyzed. These data are representative of six similar experiments. (C) The time courses of DC reconstitution in the spleen, CLNs, skin epidermis, skin dermis, and vaginal epithelium were examined. The host mice (CD45.2) injected with Depo-Provera or PBS 7 days before lethal irradiation were transplanted with donor BM cells (CD45.1). At the indicated time points, the ratio of donor vs. host-derived DCs (CD11chiMHC class IIhi) was analyzed by flow cytometry. (D) BrdU-labeling kinetics of DCs from the indicated organs was analyzed during continuous administration of BrdU in the absence of irradiation. The y axis depicts the percentages of the CD11c+MHC II+ cells that are BrdU+ within the indicated tissue.
Next, we measured the labeling kinetics of VEDCs in mice during continuous administration of BrdU (21). The labeling kinetics of VEDCs demonstrates how quickly these cells arise from precursor cells at steady state. As reported in ref. 21, skin LCs incorporated BrdU very slowly, whereas splenic DCs were rapidly repopulated by dividing precursor (Fig. 1D). Compared with their skin counterpart, VEDCs became labeled with much faster kinetics (Fig. 1D).
Furthermore, we used a recently described method of investigating DC regeneration in DC-depleted mice (22). To this end, we used the CD11c–DTR → WT BM chimeric mice (SI Materials and Methods). By removing the diphtheria toxin receptor (DTR) from the stromal compartment, we were able to carry out long-term diphtheria toxin (DT) injection and DC depletion without lethality, as described in refs. 23 and 24). Repeated DT injection eliminated CD11c+GFP+ DCs in various organs but not in the skin epidermis, because skin LCs remained of the WT origin (SI Fig. 7 A and B). Total BM cells from CD45.1 congenic mice were transferred into DC-depleted mice, and their ability to reconstitute VEDC was examined (SI Fig. 7 C). DC depletion in the CD11c–DTR → WT chimeric recipients promoted efficient repopulation of VEDCs by the donor BM cells within 10 days of transplantation (SI Fig. 7 C), and similar repopulation of lung DCs also occurred after BM transplantation, as described in ref. 22. Collectively, these data indicate that, unlike the skin LCs, VEDCs rapidly repopulate from BM precursors at steady state.
Phenotypic Analysis of the DCs in the Vaginal Epithelia.
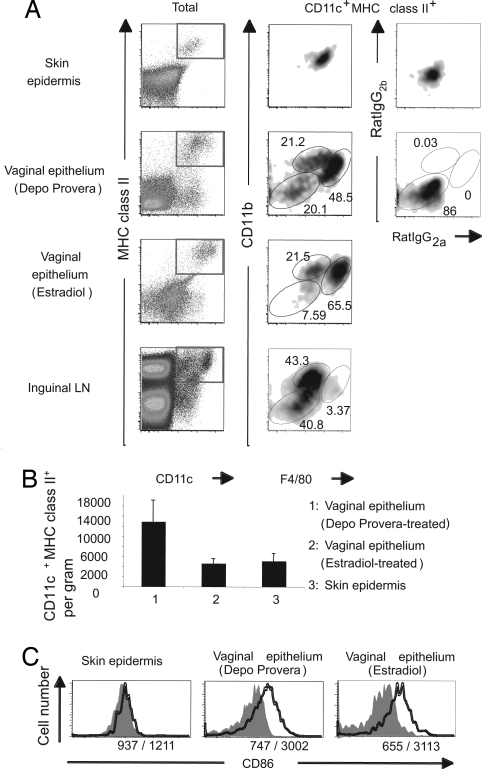
To better understand the nature of the VEDCs, we examined the expression of various phenotypic markers on VEDCs. Single-cell suspensions of the vaginal epithelial layer were stained for CD11c, MHC class II, CD11b, and F4/80. This analysis revealed that in progesterone-treated mice, VEDCs (CD11chiMHC class IIhi) could be subdivided into three distinct populations, CD11b+F4/80hi, CD11b+F4/80int, and CD11b−F4/80− (Fig. 2A). In the vaginal epithelium of mice treated with estradiol, a reduced number of the CD11b−F4/80− VEDC population was observed. In contrast to VEDCs, the LCs in the skin epidermis consisted of only one dominant population being CD11c+MHC II+F4/80+CD11b+. Furthermore, in the inguinal LNs draining the vagina and the skin, a minor population of CD11b+F4/80hi DCs was detected (Fig. 2A). Consistent with our previous report (7), the CD11c+MHC II+ population was abundantly present in the vaginal epithelium at the diestrous stage after progesterone treatment. During diestrus, the density of DCs present in the vaginal epithelium increased by >3-fold compared with the estrous stage induced by estradiol treatment (Fig. 2B). The frequency of VEDCs in estrus was similar to that of LCs present in the epidermis of the external skin (ear) of the mouse.
Fig. 2.
Phenotypic analysis of DC populations in the vaginal epithelium. The epidermis of the skin and the epithelium of vagina were separated from the dermis and lamina propria, respectively. (A) CD11chiMHC class IIhi cells were analyzed for CD11b and F4/80 expression. (B) Average numbers of DCs per gram of tissue in the vaginal epithelium after Depo-Provera or 17β-estradiol treatment or in the skin epidermis of the ear are shown. Each bar represents the mean ± SE of six independent experiments. (C) CD86 expression on DCs from the vaginal epithelium and the skin epidermis was analyzed. The histograms depict CD86 (solid lines) or isotype-matched control (gray filled area) staining of DCs. The numbers indicate the mean fluorescence intensities of isotype-matched Ab/anti-CD86 Ab. Three separate experiments were repeated and produced similar results.
To examine the activation status of the skin LCs and VEDCs under steady-state condition, we analyzed their respective expression of the costimulatory molecule CD86. Epidermal LCs of the skin displayed low levels of CD86 (Fig. 2C). In contrast, higher levels of CD86 expression were detected on the VEDCs at diestrous and estrous stages. Consistent with this expression pattern, MHC class II levels on VEDCs were also higher than on skin LCs (Fig. 2A). Thus, these results indicate that VEDCs comprise at least three distinct subsets and that they exhibit a constitutively activated phenotype in the vaginal epithelium in situ.
Langerin Expression on DCs in the Skin Epidermis Vs. Vaginal Epithelium.
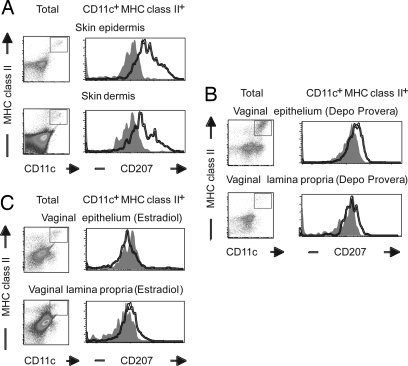
Langerin (CD207) is specifically expressed on the LC-specific intracellular organelles of skin LCs known as Birbeck granules (25). Thus, we examined whether VEDCs express CD207 by using the mAb 929F3 to detect intracellular CD207 (26). Our analyses revealed that CD207 was highly expressed in the LCs of the skin epidermis (Fig. 3A). A subpopulation of the MHC class II+ DCs in the dermis also expressed CD207, likely representing the LCs in transit. In contrast, in the vaginal epithelium and lamina propria, low levels of CD207 were detected within CD11c+MHC class II+ DCs at diestrous stage after Depo-Provera treatment (Fig. 3B). The level of CD207 on VEDCs at estrus after estradiol treatment became almost undetectable (Fig. 3C). The reduced level of CD207 protein expression correlated with the reduction in the mRNA level of CD207 by VEDCs compared with skin LCs (SI Fig. 8). Furthermore, we asked whether VEDCs were able to express high levels of CD207 if they resided within the vaginal epithelium for a much longer time period. Of the small number of VEDCs that remained in the vagina after day 34 of irradiation, none expressed CD207 at the level of the skin LCs, regardless of whether the mice received progesterone injection or not (SI Fig. 9). In the skin, the long-lived host LCs maintained CD207 expression throughout the time course. Thus, these data indicated that the VEDCs were not able to up-regulate CD207 expression despite their ability to remain within the epithelial layer for >30 d.
Fig. 3.
Reduced expression of CD207 by VEDCs. Single-cell suspensions were prepared from the epidermis and dermis of skin (A) and the epithelium and lamina propria of vagina from Depo-Provera-treated (B) or 17β-estradiol-treated (C) mice. CD11chi MHC class IIhi cells were analyzed for CD207 expression. Three separate experiments were repeated and produced similar results.
Comparison of DC Phenotype in the Skin Vs. Vagina and in Their Respective Draining Lymph Nodes.
Our results indicated that VEDCs consisted of at least three populations, all of which repopulate from the BM precursors at steady state, and express reduced levels of CD207. To understand the nature of the respective LCs upon migration to the regional LN at steady state, we examined the phenotype of tissue-derived DCs in the cutaneous (C)LNs and vagina-draining LNs. Previous studies have indicated that CD8α+ blood DCs express intermediate levels of the CD207, whereas LC-derived cells express high levels of CD207 in the CLNs (10). Upon arrival to the LN, epidermal LCs can be distinguished from other CD11c+ DC subsets by the CD4−CD8αloCD205hi phenotype and the expression of high levels of CD207 (10). Our flow cytometric analysis of the inguinal CLNs revealed the presence of CD207hi DCs (SI Fig. 10 B, arrowhead), whereas the iliac LNs, which drain the vaginal tract but do not drain external skin, contained only the CD8α+CD207int population (SI Fig. 10A). The LC-derived cells in the CLNs were CD8α−CD11bintCD205hi and CD86hi (SI Fig. 10 B), a phenotype consistent with a previous study (27).
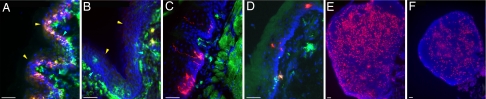
Next, we examined CD207 expression in DCs that reside within the skin and vagina in situ. Consistent with the flow cytometric data, CD207 was below the limit of detection by immunofluorescence staining on the MHC class II+ DCs within the internal vaginal mucosal epithelium (Fig. 4B), whereas, in the external vaginal skin (cornified with hair follicles), epidermal LCs expressed abundant CD207 (Fig. 4A). Correspondingly, in the CLNs, CD207hi LC-derived cells were found within the inner paracortex of the T cell area (Fig. 4C), as described in ref. 10. In contrast, although the vagina-draining iliac LNs contained CD207+ population that localized in the T cell area, the intensity of CD207, as well as the frequency of these cells, was much reduced (Fig. 4F) compared with the CLNs (Fig. 4E). These data indicate that VEDCs have reduced CD207 expression, and consequently, in the vagina-draining LNs, CD207hi DCs are absent. It was unclear whether the reduced expression of CD207 was a feature common to other mucosal stratified epithelial layers. To examine this issue, CD207 expression was assessed in the epithelial layers of the tongue (Fig. 4C) and the esophagus (Fig. 4D). In contrast to the vaginal epithelial layer (Fig. 4B), the intensity of CD207 of the LCs that reside in the tongue and esophageal epithelial layers was comparable to that of the skin LCs (Fig. 4A). Therefore, the reduced expression of CD207 is a characteristic of only the VEDCs and not other mucosal LCs.
Fig. 4.
CD207 expression in VEDCs within the internal vs. external vaginal epithelia. Frozen sections of external vaginal skin (A), the internal vaginal mucosa (B), tongue (C), and esophagus (D) of Depo-Provera-treated mice were labeled with antibodies against MHC class II (green) and CD207 (red). CLNs (E) and iliac LNs (F) were labeled with anti-CD207 Ab. Nuclei were stained with DAPI (blue). (Scale bars, 100 μm.) Yellow and blue arrowheads depict the luminal and basal edges of the epithelial layer, respectively. These images are representative of four similar experiments.
Characterization of VEDC Precursors Under Steady-State and Inflammatory Conditions.
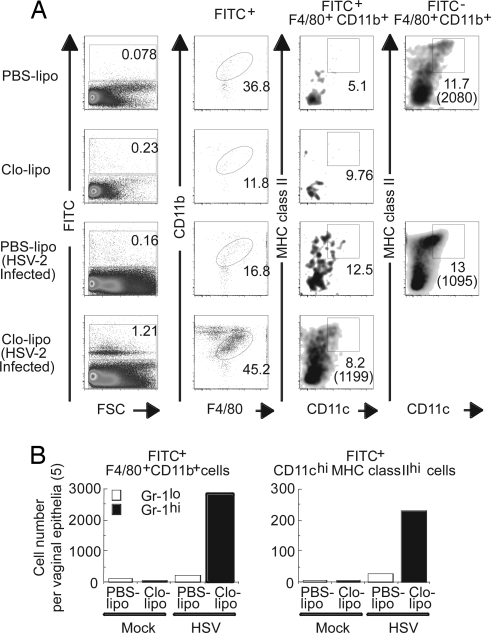
Our data thus far demonstrated distinct features of VEDCs compared with skin LCs with respect to their turnover, phenotype, and maturation status. These findings suggest that VEDCs may originate from precursors that are distinct from those of skin LCs. To examine whether Gr-1lo or Gr-1hi monocytes in the blood are the direct precursors of VEDCs, we used an established technique for labeling monocytes with i.v. injection of FITC beads. As reported in ref. 28, treatment of mice with PBS liposome (PBS-lipo) or clodronate liposome (Clo-lipo) selectively labeled Gr-1lo or Gr-1hi monocytes (CD11b+F4/80+) in the blood and spleen, respectively (SI Fig. 11A). By using this technique, we examined whether Gr-1lo or Gr-1hi monocytes extravasate into the vaginal epithelium. Under steady state, only a few Gr-1lo or Gr-1hi monocytes were recruited into the vaginal epithelia after FITC bead inoculation (Fig. 5). Monocyte-derived DCs at steady state remained only a minor population (Fig. 5A). In contrast, under an inflammatory condition elicited by intravaginal HSV-2 infection, a large number of Gr-1hi, and to a lesser extent Gr-1lo, monocytes migrated into the vaginal epithelium and gave rise to CD11c+ MHC class II+ DCs (Fig. 5 A and B). After HSV-2 infection, the total number of Gr-1hi monocytes and Gr-1hi monocyte-derived DCs in the vaginal epithelium increased by >50-fold (Fig. 5B). In addition, the percentage of Gr-1hi monocyte-derived DCs in the vagina increased by 17-fold in response to HSV-2 infection (SI Fig. 11 D), whereas the percentage of the Gr-1hi monocytes in the blood remained constant (SI Fig. 11E). Thus, these data indicated that, from the same frequency of circulating Gr-1hi monocytes, these cells selectively migrated and differentiated into VEDCs in the inflamed vagina.
Fig. 5.
Recruitment and differentiation of monocyte subsets at steady state and after vaginal HSV-2 infection. Gr-1lo or Gr-1hi monocytes were selectively labeled with FITC bead injection after PBS-lipo or Clo-lipo treatment 1 day before. (A) Recruitment of Gr-1lo and Gr-1hi monocyte populations into vaginal epithelium under normal and inflammatory conditions (3 days after intravaginal HSV-2 infection) was assessed. CD11c and MHC class II expression on monocyte-derived cells was analyzed. The numbers in parentheses indicate mean fluorescence intensity of CD11c expression. For comparison, the phenotype of vaginal F4/80+CD11b+ cells that did not incorporate FITC beads is shown. (B) Average cell numbers (n = 5) of FITC+ F4/80+CD11b+ monocytes and FITC+ monocyte-derived CD11chiMHC class IIhi DCs in the vaginal epithelial layers are depicted.
To examine whether monocytes contribute to VEDC generation at steady state in the course of a longer time period, mice were injected with Depo-Provera (SI Fig. 11 C) and treated with either PBS-lipo or Clo-lipo. VEDC populations were analyzed 12 days after FITC bead inoculation. These data indicated that Gr-1hi and Gr-1lo monocytes gave rise to a very minor but significant VEDC population in the absence of infection, regardless of whether mice were arrested in diestrus (SI Fig. 11 C) or undergoing normal sexual cycles (data not shown). Furthermore, we used another method to examine whether the Gr-1hi monocytes gave rise to DCs by using the CD11c–DTR → WT BM chimeras. DT-induced DC depletion in the CD11c–DTR → WT recipients promoted efficient repopulation of lung DCs by the grafted Gr-1hi monocytes (SI Fig. 7 D) as described in ref. 22. In contrast, monocytes gave rise to a very small number of DCs in the vaginal epithelium and undetectable levels of skin LCs in the same animal (SI Fig. 7 D). Collectively, our data demonstrated (i) that monocytes account for a small minority of the VEDCs in the absence of infection and (ii) that Gr-1hi and, to a lesser extent, Gr-1lo monocytes give rise to DCs after HSV-2 infection in the vaginal mucosa.
Discussion
LCs are a specialized DC population that occupies the epidermis of the skin and the stratified epithelium of various mucosal surfaces. After lethal irradiation and BM transplantation, the skin LCs are repopulated by the stem cells of the host origin and provide a constant reservoir for the epidermal LCs and LC-derived DCs found in the CLNs (11). In our current study, we showed that the DCs in the epithelium of the vagina are repopulated mostly by circulating, nonmonocyte precursors of the BM origin under steady-state condition. The VEDCs consisted of at least three distinct populations, namely, CD11b+F4/80hi, CD11b+F4/80int, and CD11b−F4/80−. Also, in contrast to the LCs of the external skin and other mucosal surfaces, none of the DC populations in the vaginal epithelial layer expressed high levels of CD207. VEDCs exhibited a more activated phenotype than the skin LCs in the absence of infection or inflammation. After HSV-2 infection, the Gr-1hi monocytes gave rise to DCs in the vaginal epithelium. Collectively, these results indicate that the lineage, homeostasis, and differentiation of the DCs in the vaginal epithelium are distinct from those of the LCs in the skin epidermis.
On the basis of their distinct rate of turnover, DC precursors that repopulate the vaginal epithelium are likely different from those that give rise to skin LCs. Selective recruitment of such distinct precursors could be mediated by differential chemokine expression patterns in the vagina vs. the skin. In the skin, several chemokines are known to be secreted constitutively, including CXC chemokine ligand 12 and CC ligand (CCL)27 (29, 30), whereas the chemokine CCL2 is only secreted upon inflammation (31). The chemokines responsible for the recruitment of DCs into the vaginal epithelium are unknown. Our data excluded the possibility that the short half-life of the VEDCs per se renders them incapable of expressing high levels of CD207 within the vaginal microenvironment (SI Fig. 9). It is interesting to note that the “skin resident” LC precursors capable of expressing CD207 and forming Birbeck granules (11) fail to occupy a niche in the vagina but are able to repopulate the mucosal stratified epithelial layers in the tongue and esophagus.
Our analyses revealed the heterogeneous nature of the VEDCs compared with the skin LCs. As reported in ref. 32, freshly isolated epidermal LCs were CD11c+MHC class II+ F4/80+CD86lo (Fig. 2) and thus appeared to constitute a single homogenous population. In contrast, DCs in the epithelium of the vagina were found to contain at least three distinct populations based on their F4/80 and CD11b expression. All VEDCs expressed CD11c and MHC class II but consisted of CD11b+F4/80hi, CD11b+F4/80int, and CD11b−F4/80− populations. In the lung-draining LNs, three DC populations have been reported, namely, CD11b+F4/80+, CD11b−F4/80+, and CD11b−F4/80−. Of these three, only the CD11b−F4/80+ emigrated from the lung and presented antigen to cognate CD8 T cells (33). It will be important to determine which of the three VEDC populations migrate to the draining LN and mediate immune responses to sexually transmitted pathogens. With respect to their potential ability to participate in the generation of adaptive immune responses, we found that the VEDCs expressed higher levels of CD86 and MHC class II on the cell surface compared with skin LCs. These results raise a question as to whether such phenotypically mature VEDCs still maintain the capacity to phagocytose pathogens at the frontline of mucosal surface. VEDCs, particularly during late metestrus and early diestrus, have been shown to take up apoptotic keratinocytes (17). Thus, it will be important to examine the role of the three distinct VEDC populations in the context of infectious agents and in the maintenance of immunological tolerance (34) within the reproductive organs.
In an effort to determine the origin of the VEDCs, we examined the ability of Gr-1lo and Gr-1hi monocytes to give rise to VEDCs. Our data showed that these monocyte subsets gave rise to only a minor population of the VEDC pool in the absence of infection. Even though the vaginal environment might be considered to be constitutively “inflamed” based on high expression of inflammatory cytokines and chemokines (35), our data showed that inflammatory Gr-1hi monocytes are not the major precursor of VEDCs under normal female sexual cycle. In contrast, stimuli elicited by vaginal HSV-2 infection led to the recruitment of Gr-1hi monocytes and subsequent differentiation into VEDCs. However, our data do not exclude the possibility of nonmonocyte precursors giving rise to VEDCs after HSV-2 infection. In fact, the majority of VEDCs that accumulated in the inflamed vagina did not contain FITC beads (Fig. 5 and SI Fig. 11). Previous studies have identified nonmonocyte CD11c+MHC class II− population in the BM and blood as a direct precursor of DCs (36, 37). In addition, nonmonocyte pre-DCs capable of giving rise to splenic conventional (c)DCs have been found in the spleen (38). A clonogenic BM progenitor, macrophage/DC precursor (MDP), that differentiates into monocytes, macrophages, and splenic cDCs has also been identified (39). Both MDP and monocytes have been shown to give rise to small intestinal and lung parenchymal DCs (22). Indeed, we observed the differentiation of small numbers of VEDCs from both Gr-1lo and Gr-1hi monocytes at steady state. The nature of these and other DC precursors that give rise to VEDCs in the presence or absence of inflammation needs to be addressed in future studies.
In summary, our results support the view that the DCs in the vaginal epithelia are under mucosa-specific homeostatic regulation and that they undergo recruitment and differentiation processes that are distinct from those of the LCs in the epidermis of the skin. The VEDCs renew mainly from a circulating nonmonocyte hematopoietic precursor, differentiate into three distinct populations, and are in a constitutive state of activation. So far, the role of these three LC subpopulations is unknown. It remains an intriguing possibility that the three LC populations are endowed with distinct capacity to migrate and present antigen, allowing them to serve specific roles in both defense and immunoregulation. A better understanding of the homeostasis of the VEDCs and their function in immunity and tolerance will contribute to a rational design for immunotherapy against a variety of STI agents and cancers of the reproductive organs.
Methods
Animals.
Six- to 8-wk-old female C57BL6 (CD45.2+) or congenic C57BL6 mice B6.SJL-PtprcaPep3b/BoyJ (B6.Ly5.1) (CD45.1+) were obtained from The Jackson Laboratory and the National Cancer Institute. To maintain diestrous stage, mice were injected s.c. in the neck ruff with progesterone (Depo-Provera, Amersham Pharmacia/Upjohn) at 2 mg per mouse in a 100-μl volume (7). To synchronize the mice into estrus, they received 500 ng of 17β-estradiol (Calbiochem) in a 100-μl volume for 3 consecutive days (40). Control mice were injected with 100 μl of saline alone. In some experiments, mice pretreated with Depo-Provera were inoculated with 104 pfu of HSV-2 (186syn+) intravaginally by using a previously described protocol (7). All procedures used in this study complied with federal guidelines and institutional policies of the Yale Animal Care and Use Committee.
Isolation of DCs from the Vaginal Epithelium.
The vaginal tracts of groups of mice (3–5 mice per group) were processed to obtain single-cell suspensions. Vagina was separated from urethra and cervix and incubated in 4 mg/ml Dispase II (Roche) for 60 min at 37°C to separate the epithelium from the lamina propria. These tissues were cut into small pieces and digested with 0.425 mg/ml collagenase D and 30 μg/ml DNase I at 37°C for 10 min. To analyze the population in the lamina propria of vagina, the lamina propria were cut into small pieces and were further digested with 0.425 mg/ml collagenase D and 100 units/ml hyaluronidase (Sigma) and 30 μg/ml DNase I (Sigma) for 60 min. The resulting cells were filtered through a 70-μm filter and used for FACS analysis.
BM Transplantation.
BM transplantation was conducted according to a standard method (11). Briefly, the recipient mice were irradiated with two doses of 475 rad each, 3 h apart. BM was obtained and resuspended in sterile PBS at a concentration of 5.0 × 107 cells/ml. Irradiated recipient mice were reconstituted with 107 cells of the appropriate cell suspension by i.v. injection. The reconstituted mice were maintained in a clean facility for at least 8 wk to allow for complete engraftment with donor BM, or killed at various time points for analysis of DC reconstitution in various tissues. To analyze the rate of DC turnover, CD11chiMHC class IIhi DCs from the indicated organs were isolated at the various time points after irradiation and transplantation of BM cells. These mice were injected with Depo-Provera 7 days before irradiation to arrest sexual cycle, unless indicated.
Tracking FITC-Labeled Blood Monocytes to Vaginal Tissues.
Five days before HSV-2 infection or mock inoculation, mice were injected with Depo-Provera. To deplete monocytes in the blood, liposomes containing clodronate (250 μl per mouse) were injected into the tail vein. Clo-lipos and PBS-lipos were a gift from Roche and liposomes were generated as described in ref. 41. Twenty-four hours after Clo-lipo or PBS-lipo treatment, FITC-conjugated microspheres (0.5 μm) were injected into the tail vein to label blood monocytes as described in refs. 12, 27, and 28. Forty-eight hours later, these mice were infected intravaginally with 104 pfu of HSV-2 (186syn+) (42). Epithelia of vagina were separated from the lamina propria at 3 days after HSV-2 infection and were processed and analyzed by FACS as described above.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health U.S. Public Health Service Grants AI054359, AI062428, and AI064705 (to A.I.). N.I. was supported by a postdoctoral fellowship from the Japan Antibiotics Research Association Pfizer Infectious Diseases Research Fund. A.I. holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707179104/DC1.
References
- 1.Banchereau J, Steinman RM. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 3.Miller CJ, Shattock RJ. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 4.Valladeau J, Saeland S. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Proc Natl Acad Sci USA. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Jung S, Littman DR. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 15.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parr MB, Parr EL. Biol Reprod. 1991;44:491–498. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 17.Parr MB, Kepple L, Parr EL. Biol Reprod. 1991;45:252–260. doi: 10.1095/biolreprod45.2.252. [DOI] [PubMed] [Google Scholar]
- 18.Parr MB, Kepple L, Parr EL. Biol Reprod. 1991;45:261–265. doi: 10.1095/biolreprod45.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, et al. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 22.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- 25.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, et al. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 26.Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stossel H, Kapp M, Kammerer U, Douillard P, Kampgen E, Koch F, et al. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 27.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 28.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. J Immunol. 2001;166:5749–5754. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- 30.Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 31.Gautam S, Battisto J, Major JA, Armstrong D, Stoler M, Hamilton TA. J Leukoc Biol. 1994;55:452–460. doi: 10.1002/jlb.55.4.452. [DOI] [PubMed] [Google Scholar]
- 32.Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C. Blood. 1999;93:590–598. [PubMed] [Google Scholar]
- 33.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, et al. Ann NY Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 35.De M, Sanford TR, Wood GW. Dev Biol. 1992;151:297–305. doi: 10.1016/0012-1606(92)90234-8. [DOI] [PubMed] [Google Scholar]
- 36.del Hoyo GM, Martin P, Vargas HH, Ruiz S, Arias CF, Ardavin C. Nature. 2002;415:1043–1047. doi: 10.1038/4151043a. [DOI] [PubMed] [Google Scholar]
- 37.Diao J, Winter E, Chen W, Cantin C, Cattral MS. J Immunol. 2004;173:1826–1833. doi: 10.4049/jimmunol.173.3.1826. [DOI] [PubMed] [Google Scholar]
- 38.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 39.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 40.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. J Virol. 2005;79:3107–3116. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rooijen N, Sanders A. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 42.Lund JM, Linehan MM, Iijima N, Iwasaki A. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.