Abstract
The onconeural antigens appear to serve as tumor rejection antigens in the paraneoplastic neurologic disorders. Here, we used an unbiased peptide binding screen, followed by studies in HLA-A2.1 transgenic mice to identify naturally processed HLA-A2.1 restricted epitopes of the paraneoplastic cerebellar degeneration breast/ovarian cancer antigen cdr2. These mice were used to clone high-avidity cdr2-specific CD8+ T cells that recognize human tumor cells presenting endogenously loaded MHC class I-cdr2 peptide. T cells with this specificity were detected in the peripheral blood of two HLA-A2.1+ paraneoplastic cerebellar degeneration patients. We cloned T cell receptor (TCR) α and β genes from cdr2-specific T cells; electroporation of RNA encoding this TCR turned nonreactive donor T cells into efficient killers of human cdr2-expressing tumor cells. Cloned cdr2-specific TCR genes provide a clinically relevant means for immunologic targeting of human gynecologic cancers.
Keywords: gene therapy, gynecologic cancer, paraneoplastic cerebellar degeneration
A new strategy for treating cancer patients has emerged from efforts to stimulate CD8+ cytotoxic T lymphocytes (CTL) through vaccination or adoptive T cell transfer (1–3). This effort has relied on a growing list of human tumor antigens recognized by CTL. However, few of these tumor antigens are associated with naturally robust immune responses and spontaneous tumor rejection, and their clinical utility is unclear. The paraneoplastic neurologic disorders (PNDs), perhaps the best-known examples of naturally occurring tumor immunity (4–7), offer a different approach to this problem. For example, patients with paraneoplastic cerebellar degeneration (PCD) develop an antigen-specific and clinically robust antitumor immune response directed against their breast and ovarian carcinomas (5, 8). In one series, two-thirds of PCD patients (34 of 52) presented with neurologic symptoms before the diagnosis of cancer, 87% (45 of 52) had limited oncologic disease when diagnosed, and tumor could only be found by exploratory surgery in 4 of 52; by comparison, only 50–60% of unselected breast cancer patients and 25% of ovarian cancer patients present with limited-stage disease (9).
Expression of the PCD antigen, cdr2, is believed to be largely restricted to cerebellar Purkinje neurons (10). The immune system is thought to initiate PCD when cdr2 is abnormally made in breast or ovarian tumors, triggering an antitumor immune response that can break immune tolerance in the brain. Interestingly, cdr2 is expressed by a large proportion of breast (25%) and ovarian (60%) tumors from individuals who do not develop neurological disease (11). Taken together with observations of immune responses made in other PNDs (5–7), cdr2 expression, and perhaps immune responses to it, may develop independently of autoimmune responses. These observations suggest the possibility that some gynecologic cancer patients might benefit from cdr2-directed immunotherapy. In addition, the unique expression of cdr2 in tumor cells (outside of the brain) suggests that reagents capable of recognizing cdr2-expressing cells might be of diagnostic importance.
cdr2 is the only one of several genes cloned by using PCD antisera that is expressed in PCD tumors (11), and it is a cytoplasmic protein. cdr2 peptide-specific CTL have been detected in the peripheral blood of HLA-A2.1+ PCD patients (12) but not HLA-A2.1+ normal control individuals, suggesting that CTLs are critical components of the tumor immunity in PCD. Although CTL from PCD patients were found to lyse cdr2 peptide-pulsed target cells, immunodominant cdr2 epitopes are unknown.
To explore the possibility of establishing cdr2 as a target for immunotherapy, we have identified immunodominant cdr2 epitopes recognized by PCD patients. We screened a complete cdr2 peptide library for HLA-A2.1 binding and evaluated immunogenicity and natural processing using HLA-A2.1 transgenic mice previously used to evaluate human tumor-associated antigens (13–15). Immunization of these mice with adenovirus expressing human cdr2 allowed us to isolate a high-avidity CD8-independent cdr2-specific CTL clone and the genes encoding its T cell receptor (TCR) α- and β-chains. This TCR is clinically relevant, because two A2.1+ PCD patients harbor tetramer-positive T cells of the same specificity. Moreover, transfecting RNA encoding this TCR into normal donor CD8+ T cells conferred on them the ability to lyse human tumors endogenously expressing cdr2. These studies offer the possibility of a previously undescribed approach to breast and ovarian cancer diagnosis and treatment.
Results
Identification of cdr2 Peptides That Bind to HLA-A2.1.
To identify cdr2 immunodominant epitopes, we assayed a complete library of 446 overlapping human cdr2 peptide nonamers for high-affinity binding to HLA-A2.1 plate-bound monomers. After an initial screen for peptides binding >30% of control, HLA-A2.1 binding affinity was determined over a range of peptide concentrations (10−4 to 10−9 M), and MHC/peptide stability was determined by an off-rate assay. Peptides were given a net score integrating these data [iTopia iScore; supporting information (SI) Table 1]. Among the 38 top-scoring peptides (net scores >0.1; SI Table 1) were three previously found to be recognized by PCD patient CTLs (12, 16) [cdr2 (289–297), cdr2 (273–281), and cdr2 (355–363)). The best HLA-A2.1 binder was a previously unidentified peptide, cdr2(290–298; LLEEMFLTV; termed cdr2 (290)]. Because it is believed that epitope immunogenicity is correlated with MHC class I binding affinity and off-rate (15) for nonself- (17, 18) and self-antigens (19–21), we evaluated the cdr2 (290) peptide in greater detail.
Generation of High-Affinity Human cdr2 (290)-Specific CTLs in A2.1 Transgenic Mice.
We used HLA class I transgenic mice to screen for high-affinity cdr2-specific CTLs, because such mice have been used to study immune responses to human tumor antigens (13, 15). Immunizing AAD HLA-A2.1 transgenic mice with recombinant adenovirus-expressing full-length human cdr2 (Ad-hcdr2) yielded robust ex vivo CD8+ T cell responses (frequency >0.3%) specific for cdr2 (290); weaker responses were seen to six other peptides, and these were not pursued further. We observed poor reactivity to several A2.1 restricted peptides that were highly homologous or identical between human and mouse [for example cdr2 (289–297), which differs only in the C-terminal anchor residue; Fig. 1 A and B and data not shown], suggesting the possibility of self-tolerance to mouse cdr2. Because the human cdr2 (290) peptide sequence (LLEEMFLTV) is not identical to mouse cdr2 (290) sequence (LLEEMFLAA; Fig. 1B) in a region involving TCR recognition, we reasoned that high-affinity CTL might be more readily generated in HLA-A2.1 transgenic mice against the human cdr2 (290) epitope.
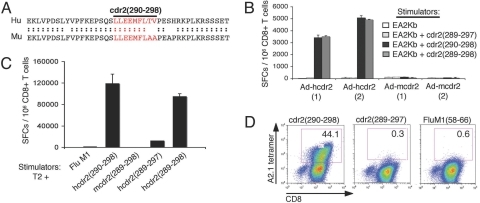
Fig. 1.
Characterization of cdr2 (290)-specific CTLs from Ad-hcdr2 immunized A2.1 transgenic mice. (A) Sequence homology of murine (Mu) and human (Hu) cdr2 surrounding residues 290–298. (B) AAD A2.1 transgenic mice were immunized with 109 pfu of Ad-hcdr2 or Ad-mcdr2 (two animals per group), and 12 days later splenic CD8+ T cells were cocultured with EA2Kb cells pulsed with human cdr2 (289–297), cdr2 (290), or cdr2 (289–298) peptide (10−5 M) in an 18-h IFN-γ ELISPOT assay. Spot-forming cells (SFCs) per 106 CD8+ T cells are shown (the average of triplicate wells, error bars indicate standard deviations). (C) Splenocytes from Ad-hcdr2-immunized AAD mice were stimulated with human cdr2 (290) for two rounds in vitro and assayed by IFN-γ ELISPOT using indicated peptide-pulsed T2 cells as stimulators. (D) CD8-independence of AAD 290 CTL. AAD 290 CTL were purified by CD8-negative selection and stained with the indicated HLA-A2.1 tetramers.
A cdr2 (290) peptide-specific CTL line (designated AAD 290) was generated from an Ad-hcdr2-immunized AAD mouse. The AAD 290 CTL recognized human cdr2 (290) but not murine cdr2 (289–298), murine cdr2 (290) (data not shown) or FluM1 peptide (derived from the influenza matrix protein) pulsed T2 cells in IFN-γ ELISPOT assays (Fig. 1C). Murine cdr2 (289–298) demonstrates strong binding to HLA-A2.1 (t1/2 = 8.7 h; ED50 = 5.93 E-07), suggesting that the inability of AAD 290 CTL to cross-react with the murine sequences is most likely due to the restriction of the AAD 290 CTL rather than inability of the murine peptides (harboring alanine residues at positions 8 and 9) to bind to HLA-A2.1. The human decapeptide cdr2 (289–298), which overlaps cdr2 (290) but is extended N-terminally by one residue, was recognized by AAD 290 CTL as efficiently as the nonamer (Fig. 1C). Small but reproducible cross-reactivity to human cdr2 (289–297) was also observed, but this response was 100-fold lower than the response to cdr2 (290) and was absent at concentrations of peptide <10−6 M.
AAD 290 CTL avidity was assessed by assessing binding to a cdr2 (290) A2.1 tetramer. A large proportion (≈40%) of AAD 290 CTL showed specific binding to A2.1/cdr2 (290) tetramers, with no detectable binding to cdr2 (289–297) or FluM1 A2.1 tetramers (Fig. 1D). Binding of murine CTL to human A2.1 tetramers is CD8-independent, because murine CD8 does not interact with the α3 domain of the A2.1 molecule, and such binding suggests high-affinity TCR-MHC/peptide interactions (22, 23). Taken together, these results indicate that high-avidity CTL specific for nonself human cdr2 epitopes can be generated in HLA-A2.1 transgenic mice.
cdr2 (290) Peptide Is Naturally Processed and Recognized in Tumor Cells.
Generating cdr2 (290)-specific T cells after Ad-hcdr2 immunization suggests that this epitope may be naturally processed. We assessed whether the AAD 290 CTL line responds to Ad-hcdr2 transduced HLA A2.1 transgenic kidney epithelial cells (KECs), which make no endogenous cdr2 protein (see below). AAD 290 CTL produced IFN-γ in ELISPOT assays after coculture with Ad-hcdr2-transduced KECs but not control KECs (Fig. 2A). Recognition of Ad-hcdr2-transduced HHD KECs, which have no mouse MHC molecules (Fig. 2A), indicated that this response was A2.1 restricted. Moreover, AAD 290 CTL responded to Ad-hcdr2 transduced AAA KECs, which have a human α3 domain, demonstrating that they were capable of CD8-independent recognition of endogenous human cdr2. Taken together, these results confirm that AAD 290 CTL recognize naturally processed cdr2 protein presented on human HLA-A2.1 molecules.
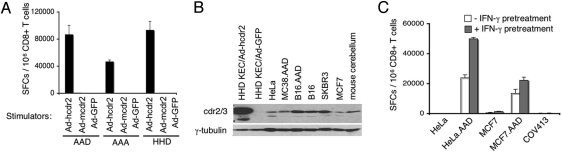
Fig. 2.
AAD 290 CTL recognize endogenous cdr2. (A) AAD 290 CTL were cocultured with KECs isolated from AAD, AAA, or HHD mice; KECs were infected with the indicated AdV constructs and used as stimulators in IFN-γ ELISPOT assay. (B) Western blot analysis of cdr2 protein expression tumor cell lines and normal tissue. The antibody used detects cdr2 and cdr3 as discussed (10); additional IP-Western blot analysis with a cdr2-specific antibody confirmed cdr2 expression from Ad-hcdr2, HeLa, COV413, and mouse cerebellum (data not shown). (C) AAD 290 CTL recognizes endogenous cdr2 in tumors. AAD 290 CTL were cocultured with the indicated tumor cells with (gray) or without (white) pretreatment with IFN-γ for 40 h before ELISPOT assay.
All A2.1 transgenic KECs have murine antigen processing machinery, raising the question of whether cdr2 is processed the same in human and mouse A2.1+ cells. We tested AAD 290 CTL for recognition of physiologic levels of endogenous cdr2 in human tumor cells. Several tumor cell lines were assessed for cdr2 expression [the breast cancer cell lines MCF7 (A2.1+) and SKBR3, HeLa cells (A2.1−), and an ovarian carcinoma cell line COV413 (A2.1+)]. Each appeared to express cdr2 by Western blot analysis, as did mouse cerebellum and KECs transduced with Ad-hcdr2, whereas primary KECs did not (Fig. 2B and data not shown).
We examined whether human tumor cells could stimulate the murine AAD 290 CTL in an IFN-γ ELISPOT assay. AAD 290 CTL showed small but consistent responses to MCF7 cells (1350 ± 212 SFCs) but not HeLa cells (150 ± 212 SFCs) or COV413 cells (350 ± 70 SFCs) (Fig. 2C). To facilitate recognition of these cells by the murine AAD 290 CTL, the murine α3 molecule was supplied by stably transfecting MCF7 and HeLa with the AAD molecule. The resulting cell lines, HeLa.AAD and MCF7.AAD, induced significant IFN-γ production in AAD 290 CTL (Fig. 2C), whereas KECs expressing the AAD molecule without the cdr2 antigen did not (Fig. 2A and data not shown). Moreover, specific recognition of cdr2 in tumor cells was enhanced by pretreating them with IFN-γ (Fig. 2C), a cytokine that increases MHC class I expression, antigen processing, and ICAM expression (24, 25). Neither treated nor untreated A2.1− HeLa cells were recognized, confirming that target recognition was A2.1-restricted. The relative lack of recognition of MCF7 cells and COV413 cells (albeit at levels that appeared higher than recognition of HeLa cells) is consistent with the inability of the murine CD8 molecule present on AAD 290 CTL to interact with the human α3 region of A2.1 on these cells. In conclusion, AAD 290 CTL are capable of recognizing endogenous cdr2 expressed in tumor cells, suggesting that these CTL may be a good source of cdr2-specific TCR.
Tetramer Analysis of Human CD8+ T Cells.
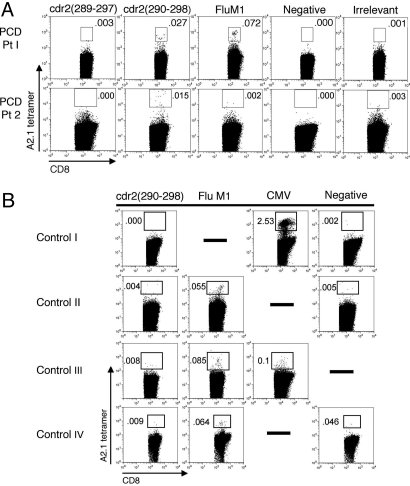
To examine whether our findings with the AAD 290 CTL line were relevant to human tumor immune responses, A2.1/cdr2 (290) tetramers were used to examine CD8+ T cells obtained from the peripheral blood of two A2.1+ PCD patients (clinical case histories in SI Methods). As controls, we used negative tetramers, HLA-A2.1/FluM1 (58–66) tetramers, or irrelevant tetramers. Peripheral blood lymphocytes (PBLs) of these A2.1+ PCD patients harbored T cells able to bind the cdr2 (290) tetramer (0.027 to 0.015% of CD8+ T cells) but not control tetramers (Fig. 3A). The cdr2 tetramer staining was specific to our PCD patients; it was not seen in a normal donor, two patients with the paraneoplastic Hu syndrome, and a neurologically normal ovarian cancer patient (Fig. 3B). PBLs from a third A2.1+ PCD patient obtained 4 months after neurologic deterioration gave ambiguous staining, and a fourth A2.1+ PCD patient had low but detectable cdr2 (290) tetramer staining (0.02%, vs. 0.005% with control tetramer, data not shown). These studies demonstrate that cdr2 (290) is a naturally processed and presented CTL epitope in humans and suggest that cdr2 (290) tetramer-positive T cells may correlate with the immunity to ovarian cancer.
Fig. 3.
Tetramer analysis of human CD8+ T cells in HLA-A2.1+ PCD patients. (A) PBLs from two A2.1+ PCD patients were stained with tetramers specific to cdr2, FluM1 (58–66), control [PSMA (4–12) in Pt 1, HuD (157–165) in Pt 2], or negative (Beckman Coulter) tetramer and analyzed by flow cytometry. Results are gated on CD8+ T cells. (B) Tetramer analysis of control patients: normal donor (I), two Hu PND patients (II and III), and a neurologically normal ovarian cancer patient (IV). Controls are FluM1 (Pt 1 harbored FluM1 tetramer-positive CD8+ T cells confirmed by IFN-γ ELISPOT assay; Pt 2 had no flu response), CMVpp65 (495–503), or negative A2.1 tetramers.
Cloning and Characterization of cdr2 (290)-Specific TCRs.
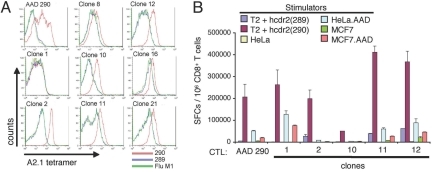
We cloned and characterized the genes encoding the cdr2-specific TCR from AAD 290 CTLs. Individual clones from the AAD 290 CTL line were screened by assessing their avidity and were isolated by limiting dilution. We assessed ability of clones to bind cdr2 (290) tetramer (Fig. 4A) and to produce IFN-γ in response to cdr2 (290) peptide-pulsed T2 cells or human cdr2-expressing tumor cell lines (HeLa or MCF7 cells; Fig. 4B). Clones 11 and 12 had both high binding affinity and functional avidity, and an identical TCR (SI Fig. 6) that was analyzed in greater detail.
Fig. 4.
Structural and functional avidity of cdr2-specific T cell clones. (A) CD8+ T cells from the AAD 290 CTL line (after the 6th in vitro restimulation) and eight daughter CTL clones were stained with A2.1/cdr2 (290) tetramer (red line) or control [A2.1/cdr2 (289–297) (blue line), or FluM1 (green line)] tetramers and analyzed by FACS. (B) The ability of bulk AAD 290 CTL or the indicated clones to recognize human cdr2 (289–297) or cdr2 (290) peptide-pulsed T2 cells (10−6 M) or the indicated cdr2-expressing tumor cells (pretreated with IFN-γ for 40 h) was evaluated in an 18-h IFN-γ ELIPSOT assay.
We tested the ability of the α-and β-chains encoded by clone 11/12 to form functional TCR αβ heterodimers. In vitro-transcribed α- and β-chain mRNA was coelectroporated into CD8+ normal human donor PBLs. More than 32% of CD8+ PBLs formed cell surface heterodimers capable of binding HLA-A2.1/cdr2 (290) tetramers (Fig. 5A). T cells electroporated in parallel with RNA encoding GFP did not show any tetramer staining but demonstrated that our electroporation was highly efficient (≈98% cells were GFP-positive; Fig. 5A). A control tetramer did not stain either α- and β-chain TCR or GFP mRNA electroporated T cells (Fig. 5A).
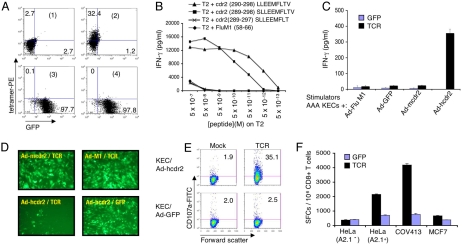
Fig. 5.
Characterization of a cdr2-specific T cell receptor. (A) Tetramer analysis of CD8+ PBL electroporated with cdr2-TCR mRNA. cdr2 TCR α- and β-chain (1 and 2) and GFP RNAs (3 and 4) were electroporated into 106 OKT3-stimulated CD8+ PBL. FACS analysis with negative (1 and 3) or cdr2 (290) tetramer (2 and 4) and percentage staining cells is shown. (B–F) Functional analysis of cdr2 TCR. Human CD8+ T cells were electroporated with cdr2 TCR, cocultured for 18 h with target cells [T2 cells pulsed with the indicated peptide (B), or AAA KECs-transduced with the indicated AdV constructs (C)], and IFN-γ secretion measured by ELISA. Values are the average of duplicates. In B, IFN-γ secretion in cocultures with GFP-electroporated CD8+ T cells was <15 pg/ml for all peptides (data not shown). (D) The cells in C were assessed for cell lysis by fluorescence microscopy. (E) FACS analysis for surface expression of CD107a after mock- or TCR-electroporated human CD8+ T cells were cocultured with Ad-hcdr2 or Ad-GFP-transduced AAA (A2.1) KECs. (F) cdr2 TCR-electroporated CD8+ T cells recognize cdr2-expressing gynecologic tumor cells. Human CD8+ T cells were electroporated with cdr2 TCR α- and β-chain (black bar) or GFP (blue bar) RNA and cocultured with the indicated tumor cells (5 × 104) in an 18-h IFN-γ ELISPOT assay; average of duplicate wells and SD are shown.
We next determined whether cdr2-specific TCR-electroporated CD8+ PBLs were functional by culturing them with target cells and measuring IFN-γ release. CD8+ T cells were electroporated with the cdr2 TCR α- and β-chain RNAs and cocultured with T2 cells pulsed with specific [cdr2 (290) and cdr2 (289–298)] or control [FluM1 and cdr2 (289–297)] peptides. IFN-γ release was detected when cdr2-specific TCR-electroporated CD8+ PBL were cocultured with T2 cells pulsed with cdr2 epitopes but not with control peptides (Fig. 5B). The cdr2-specific TCR electroporated CD8+ PBL populations were capable of releasing IFN-γ 20-fold above background at cdr2 (290) concentrations as low as 0.5 pM, with half-maximal IFN-γ secretion occurring at peptide concentrations of 35 pM. This represents a >3-log improvement in avidity from the original AAD 290 CTL line (6.8 nM, measured by ELISPOT, data not shown). The ability of the cdr2 α- and β-chain to confer specific recognition for both cdr2 (290) and cdr2 (289–298) (Fig. 5B) indicates that promiscuous recognition of these epitopes in the parental AAD 290 CTL line is due to cross-reactivity by a single TCR.
We investigated whether human T cells expressing the cloned cdr2 TCR could recognize cells expressing endogenous cdr2. cdr2-specific TCR-electroporated CD8+ PBL were cocultured with AAA A2.1 transgenic KECs (with a human α3 domain) transduced with Ad-hcdr2 or control vectors. IFN-γ release was detected in cocultures with Ad-hcdr2 but not control transduced cells (Fig. 5C). No IFN-γ release was seen in cocultures with GFP-transfected T cells (Fig. 5C).
We assessed the functional lytic activity of the TCR-electroporated T cells by fluorescence microscopy of targets and by FACS analysis of surface CD107a (LAMP1) mobilization in CD8+ T cells (a means of enumerating activated lytic CD8+ T cells). When cocultures of cdr2-specific TCR-electroporated CD8+ PBL and targets were observed by fluorescence microscopy, cdr2 TCR-PBLs, but not control GFP-PBLs, killed Ad-hcdr2 KECs but not control-transduced KECs (Fig. 5D). Moreover, 35% of cdr2 TCR-PBLs cocultured with Ad-hcdr2-transduced KECs became positive for surface CD107a expression (Fig. 5E), whereas only ≈2% of cdr2 TCR-PBLs cocultured with KEC-GFP or mock electroporated CD8+ T cells mobilized CD107a (Fig. 5E). The percentage of cdr2 TCR-PBL detected by tetramer analysis (32%; Fig. 5A) correlated well with the percentage that mobilized CD107a after detection of cdr2-expressing target cells (Fig. 5E), suggesting that the majority of cdr2-TCR-transfected T cells were functional.
cdr2 TCR-Expressing Human CD8+ T Cells Recognize cdr2-Expressing Gynecologic Tumor Cells.
Up to 60% of ovarian tumors and 25% of breast tumors from neurologically normal cancer patients express cdr2 (11). To determine whether cdr2-specific TCR might be suitable for targeting cdr2-expressing human tumors, we evaluated whether cdr2-specific TCR-electroporated CD8+ PBLs can recognize physiologic levels of endogenous cdr2 antigen in human tumor cell lines. cdr2 TCR-PBL, but not control GFP-PBL, were able to recognize A2.1− HeLa cells by IFN-γ ELISPOT only after they had been stably transfected with A2.1 (HeLa.A2.1+; Fig. 5F). In addition, a significant, but lower, number of cdr2 TCR-PBLs responded to the A2.1+ breast cancer cell line MCF7 (Fig. 5F). This level of response may relate to the fact that MCF7 have relatively low levels of A2.1 expression (e.g., one log lower than COV413, data not shown). Finally, cdr2 TCR-PBLs, but not GFP-PBL controls, were capable of robust recognition of A2.1+ COV413 ovarian tumor cells (Fig. 5F). This contrasts with the failure of the murine AAD 290 CTL line to recognize COV413 cells (Fig. 2C) and indicates that an A2.1 mouse-derived TCR in a human CD8 T cell has an enhanced ability to recognize human tumor cells relative to the parental murine clone, likely because of the human CD8 molecule adding to the avidity of the TCR:MHC-peptide interaction. Taken together, our results indicate that the cloned cdr2-specific TCR confers normal CD8+ T cells with the ability to become functional CTL able to recognize cdr2-expressing gynecologic tumor cells.
Discussion
We identified and characterized a CTL-MHC peptide interaction present in patients with naturally occurring tumor immunity to breast or ovarian cancers, leading to the isolation of a potentially diagnostic and therapeutic TCR specific for a human breast and ovarian tumor-associated antigen. Current interest in identifying such TCR derive from studies describing human tumor antigens recognized by CD8+ CTL. The use of CTL by vaccination or adoptive T cell transfer protocols is a major current strategy for treating cancer patients (1–3, 26). These include the use of TCRs specific to human-derived MART-1 (27, 28), NY-ESO-1 (29), and gp-100 (30) and the murine-derived MDM2 (31) and p53 (32, 33). Although these antigens are not generally believed to be associated with naturally occurring robust immune responses and spontaneous tumor regressions, evaluation of their clinical utility is ongoing.
cdr2 has attracted attention as a tumor rejection antigen for breast and ovarian cancer immunotherapy after three sets of observations. First, a series of clinical studies have shown that most PCD patients present with their neurologic disease unaware that they have gynecologic tumors, and that, when found, ≈90% of these cancers are at a limited stage and, in ≈8%, they can only be found after empiric surgery and microscopic examination (5, 8, 9), a profile reflected in the two case reports presented here. These initial studies were complemented by the discovery of cdr2 peptide-specific CTL in the peripheral blood of PCD patients (12) and by the observation that cdr2 is expressed by a large proportion of gynecologic tumors from individuals who do not develop neurologic disease (11). Taken together with clinical data from other PNDs suggesting frequent uncoupling of tumor immunity and autoimmunity (5, 6, 34), these observations encouraged us to search for naturally processed PCD-specific CTL epitopes relevant to gynecologic cancer.
Immunodominant PCD epitopes have not previously been identified, either from our own previous screens or from other studies (35–37). We find that previously identified candidates, as well as five new peptides, are naturally processed and presented by Ad-cdr2-immunized HLA-A2.1 transgenic mice. One of these, peptide 290, was studied in greater detail and found to be naturally processed and presented by human tumors. Furthermore, we found circulating CD8+ T cells specific for this epitope in the peripheral blood of A2.1+ PCD patients but not in A2.1+ normal and diseased control individuals, suggesting that cdr2 (290) is a tumor epitope associated with gynecologic tumor immunity. In our analysis of a limited number of PCD patients, we have found the signal-to-noise ratio for tetramer staining of PBL to be low. This may relate to low precursor frequencies present in the peripheral blood in a disease where the relevant cell population may have trafficked to the tumor and/or central nervous system and may vary according to disease state (the patients analyzed had chronic PCD). Moreover, in PCD Pt 2, CD8+ T cells specific for another cdr2 epitope, cdr2 (289–297), were detected in this patient's peripheral blood by tetramer staining (data not shown), indicating that the presence of cdr2 (290)-specific CD8+ T cells does not exclude the existence of other cdr2-specific CD8+ T cells.
HLA-A2.1 transgenic mice may generally serve as a source of clinically useful TCRs. Murine TCRs are sufficiently homologous to human TCRs that they can be incorporated into the human CD3 complex (31) and rescue surface expression in mutant T cells (38). Gene transfer of HLA-A2.1-transgenic mice-derived TCRs into human T cells can circumvent self-tolerance to the tumor-associated self/tumor antigens murine double minute 2 (MDM2) (31) and p53 (32, 39). High-affinity TCRs specific for self/tumor antigens can be generated by immunizations that take advantage of nonhomology between human and mouse sequences (31, 32). cdr2 (290) is different in mouse and human, and we took advantage of this observation to immunize HLA-A2.1 transgenic AAD mice and clone a cdr2 (290)-reactive T cell and TCR able to recognize MHC-peptide without CD8 coreceptor binding. Although we have not directly compared the affinity of human cdr2-specific TCRs with the mouse-derived TCRs cloned here, we identified a high-affinity TCR as assessed by tetramer staining and IFNγ production after coculture with targets presenting low concentrations of cdr2 peptide (Fig. 5 A and B). The affinity of this cdr2-specific TCR compares favorably with analysis of cloned TCRs generated against melanoma peptides isolated from human tumor-infiltrating lymphocytes (27).
Although the pathogenesis of PCD remains unproven, the observation that CD8+ T cells in two HLA A2.1+ patients with PCD cells were specific for the cdr2 (290) TCR strongly supports a model in which these T cells mediate PCD tumor immunity. Given the small number of patients analyzed in this study, the correlation between the presence of cdr2 (290) tetramer-positive cells and effective tumor immunity needs to be validated with greater numbers of patients. Nonetheless, the clinical relevance of this work is underscored by finding that RNA encoding the cdr2-specific TCR can reprogram donor CD8+ T cells to specifically recognize human breast and ovarian tumor cell lines expressing physiologic levels of endogenous cdr2. Notably, these TCR-electroporated lymphocytes exhibited antigen-specific lytic function as well as IFNγ production.
In conclusion, this study offers the intriguing possibility that cdr2-specific TCR may hold therapeutic promise for breast and ovarian cancer immunotherapy. Recent studies have shown that adoptive transfer of autologous T cells transfected with genes encoding antigen-specific TCRs may provide potent tumor immune responses, although such studies have been limited to special cases, particularly melanoma, where tumor-expressed antigens have been identified. The current work offers the possibility of extending this strategy to gynecologic tumors and, moreover, offers the chance to use a naturally occurring tumor rejection antigen as the target for adoptive TCR therapy. Autoimmunity would be a paramount concern for such studies. Before considering the use of this TCR for adoptive immunotherapy, it will be critical to screen multiple primary human tumors and normal human tissue for cdr2 expression, expanding initial studies suggesting frequent expression of cdr2 in human breast and ovarian tumors (11) and the paucity of cdr2 expression in normal mouse tissue (10). Nonetheless, recent data suggest that T cells do not survey the immune-privileged brain in the same way they do other areas of the body. Forced overexpression of MHC-I molecules in neurons of transgenic mice do not provide a sufficient signal for CTLs to cause destruction of neurons (40), although forced expression in other tissues has been associated with autoimmunity (41); in our own studies we have been unable to trigger autoimmune neurologic disease in animal models of PND (B.D.S., N.E.B., and R.B.D., unpublished data). In addition, one might consider, if necessary, the use of transfected T cells of limited half-life, perhaps together with adjuvants used to prevent neurologic disease [FK506 (16), nataluzimab (42)]. These observations, together with the apparent uncoupling of relatively frequent tumor expression of PND antigens, such as cdr2 and Hu (6, 34) from the extremely rare development of autoimmune disease, offer the possibility that adoptive onconeural antigen-specific TCR therapy may have a safe therapeutic window.
Materials and Methods
Clinical Case Histories.
Clinical samples were obtained, and tacrolimus treatment was undertaken under Institutional Review Board-approved protocols after obtaining informed consent. Detailed information is provided in SI Methods.
Peptide Screens and CTL Cloning.
Jerini Peptide Technologies generated the human cdr2 peptide library (peptides >80% purity). iTopia screening assays were performed according to the manufacturer's protocol (Beckman Immunomics) and analyzed with iTopia software using Prism (GraphPad).
DNA Constructs, Western Blots, Cells, and Mice.
Recombinant adenovirus (E1/E3-deleted) were constructed by using the AdEasy vector system (45) and purified as described (46). Details are provided in SI Methods.
Cloning of Murine cdr2-Specific, TCR-α and TCR-β cDNA and RNA Transfection.
Total RNA was extracted with the RNeasy kit (Qiagen) from 2 × 105 CD8-purified (MACS; Miltenyi Biotech) cdr2-290 clone T cells. RNA (1 μg) was amplified by 5′ RACE PCR using the GeneRacer kit (Invitrogen). In vitro TCR RNA transcription and PBL expression was performed as described (27). Details are provided in SI Methods.
T Cell Cytokine Release Assays and IFN-γ ELISPOTs.
Cytokine release assays were performed with 1 × 105 each of responder cells and stimulator cells incubated for 18–20 h. Cytokines were measured by using an IFN-γ ELISA kit (Pierce Endogen). Details are provided in SI Methods.
Tetramer Staining and CD107 Assay.
Data using PE-labeled tetramers (Immunomics iTAg MHC Tetramer; Beckman Coulter) was acquired with a FACScaliber (Becton Dickinson) and analyzed by using FloJo software (Treestar). FITC-conjugated CD107a antibody and GolgiStop (Becton Dickinson) were added to tumor cell-CD8+T cell cocultures, and cells were analyzed by flow cytometry. Details are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank members of the laboratory and Laura Johnson and Victor Engelhard for helpful discussions and critical review of the work, Francois Lemonnier for supplying mice, and Victor Engelhard and Paul Robbins for supplying cell lines and plasmids. Supported by the National Institutes of Health (NIH) Grant R01 CA85784 (to R.B.D.), the Ovarian Cancer Research Fund, and The Rockefeller University Hospital General Clinical Research Center and CTSA Grants M01-RR00102 and 1UL1RR024143 (from the NIH). B.D.S. was supported by NIH Medical Scientist Training Program Grant GM07739. R.B.D. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- CTL
cytotoxic T lymphocytes
- KEC
kidney epithelial cell
- PBL
peripheral blood lymphocyte
- PCD
paraneoplastic cerebellar degeneration
- PND
paraneoplastic neurologic disorder
- SFC
spot-forming cell
- TCR
T cell receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704336104/DC1.
References
- 1.Rosenberg SA. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Dudley ME, Rosenberg SA. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 4.Darnell RB. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell RB, Posner JB. Nat Immunol. 2003;4:201. doi: 10.1038/ni0303-201. [DOI] [PubMed] [Google Scholar]
- 6.Darnell RB, Posner JB. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 7.Darnell RB, Posner JB. Semin Oncol. 2006;33:270–298. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Albert ML, Darnell RB. Nat Rev Cancer. 2004;4:36–44. doi: 10.1038/nrc1255. [DOI] [PubMed] [Google Scholar]
- 9.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Neurology. 1992;42:1931–1937. doi: 10.1212/wnl.42.10.1931. [DOI] [PubMed] [Google Scholar]
- 10.Corradi JP, Yang CW, Darnell JC, Dalmau J, Darnell RB. J Neurosci. 1997;17:1406–1415. doi: 10.1523/JNEUROSCI.17-04-01406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnell JC, Albert ML, Darnell RB. Cancer Res. 2000;60:2136–2139. [PubMed] [Google Scholar]
- 12.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 13.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Proc Natl Acad Sci USA. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhard VH, Bullock TN, Colella TA, Mullins DW. Cancer J. 2000;6(Suppl 3):S272–S280. [PubMed] [Google Scholar]
- 15.Wentworth PA, Vitiello A, Sidney J, Keogh E, Chesnut RW, Grey H, Sette A. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- 16.Albert ML, Austin LM, Darnell RB. Ann Neurol. 2000;47:9–17. [PubMed] [Google Scholar]
- 17.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM, Melief CJ, Oseroff C, Yuan L, Ruppert J, et al. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 18.Vitiello A, Marchesini D, Furze J, Sherman LA, Chesnut RW. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 20.Keogh E, Fikes J, Southwood S, Celis E, Chesnut R, Sette A. J Immunol. 2001;167:787–796. doi: 10.4049/jimmunol.167.2.787. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez J, Schoeder K, Blondelle SE, Pons FG, Lone YC, Simora A, Langlade-Demoyen P, Wilson DB, Zanetti M. Eur J Immunol. 2004;34:2331–2341. doi: 10.1002/eji.200425134. [DOI] [PubMed] [Google Scholar]
- 22.Sherman LA, Hesse SV, Irwin MJ, La Face D, Peterson P. Science. 1992;258:815–818. doi: 10.1126/science.1439792. [DOI] [PubMed] [Google Scholar]
- 23.Choi EM, Chen JL, Wooldridge L, Salio M, Lissina A, Lissin N, Hermans IF, Silk JD, Mirza F, Palmowski MJ, et al. J Immunol. 2003;171:5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 24.Fisk B, Ioannides CG, Aggarwal S, Wharton JT, O'Brian CA, Restifo N, Glisson BS. Lymphokine Cytokine Res. 1994;13:125–131. [PubMed] [Google Scholar]
- 25.Fady C, Gardner A, Gera JF, Lichtenstein A. Cancer Immunol Immunother. 1993;37:329–336. doi: 10.1007/BF01518456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Yang JC, Restifo NP. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, et al. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, Ruppert T, Bolhuis RL, Melief CJ, Huber C, et al. Nat Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 32.Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, Strand S, Romero P, Huber C, Sherman LA, et al. Immunity. 2005;22:117–129. doi: 10.1016/j.immuni.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, Morgan RA. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darnell RB. N Engl J Med. 1999;340:1831–1833. doi: 10.1056/NEJM199906103402311. [DOI] [PubMed] [Google Scholar]
- 35.Plonquet A, Garcia-Pons F, Fernandez E, Philippe C, Marquet J, Rouard H, Delfau-Larue MH, Kosmatopoulos K, Lemonnier F, Farcet JP, et al. J Neuroimmunol. 2003;142:93–100. doi: 10.1016/s0165-5728(03)00269-8. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau A, Benyahia B, Dalmau J, Connan F, Guillet JG, Delattre JY, Choppin J. J Neurooncol. 2005;71:231–236. doi: 10.1007/s11060-004-1723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laugel B, Boulter JM, Lissin N, Vuidepot A, Li Y, Gostick E, Crotty LE, Douek DC, Hemelaar J, Price DA, et al. J Biol Chem. 2005;280:1882–1892. doi: 10.1074/jbc.M409427200. [DOI] [PubMed] [Google Scholar]
- 38.Gouaillard C, Huchenq-Champagne A, Arnaud J, Chen CL, Rubin B. Eur J Immunol. 2001;31:3798–3805. doi: 10.1002/1521-4141(200112)31:12<3798::aid-immu3798>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Kuball J, Schuler M, Antunes Ferreira E, Herr W, Neumann M, Obenauer-Kutner L, Westreich L, Huber C, Wolfel T, Theobald M. Gene Ther. 2002;9:833–843. doi: 10.1038/sj.gt.3301709. [DOI] [PubMed] [Google Scholar]
- 40.Rall GF, Mucke L, Oldstone MBA. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, et al. Proc Natl Acad Sci USA. 2000;97:9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, et al. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 43.Engelhard VH, Yannelli JR, Evans GA, Walk SF, Holterman MJ. J Immunol. 1985;134:4218–4225. [PubMed] [Google Scholar]
- 44.Bernhard EJ, Le AX, Barbosa JA, Lacy E, Engelhard VH. J Exp Med. 1988;168:1157–1162. doi: 10.1084/jem.168.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanegae Y, Makimura M, Saito I. Japanese J Med Sci Biol. 1994;47:157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.