Abstract
Whereas the zebrafish retina has long been an important model system for developmental and genetic studies, little is known about the responses of the inner retinal neurons. Here we report single-unit ganglion cell recordings from 5- to 6-day-old zebrafish larvae. In wild-type larvae we identify at least five subtypes of ganglion cell responses to full-field illumination, with ON-OFF and ON-type cells predominating. In the nrc mutant retina, in which the photoreceptor terminals develop abnormally, we observe normal OFF responses but abnormal ON-OFF responses and no ON responses. Previously characterized as blind, these mutants lack an optokinetic reflex (OKR), but in another behavioral assay nrc mutant fish have near-normal responses to the offset of light and slow and sluggish responses to the onset of light. Pharmacological block of the ON pathway mimics most of the nrc visual defects. We conclude that the abnormal photoreceptor terminals in nrc mutants predominantly perturb the ON pathway and that the ON pathway is necessary to drive the OKR in larval zebrafish.
Keywords: extracellular recordings, ON and OFF retinal pathway, optokinetic response, retina, retinal ganglion cells
Zebrafish are highly visual animals whose retinas contain numerous rods and four types of cones (1). By 3 days postfertilization (dpf) zebrafish retinas have largely differentiated (2), and by 4 or 5 days of age, visual responses can be reliably elicited (3, 4). Mutations affecting retinal development and function are easily induced by using forward genetic techniques, and the visual abilities of potential mutants are often behaviorally tested by using the optokinetic reflex (OKR) (3, 5). The OKR is a basic visual reflex exhibited by most vertebrates and plays an important role in stabilizing the eye relative to the visual scene (6, 7). It is easily elicited by moving vertical stripes through the visual field. The eyes follow the stripes with a smooth pursuit movement, followed by a rapid (saccade) eye movement in the opposite direction.
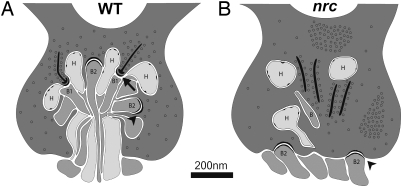
One zebrafish mutant, no optokinetic response c (nrc), originally identified in a OKR behavioral screen, has no OKR under any conditions tested and was reported to be completely blind (8). The nrc mutant has a premature stop codon in the synaptojanin1 gene (9), which encodes a polyphosphoinositide phosphatase that regulates clathrin-mediated endocytosis and actin cytoskeletal rearrangement at synapses (10, 11). In the nrc mutant, the photoreceptor synaptic terminals do not form properly and appear abnormal when viewed by electron microscopy (8). In WT fish, horizontal and bipolar cell processes invaginate into the photoreceptor terminals which form two types of synapses, ribbon synapses and flat contacts that relate to the separation of visual input into ON and OFF channels (Fig. 1A) (12). The ON channel underlies the ability to see light increments; the OFF channel, to see the light decrements (13). Ribbon synapses are made mainly on ON bipolar and horizontal cells, whereas the flat contacts are made mainly on OFF bipolar cells (14–16). In the nrc mutant, few processes invaginate the photoreceptor terminals, and the ribbons appear “free-floating” in the terminals and unrelated to any postsynaptic elements (Fig. 1B). Flat synaptic contacts are observed, but they are displaced to the base of the photoreceptor terminal (8). On the other hand, the inner retina of the nrc mutant appears normal anatomically, including proper bipolar cell ribbon synapses with amacrine and ganglion cell processes, as well as conventional amacrine cell synapses.
Fig. 1.
Diagram of photoreceptor terminals in WT and nrc mutant zebrafish at 5 dpf. (A) In the WT retina, bipolar and horizontal cell processes invaginate into the pedicle in a tight bundle to make two types of junctions: ribbon synapses (arrow) and flat contacts (arrowhead). Ribbon synapses are made onto presumed ON bipolar (B1) and horizontal (H) cell dendrites. Synaptic vesicles surround the synaptic ribbon. Flat contacts are found between the ribbon synapses and have dense cytoplasmic material on both sides of the junction. (B) In the nrc retina, few processes invaginate into the photoreceptor terminals. However, when present, many of these processes have small membrane densities, characteristic of horizontal cell processes. Synaptic ribbons in most of the pedicles are unassociated with postsynaptic processes and appear to be floating. However, flat contacts (arrowhead) are seen onto presumed OFF bipolar cell (B2) dendrites. However, they are displaced and make junctions at the photoreceptor base, rather than within the photoreceptor terminal. Synaptic vesicles often clump and fail to distribute evenly in nrc pedicles, but they surround synaptic ribbons as they do in WT pedicles. Based on Allwardt et al. (8).
To understand better the functional consequences of the nrc mutation, we have recorded from the retinas of WT and nrc mutant larvae. The electroretinogram (ERG) is readily recorded from larvae and provides information about the function of the outer retina–photoreceptors (ERG a wave) and bipolar cells (ERG b and d waves) (3, 17). However, recording inner retinal responses—the amacrine and ganglion cells—is more challenging and only a few reports of massed ganglion cell recordings in larval zebrafish have appeared (18). At 5–6 days of age, zebrafish are 3 mm long and their eyes are only 200–300 μm in diameter, making single-cell recordings from the intact eye technically difficult. By using an isolated eye preparation (17), we have succeeded in recording single-unit ganglion cell responses from WT and nrc mutant zebrafish larvae. Here, we report that nrc mutants have OFF ganglion cells but lack ON ganglion cells and that many of the nrc mutant visual deficits can be mimicked by pharmacological block of the ON pathway. Furthermore, a behavioral test demonstrates that the nrc mutants respond normally to the offset of light and are not totally blind.
Results
Characterization of WT Retinal Ganglion Cells.
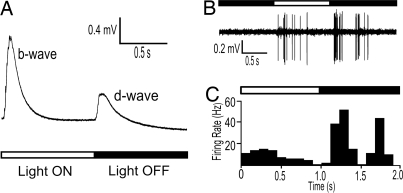
We recorded ganglion cell (GC) responses from either the surface of the retina or from the optic nerve, and the recordings appear equivalent. The former method, with the cornea facing up, allows for simultaneous recordings of the ERG and single-ganglion cell responses (Fig. 2). A typical GC recording is shown in Fig. 2B; in this case an ON-OFF response. The recording and poststimulus time histogram (PSTH) for the cell of Fig. 1B show that the OFF response was more vigorous and had a higher firing rate than the ON response (Fig. 2C). Once a single-unit response was isolated from either the retinal surface or optic nerve, the recordings typically remained stable for 20 min or longer.
Fig. 2.
ERG and single-unit recordings from the same eye of a WT zebrafish at 5 dpf. (A) ERG recording averaged from six responses to a 1-s light ON stimulus. The ERG b wave originates from the ON -bipolar cells, whereas the d wave originates mainly from the OFF bipolar cells (20, 35, 36). In zebrafish (and most animals), the b wave is significantly larger than the d wave as in this article (12). (B) Extracellular spike recording from a single ON-OFF retinal ganglion cell in response to 1-s full-field illumination. (C) PSTH of the ON-OFF ganglion cell (12 repeats of 1 s of light ON and 9 s of dark). (Bin width, 100 ms.) The bars above the trace in B and PSTH in C indicate light ON (open bar) or light OFF (filled bar).
We first characterized the types of GCs found in the zebrafish retina. Action potentials from 156 GCs in WT zebrafish larvae were recorded in response to full-field illumination. The majority of cells (56%) responded at both light ON and OFF, that is, they were ON-OFF cells (Fig. 3A). Other types of GC activity that were frequently observed include transient ON responses (16%), sustained ON responses (13%), and transient OFF responses (11%) (Fig. 3 B, C, and D, respectively). In addition, some GCs responded vigorously during darkness and stopped firing only after the introduction of a light flash (Fig. 3E). These sustained OFF cells resemble dimming detectors that were first discovered in the frog (19), but we rarely observed them in zebrafish (1%). Another infrequently encountered type of ganglion cell fired independently of the light stimulus (3%). These “spontaneous” cells are unlikely to be injured cells because the recordings typically lasted for >20 min. They may be immature GCs. In contrast to these two rare cell types, most light-driven cells do not fire spontaneously during darkness. The frequency distribution of the six major types of GCs observed in the WT zebrafish larvae is shown in Fig. 3F.
Fig. 3.
Types of GC responses in the WT retina elicited by 1 s of full-field illumination. (A) ON-OFF response. (B) Transient ON. (C) Sustained ON. (D) Transient OFF response. (E) Sustained OFF (dimming detector). Upper traces show representative examples of a ganglion cell spike output to the light ON and OFF stimulus. Lower histograms show the corresponding PSTHs of the single-unit recordings. (Bin width, 100 ms for all PSTHs.) The bars above the traces and PSTHs indicate light ON (open bar) or light OFF (filled bar). (F) Distribution of retinal GC types observed in the WT retina.
Characterization of nrc Mutant Retinal Ganglion Cells.
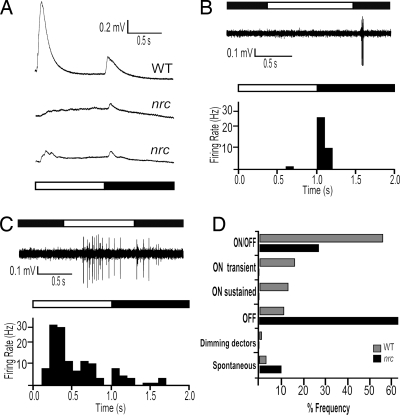
As noted above, the nrc mutant was found to give no visual responses as measured by the OKR and was thought to be completely blind (8). However, the nrc mutant does show ERG activity, but it was highly variable in the anesthetized animals in the previous study (8). By using the isolated eye preparation, we found that the nrc ERG is much more consistent. It consists of an a wave, a small or absent b wave and a reduced d wave (Fig. 4A). Some oscillations are typically seen after the a wave in accord with the earlier study. The d wave, which reflects the activity of the OFF bipolar cells (20), was consistently observed in all nrc mutants tested, although it was almost always smaller than in the WT retina (Fig. 4A). This finding suggests that at least some OFF bipolar cells are receiving synaptic input from the photoreceptors in response to the cessation of light.
Fig. 4.
The nrc mutant has predominantly OFF GCs and some ON-OFF cells. (A) ERG traces (average from six responses each) from a WT and two nrc mutants in response to a 1-s full-field illumination. Note missing or reduced b wave response amplitude in nrc mutants during light ON. OFF responses (B) and ON-OFF responses (C) in nrc retinal GCs are shown in the upper traces of B and C, and PSTHs are shown in the histograms of B and C. (Bin width, 100 ms.) (D) Histogram of the frequency of retinal GC type in WT and nrc retinas.
Single-unit recordings from nrc mutant eyes revealed that the majority of cells (63%) responded only to the offset of light (OFF cells, Fig. 4B), and no pure ON cells were recorded from any of the 49 cells. This result is in striking contrast to the findings in WT animals where the great majority of cells (≈85%) are ON-OFF or ON cells. Some nrc ganglion cells responded at both light ON and OFF (27%) (Fig. 3C). However, the ON cssomponent of the ON-OFF response from the nrc mutants was usually delayed as compared with WT ON-OFF cells (compare Figs. 3A and 4C). As in the WT animals, spontaneous GCs were observed in the nrc mutants, but more frequently (10%). Whereas spiking activity of GCs was easily detected in WT retinas, it was more difficult to find spiking cells in the nrc mutants, suggesting that nrc mutants have a reduced number of light-driven GCs. Fig. 4D compares the frequency distribution of GC types between nrc and WT retinas.
nrc Mutants Can Detect Differences in Light Intensities.
The OKR assay is thought to provide a general measure of visual responsiveness. However, the OKR requires that an animal detect moving stripes and it is possible that fish that fail to exhibit an OKR may not be completely blind. To determine whether the nrc mutant zebrafish can detect simple light increments and decrements we developed a visual-motor behavioral assay based on the recent observations of Prober et al. (21). In this assay, single zebrafish larvae are placed in 80 individual wells of a 96-well plate, which allows simultaneous monitoring of each larva by using an automated video-tracking system. The motor output of the zebrafish larvae in response to periods of 30 min of light ON and 30 min of light OFF was recorded and quantified per second.
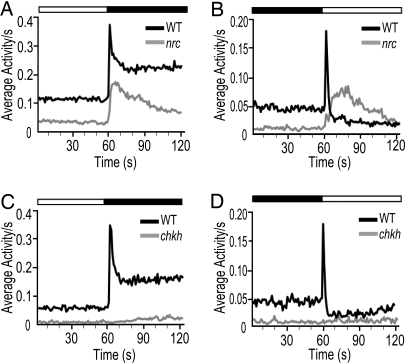
Both WT and nrc mutant fish responded similarly to the offset of light, as shown in Fig. 5A. WT and mutant genotypes sharply increased their activity immediately after the cessation of light. This increased activity gradually returned to baseline, although the nrc mutants returned to baseline faster than WT fish. On average, there was a slight delay of ≈2 s in the nrc mutant response to the offset of light (n = 120 fish for each genotype, averaged from four light-OFF responses per experiment for three experiments).
Fig. 5.
Nrc mutants increase their activity in response to light decrements and increments. The locomotor behavior of zebrafish larvae in response to 30 min of light ON and 30 min of light OFF is recorded per second. Each trace represents an average of 480 responses from 120 individual WT or nrc mutant larvae recorded over three experiments. (A) Behavioral responses after cessation of light in WT and nrc mutants. The average locomotor behavior of nrc mutants is slightly reduced as compared with WT fish but remains vigorous following the lights OFF stimulus. (B) Motor activity in response to light ON in nrc mutants is delayed and sluggish. Note the slow rise time of the nrc mutant response to light ON compared with the light ON response of the WT fish. (C and D) Analogous experiments performed with WT and eyeless chk mutants. The chk mutants do not increase their activity to either light increments or decrements and have a low baseline of activity.
The nrc mutants also responded to the onset of light, but quite differently from WT fish (Fig. 5B). WT fish had a dramatic spike of motor activity immediately at light onset, known as a startle response, after which they returned to lower-than-baseline activity, called a freeze (Fig. 5B). In nrc mutant fish, the ON behavioral response was delayed by ≈12 s, and the average increase in motor activity was more gradual and weaker than with WT fish. These observations correlate well with the slow and sluggish ON response seen electrophysiologically in the ON-OFF cells of nrc mutants. Our results indicate that the nrc mutant fish readily detect light offsets and they even respond weakly at light onset. They are, therefore, not completely blind.
Because teleosts can detect light through nonretinal tissues [e.g., the pineal gland (22)], we confirmed that the behavioral responses to light-intensity changes require intact eyes by using chokh mutants. The chokh (chk) mutant zebrafish has a mutation in the Rx3 homeodomain-containing transcription factor and completely lacks eyes from the earliest stages of development (5, 23). They are otherwise morphologically normal and have normal touch responses and swimming behavior. Unlike both WT and nrc mutants, the chk mutants did not increase their activity at either light decrement (Fig. 5C) or at light increment (Fig. 5D). These fish appear to be completely blind to light-intensity changes.
Pharmacological Block of the ON Pathway.
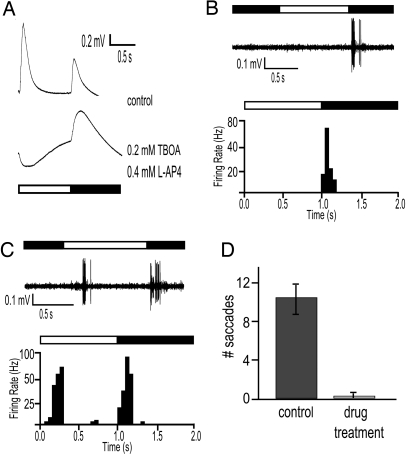
To mimic the nrc mutation and to explore further the effects of blocking the ON retinal pathway in zebrafish, WT larvae at 5 dpf were incubated in fish water containing 0.4 mM l (+)-2-amino-4-phosphonobutyric acid (L-AP4) and 0.2 mM dl-threo-β-benzyloxyaspartate (TBOA) for 2 h. TBOA (an excitatory amino acid transporter blocker) and L-AP4 (a metabotropic glutamate receptor agonist) have been shown to completely eliminate the b wave in WT zebrafish (17). Control animals (n = 25) displayed normal ERGs, whereas animals treated with the drug mixture (n = 25) did not show any b wave activity (Fig. 6A), suggesting that the activity of ON bipolar cells was essentially abolished. The drug mixture did not perturb the d wave amplitude, although it was on average slightly more prolonged. The drug treatment also effectively abolished residual b wave activity in nrc mutants (data not shown).
Fig. 6.
Pharmacological block of the ON pathway. (A) ERG traces from control and drug-treated zebrafish larvae at 5 dpf. The b wave is completely abolished in retinas of the fish treated with the drug mixture. The d wave amplitude is not significantly increased after the drug mixture treatment but is prolonged. Example of an OFF type (B) and ON-OFF type GC (C) in response to a 1-s full-field illumination after drug treatment. Note the long latency of the ON component of the ON-OFF GC response. (D) OKR assay performed on control animals (n = 30) and animals treated with the drug mixture (n = 30). The fish treated with the drug mixture lose their ability to respond in the OKR test.
To examine the effects of the drug treatment on inner retinal cells, we recorded spike activity of GCs in animals treated with the drug mixture. OFF ganglion cells were readily recorded (n = 10) (Fig. 6B), as well as some ON-OFF cells (n = 5) (Fig. 6C). No pure ON GCs were found. The ON component of the ON-OFF GC responses in these drug-treated animals had a longer latency than the ON response in WT ON-OFF cells, similar to the ON component of most of the ON-OFF-type GCs in nrc mutants (compare Figs. 4C and 6C). The latency in the ON component ranged from 150 to 600 ms, as compared with control eyes in which latencies were always <150 ms. Furthermore, zebrafish larvae lost much of their ability to respond in the OKR assay after the drug treatment (n = 30) (Fig. 6D). Some animals did display some weak and/or spontaneous eye movements indicating that the drug treatment did not affect their ability to move their eyes, and they also swam normally. Surprisingly, when tested in our visual-motor assay, the drug-treated animals displayed essentially normal responses at both the offset and onset of light suggesting that some residual ON pathway activity persisted.
Discussion
Much information about the visual world is processed within the retina and coded by the ganglion cells. In the vertebrate retina, the ON and OFF parallel pathways are two fundamental information streams that relay visual signals from photoreceptors to bipolar and ganglion cells and ultimately to higher visual centers (24). Studies by Roska and colleagues (25) have shown how spatial and temporal patterns of excitation and inhibition on ganglion cells generate the spiking output pattern for each ganglion cell type. They distinguished 10 physiological GC subtypes in rabbits and correlated these with the dendritic morphology of ganglion cells. In the present study, single-unit recordings in WT zebrafish retinas revealed a variety of GC subtypes in response to full-field illumination. For example, we observed two types of ON responses, transient and sustained, as well as transient and sustained OFF responses. We also recorded ON-OFF GCs, and examination of the poststimulus time histograms suggests several subtypes of these cells may exist (compare Figs. 2B and 3A). It is likely that these distinct types of GC responses represent different morphological subtypes of GCs. Morphological analysis of the GCs in adult zebrafish retina has revealed eleven different types (26). Five of these eleven subtypes have bistratified or multistratified dendrites within the inner plexiform layer (IPL), suggesting that they are ON-OFF cells. The six remaining subtypes of GCs have dendritic arbors that stratify exclusively in either the ON or OFF laminae in the IPL, and are therefore likely to be pure ON- or OFF-type cells, respectively. It is unknown whether the larval retina of zebrafish contains the same morphological GC types, but our data suggest that larval retinas do contain distinct subtypes of GC responses, which are likely represented by morphologically distinct classes.
Can the OFF Channel Detect Movement?
The nrc mutant was isolated because it failed completely in the optokinetic behavioral test (8). We show here that the nrc mutant does have a remaining OFF pathway and demonstrate using another behavioral test that nrc mutants can detect decreases of illumination similar to WT fish. They also respond to the onset of illumination, but sluggishly and quite differently from WT fish. Thus, contrary to previous conclusions, the nrc fish do have some visual function, although it is clearly abnormal for light onset.
Optokinetic responses depend on an animal being able to detect movement, and because nrc fish fail the OKR test, this suggests that they are unable to perceive moving stimuli. That the mutants have mainly OFF ganglion cells that appear normal suggests that OFF ganglion cells are insufficient to cause a response to movement. Pharmacological experiments in which the ON responses to bipolar cells are blocked support this view. These observations indicate that the retinal OFF pathway in zebrafish cannot detect the movement required for the optokinetic reflex.
Which Ganglion Cells Mediate Movement Perception?
Both the nrc mutant fish and WT fish with pharmacologically blocked ON bipolar cell responses suggest that ON ganglion cell activity is responsible for movement detection in zebrafish, at least the movement required for the OKR. In rabbit, ON transient, directionally sensitive (ON-DS) ganglion cells have been observed and proposed to be the cell type that detects OKR movement (27). However, ON-OFF ganglion cells in a number of species have been shown to be movement- and directionally sensitive and these, too, could be candidates (28, 29). Although ON-OFF ganglion cells have been recorded in both nrc mutant fish and in fish in which the ON pathway from photoreceptors to ON bipolar cells has been blocked, the ON responses are typically delayed. Thus, these ON-OFF ganglion cell responses are clearly abnormal, suggesting that the ON responses of these cells are not sufficient to detect moving stimuli.
Our observations in zebrafish are similar to experiments in the rabbit where pharmacological block of the ON pathway also eliminated the OKR (27). Pharmacological block of the ON pathway in cat and monkey retinas, on the other hand, does not affect the OKR (27). In zebrafish and rabbit, movement sensitivity, at least as far as the OKR is concerned, appears to depend on normal ON retinal ganglion cell activity.
Generation of Delayed ON Responses in ON-OFF Ganglion Cells.
How might the OFF pathway generate ON responses in ON-OFF ganglion cells? Pharmacological experiments that block responses in the second-order ON bipolar cells completely eliminate any b wave activity yet do not eliminate delayed ON responses in the ON-OFF ganglion cells. This suggests that these ON responses arise from laterally oriented pathways in the inner nuclear layer, because b wave activity depends on activation of the radially oriented bipolar cells. The obvious pathway, therefore, would be via an inhibitory, sign-reversing amacrine cell pathway. This needs to be tested.
In a recent study in mice in which the ON pathway to the bipolar cells was blocked pharmacologically, ON responses in ON-OFF ganglion cells were observed, but these ON responses also occurred with long latencies. These authors also proposed that ON responses in ON-OFF ganglion cells were generated by the OFF pathway (30).
Generation of OFF Bipolar Cell Activity.
It has long been recognized that photoreceptors make two types of synapses on bipolar cells: ribbon synapses and flat or basal junctions (12). Ribbon synapses are made mainly on bipolar cells that give ON responses, whereas flat junctions are made mainly on bipolar cells that give OFF responses (14–16). In teleosts, the rod-driven light responses are exclusively mediated by mGluR6 receptors, whereas the cone-driven light responses on ON bipolar cells are mediated mainly by a glutamate transporter, also known as the excitatory amino acid transporters (20, 31, 32). The OFF bipolar cell responses, on the other hand, are mediated by AMPA/kainate receptors.
The flat junctions in all retinas have long been an enigma. Although some increased membrane density has been observed at the flat junctions, no aggregation of synaptic vesicles has been seen at these junctions nor are they related to the synaptic ribbons. Thus, it has been proposed that the photoreceptor synaptic transmitter, glutamate, is released only at the ribbon synapses and the postsynaptic processes at the flat junctions are activated by transmitter diffusing from the ribbon release sites (33). This could help explain, perhaps, why photoreceptor synapses are typically invaginated.
If the ideas above are correct, then the nrc mutant presents a puzzle. In most photoreceptor terminals in this mutant, few if any ribbon synapses form (Fig. 1B). Yet flat junctions are observed, displaced to the basal surface of the terminals. These junctions are likely functional because both ERG d wave activity and OFF ganglion cells are consistently recorded from the nrc mutant. Thus, how the postsynaptic processes at the flat/basal junctions are activated in the nrc mutant remains an open question.
Materials and Methods
Zebrafish Maintenance and Behavioral Screening of nrc Mutants.
Zebrafish were maintained on a 10-h dark and 14-h light cycle (34). The nrc mutant larvae were screened at 5 dpf by using the OKR test (3). Larvae that failed to show any OKR activity were classified as mutants. Control animals were either OKR-responsive siblings or AB WT strain fish.
Eyeless (chk) Mutants.
The chokh mutants were screened at 5 dpf by using morphological markers; that is, no eye development and darker body pigmentation. The chk mutation is recessive and fully penetrant and is maintained in a TL background (23).
Isolated Eye Preparation.
One eye was gently separated from an anesthetized animal by means of a fine tungsten wire loop, severing the optic nerve and ocular muscles. The rest of the animal was pulled away with a pair of forceps and euthanized. The isolated eye was placed on 2% agarose and covered with Ringer's solution. The Ringer's solution contained 130 mM NaCl, 2.5 mM KCl, 20 mM NaHCO3, 0.7 mM CaCl2, 1.0 mM MgCl2, and 20 mM glucose. The pH of the Ringer's solution was maintained at pH 7.8 by continuously gassing it with ≈97% O2 and ≈3% CO2. Animals were incubated under dim-light conditions for at least 10 min before the eye surgery, which was also performed under dim light. Experiments were performed at room temperature (22–26°C) during the light cycle.
Retinal Ganglion Cell Recordings.
Responses were recorded by placing an electrode on the surface of the retina or within the optic nerve, and the recordings were similar. In the latter method, the isolated eye was positioned with the cornea facing downward on 2% agarose. Visual stimuli were presented at an approximate intensity of 0.35 μW/cm2 by using an LCD monitor (SyncMaster 740N, Samsung) placed directly underneath the recording chamber. For full-field stimulation, a circular spot with a diameter of 1 cm was used to ensure even illumination of the entire retina.
For recording, a glass pipette with a tip diameter of 2 μm containing a chloride-coated silver wire and filled with Ringer's solution was inserted into the back of the eye at the optic nerve head by means of a micromanipulator. The reference electrode was placed within the agarose in the recording chamber. Spikes were amplified (total gain of 1,000) and bandpass filtered at 0.3–2 kHz. Spike waveform data were acquired at 10 kHz through a NI-DAQ board to a PC by using a data-acquisition program custom-written in LabVIEW (National Instruments). To ensure single-unit isolation, recordings were monitored over a loudspeaker, and electrode placement was carefully adjusted by using the micromanipulator. Single-unit spike recordings were separated from background noise by thresholding and sorted offline. Time stamps for each action potential of each sorted unit were used to generate PSTHs with custom-written software in IGOR Pro (WaveMetrics ).
ERG or Simultaneous Recording of ERGs and Retinal Ganglion Cells.
ERG and single-unit responses either singly or together could be recorded by placing an electrode with a tip diameter of 2–6 μm under the lens on the surface of the retina by using an anterior transscleral approach. A stable ERG recording was typically achieved immediately, but it often took several minutes to isolate a single-unit recording from a ganglion cell.
The isolated eye was placed with the cornea facing up at the center of the stimulus light spot (diameter = 5 mm) from a halogen light source. ERGs were amplified at 1,000 total gain, and low-pass filtered at 300 Hz. The ERG traces and action potentials were sorted by using custom-written software in IGOR Pro (WaveMetrics ). No differences in the GC spike patterns recorded from the optic nerve versus those recorded by the transscleral approach were noted. Most of our single-unit recordings were made from the optic nerve head.
Drug Treatments.
L-AP4 and TBOA were purchased from Sigma and Tocris, respectively. Stock solutions of 100 mM were prepared by dissolving L-AP4 in 0.1 M NaOH and TBOA in DMSO and were added to fish water to achieve final concentrations. Larval fish at 5 dpf were incubated in fish water containing 0.2 mM TBOA and 0.4 mM L-AP4 for 2 h.
Visual-Motor-Behavioral Assay.
Mutants identified by OKR testing were each placed in one of 80 wells of a 96-well plate (Whatman 7701-1651). Locomotor activity was monitored for up to 10 h by using an automated video-tracking system (Videotrack; ViewPoint Life Sciences) by employing a DinionXF 1/3-inch Monochrome camera (model LTC0385, Bosch) fitted with a fixed-angle megapixel lens (M5018-MP, Computar) (21). The 96-well plate, camera, and infrared and white lights were housed inside a custom-modified Zebrabox (Viewpoint Life Sciences). The box was constantly illuminated with infrared light that the animals do not see. All calculations were based on the “middur” dataset in the Videotrack quantization mode, which, in our setup conditions, is approximately equal to the total duration per second that an individual larva is moving. The data were processed and analyzed by using custom PERL software and Visual Basic Macros for Microsoft Excel.
Nrc, WT siblings, and drug-treated WT fish were placed in the Zebrafish Monitors at 5 dpf and were dark- or light- adapted for 2 h for acclimatization to the experimental apparatus. The lights (69–83 μW/cm2 measured at 495λ) were turned on or off every 30 min during the afternoon. The transition from full lights on to full lights off occurs in <1 s. A total of 120 WT and 120 nrc larvae were tested with 12 on and 12 off stimuli.
Acknowledgments
We thank Dr. Edward Soucy for his generous technical and intellectual help and Manija Emran for her help with the art work. This work was supported by National Institutes of Health Grants EY000811(to J.E.D.) and 5T32UY07145 (to F.E.) and by Knights Templar Eye Foundation (F.E.). J.R. is a Bristol-Myers Squibb Fellow of the Life Sciences Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE. Proc Natl Acad Sci USA. 1993;90:6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt EA, Dowling JE. J Comp Neurol. 1999;404:515–536. [PubMed] [Google Scholar]
- 3.Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Easter SS, Jr, Nicola GN. Dev Biol. 1996;180:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- 5.Geisler R, Rauch GJ, Baier H., van Bebber F, Bross L, Dekens MP, Finger K, Fricke C, Gates MA, Geiger H, et al. Nat Genet. 1999;23:86–89. doi: 10.1038/12692. [DOI] [PubMed] [Google Scholar]
- 6.Oyster CW, Takahashi E, Collewijn H. Vision Res. 1972;12:183–193. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- 7.Roeser T, Baier H. J Neurosci. 2003;23:3726–3734. doi: 10.1523/JNEUROSCI.23-09-03726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allwardt BA, Lall AB, Brockerhoff SE, Dowling JE. J Neurosci. 2001;21:2330–2342. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakisaka T, Itoh T, Miura K, Takenawa T. Mol Cell Biol. 1997;17:3841–3849. doi: 10.1128/mcb.17.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WT, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Proc Natl Acad Sci USA. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling JE. The Retina: An ApproachablePart of the Brain. Cambridge, MA: Harvard Univ Press; 1987. [Google Scholar]
- 13.Schiller PH, Sandell JH, Maunsell JH. Nature. 1986;322:824–825. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- 14.Raviola E, Gilula NB. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stell WK, Ishida AT, Lightfoot DO. Science. 1977;198:1269–1271. doi: 10.1126/science.201028. [DOI] [PubMed] [Google Scholar]
- 16.Vardi N, Dhingra A, Zhang L, Lyubarsky A, Wang TL, Morigiwa K. Keio J Med. 2002;51:154–164. doi: 10.2302/kjm.51.154. [DOI] [PubMed] [Google Scholar]
- 17.Wong KY, Gray J, Hayward CJC, Adolph AR, Dowling JE. Zebrafish. 2004;1:121–131. doi: 10.1089/zeb.2004.1.121. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Dowling JE. J Neurosci. 2000;20:1893–1903. doi: 10.1523/JNEUROSCI.20-05-01893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maturana HR, Lettvin JY, McCulloch WS, Pitts WH. J Gen Physiol. 1960;43(6 Suppl):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong KY, Adolph AR, Dowling JE. J Neurophysiol. 2005;93:84–93. doi: 10.1152/jn.00259.2004. [DOI] [PubMed] [Google Scholar]
- 21.Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allwardt BA, Dowling JE. J Neurocytol. 2001;30:493–501. doi: 10.1023/a:1015689116620. [DOI] [PubMed] [Google Scholar]
- 23.Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. EMBO Rep. 2003;4:894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller PH. Nature. 1982;297:580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- 25.Roska B, Molnar A, Werblin FS. J Neurophysiol. 2006;95:3810–3822. doi: 10.1152/jn.00113.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mangrum WI, Dowling JE, Cohen ED. Visual Neurosci. 2002;19:767–779. doi: 10.1017/s0952523802196076. [DOI] [PubMed] [Google Scholar]
- 27.Knapp AG, Ariel M, Robinson FR. J Neurophysiol. 1988;60:1010–1021. doi: 10.1152/jn.1988.60.3.1010. [DOI] [PubMed] [Google Scholar]
- 28.Barlow HB, Hill RM, Levick WR. J Physiol. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchiafava PL, Weiler R. Proc R Soc London Ser B. 1980;208:103–113. doi: 10.1098/rspb.1980.0044. [DOI] [PubMed] [Google Scholar]
- 30.Renteria RC, Tian N, Cang J, Nakanishi S, Stryker MP, Copenhagen DR. J Neurosci. 2006;26:11857–11869. doi: 10.1523/JNEUROSCI.1718-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong KY, Cohen ED, Dowling JE. J Neurophysiol. 2005;93:94–107. doi: 10.1152/jn.00270.2004. [DOI] [PubMed] [Google Scholar]
- 32.Grant GB, Dowling JE. J Neurosci. 1995;15:3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterling P, Demb JB. In: The Synaptic Organization of the Brain. Shepherd GM, editor. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 34.Westerfield M. The Zebrafish Book: A Guide for the laboratory use of Zebrafish. Eugene: Univ of Oregon Press; 2000. [Google Scholar]
- 35.Chappell RL, Rosenstein FJ. J Gen Physiol. 1996;107:535–544. doi: 10.1085/jgp.107.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sieving PA, Murayama K, Naarendorp F. Visual Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]