Abstract
Anecdotal evidence suggests that birds have smaller intestines than mammals. In the present analysis, we show that small birds and bats have significantly shorter small intestines and less small intestine nominal (smooth bore tube) surface area than similarly sized nonflying mammals. The corresponding >50% reduction in intestinal volume and hence mass of digesta carried is advantageous because the energetic costs of flight increase with load carried. But, a central dilemma is how birds and bats satisfy relatively high energy needs with less absorptive surface area. Here, we further show that an enhanced paracellular pathway for intestinal absorption of water-soluble nutrients such as glucose and amino acids may compensate for reduced small intestines in volant vertebrates. The evidence is that l-rhamnose and other similarly sized, metabolically inert, nonactively transported monosaccharides are absorbed significantly more in small birds and bats than in nonflying mammals. To broaden our comparison and test the veracity of our finding we surveyed the literature for other similar studies of paracellular absorption. The patterns found in our focal species held up when we included other species surveyed in our analysis. Significantly greater amplification of digestive surface area by villi in small birds, also uncovered by our analysis, may provide one mechanistic explanation for the observation of higher paracellular absorption relative to nonflying mammals. It appears that reduced intestinal size and relatively enhanced intestinal paracellular absorption can be added to the suite of adaptations that have evolved in actively flying vertebrates.
Keywords: digestion, gut morphometrics, nutrient absorption, paracellular uptake
Birds have structural, physiological, and biochemical refinements that adapt them for flight (1), but basic differences in digestive processing between flying and nonflying vertebrates have never been described to our knowledge. The phrase “eating like a bird” wrongly suggests that birds have relatively small appetites, whereas in fact the typical wild bird eats about one-third more dry matter each day than does the typical nonflying mammal (2). Flight, a very energetically demanding activity, contributes to high daily energy demands, but its structural prescription for low weight also may shape an aspect of fliers' digestive apparatus in a way that runs counter to that system's role in providing fuel to meet high energy demands.
There is anecdotal evidence that birds have relatively shorter intestines than mammals (3), and shorter intestines are associated with less surface area and volume, parameters directly correlated with digestive capacity. Indeed, in both birds and mammals, digestive adjustments to higher feeding rates almost always include an increase in gut size and thus an increase in digestive enzymes and nutrient transporters (4). For birds that fly, however, the size of the digestive tract and consequently the mass of digesta it carries may need to be minimized because the cost of flight increases with load carried, and take-off and maneuverability can be impaired at heavier masses (5–7). Individual flying vertebrates do appear to reduce the mass of digestive organs during times when capacity is not needed (e.g., during long migratory flights), suggesting that minimizing gut size might have adaptive value for increasing flight performance by reducing weight and/or the energy cost of maintaining tissue (8–13).
To test rigorously for differences in gut morphometrics, we surveyed the literature for measures of intestine length and nominal surface area (the area of an equivalent smooth bore tube) in birds, bats, and eutherian nonflying mammals. We excluded foregut fermenters, in which many substrates are fermented by microbial symbionts proximal to the small intestine and the energy-bearing products are absorbed across the foregut epithelium. In contrast, the avian and eutherian species we compared rely mainly on their small intestines for absorption of the majority of energy-bearing compounds. We excluded marsupial endotherms because we did not have measures of intestinal absorption in any species in this taxon. We focused on omnivorous species (a species was considered such if it was noted to consume both arthropods and a plant part) to control for likely effects of diet on gut morphometrics (14, 15).
A central dilemma that emerges in this consideration of birds and bats vs. nonflying mammals is how the former satisfy relatively high energy needs if they indeed possess relatively smaller intestines. Water-soluble compounds, such as glucose and amino acids, are absorbed in the small intestine mainly by two pathways, the transcellular and paracellular pathways. The transcellular absorption of sugars and amino acids is mediated primarily by membrane-bound transporter proteins that take them up from the gut lumen into the enterocyte across the apical membrane and hasten their exit from enterocyte to blood across the basolateral membrane. There do not appear to be fundamental differences between birds and mammals in the nutrient transporters of the intestinal brush border or significant differences in mediated uptake rate of d-glucose or amino acids per unit nominal area (16). Paracellular absorption involves movement of solutes through the tight junctions adjoining cells by diffusion or by the process of solvent drag (17). In both mammalian and avian species, the paracellular route of absorption of water-soluble compounds has been visualized by either autoradiography (18) or confocal laser microscopy (19), its molecular size selectivity has been characterized by using a series of nonelectrolyte water-soluble probes that differ in molecular dimension (20, 21), and its charge selectivity has been characterized by using relatively inert charged peptides (22, 23). In this study, which is focused on patterns across species, we tested the hypothesis that paracellular absorption (measured as fractional absorption, or bioavailability, of metabolically inert water-soluble probes not transported by mediated mechanisms) is elevated in birds, relative to nonflying mammals, and thus may serve as compensation for smaller intestines.
Results and Discussion
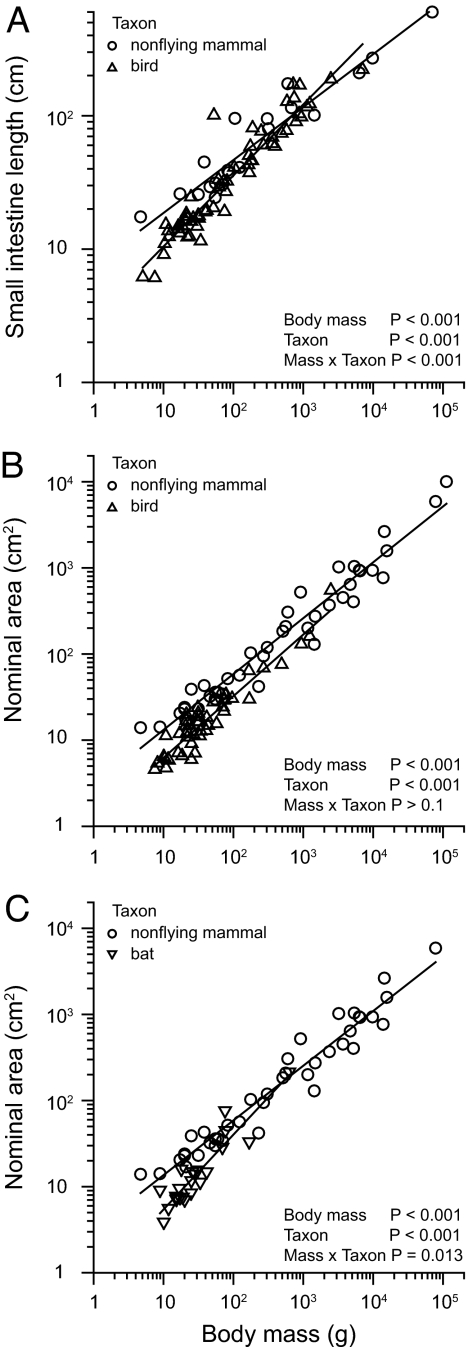
Analysis of our gut morphometrics dataset [see supporting information (SI) Table 2] confirmed that birds have significantly shorter small intestine lengths than nonflying mammals, although the difference diminishes as body size increases (Fig. 1A). Birds also have significantly lower, by 36%, small intestine nominal surface area (Fig. 1B). Surface area is important because it determines the area over which nutrients can be digested and absorbed. Small intestine volume, a direct function of tube length and area, and consequently the potential mass of digesta carried, is thus relatively smaller in birds, by ≈57% (24). Our finding of lower small intestine area in birds is actually an underestimate of the difference from mammals in absorptive area for fueling metabolic demands. Commonly in mammals, but rarely in small birds, there is additional surface area in the cecum or colon where products from microbial fermentation, such as short-chain fatty acids, may be absorbed and account for up to a third of the host's energetic demand (16). Differences in total intestinal nominal surface area among birds and nonflying mammals are not likely to be counterbalanced by greater digestive surface amplification by villi in birds, at least in the case of mediated nutrient uptake. Measurements of the latter standardized per nominal surface area inherently take differences in surface amplification into account and do not differ significantly among birds and mammals (16), suggesting that total capacity for mediated uptake is indeed lower in birds.
Fig. 1.
Small intestine length and nominal (smooth bore tube) surface area in omnivorous birds and mammals. (A and B) A comparison of birds and nonflying mammals. (A) For length, the slopes of these relationships differed significantly (F1,78 = 14.5; n = 61 species and n = 21 species for birds and nonflying mammals, respectively). Birds <195 g have significantly shorter small intestines, according to the Johnson–Neyman technique (50). (B) For nominal surface area, there was no significant difference in slope between birds and nonflying mammals (F1,83 = 2.11; n = 46 species and 41 species in birds and mammals, respectively). When the lines were fit to the common slope of 0.73, the calculated proportionality coefficients (intercept at unity) were 1.14 for birds and 1.79 for mammals (F1,84 = 47.31). Hence, small intestine nominal surface area in birds is ≈36% lower than that in nonflying mammals. (C) Nominal surface area of bat species compared with the nonflying mammals. The slopes of these relationships differed significantly (F1,60 = 7.4; n = 23 species and n = 41 species for bats and nonflying mammals, respectively).
These patterns held up in a broader analysis of >200 species of mammals and birds in which both different diets (species were classified as omnivore, carnivore, herbivore, nectarivore, or frugivore) and phylogeny (25) were taken into account (24). This analysis confirmed that small birds have significantly shorter small intestines, and avian species generally have less small intestine nominal surface area and volume than nonflying mammals. Small intestine wet mass, however, is not significantly different between taxa. Our analysis also showed that birds have significantly greater villous amplification of small intestine surface area than mammals (≈25% more amplification, F1,16 = 7.12, P = 0.0096), with no effect of body size. As mentioned above, greater villous surface amplification is not likely to counterbalance differences in intestine size (nominal surface area and length) in the case of mediated nutrient uptake, but it may provide one mechanistic explanation for the observation of higher paracellular absorption in birds.
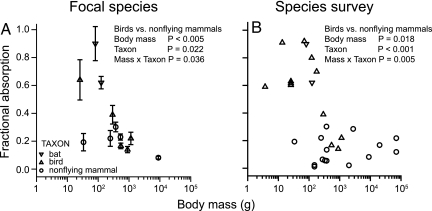
In our focal species (Table 1), absorption of water-soluble compounds by the paracellular pathway (specifically, fractional absorption or bioavailability of the metabolically inert, nontransported monosaccharide l-rhamnose) differed significantly between the avian species and the nonflying mammals, but the difference depended on body mass (Fig. 2A). We included body mass as a covariate in analysis of covariance (ANCOVA), because Pappenheimer (26) suggested that paracellular absorption might become increasingly important at larger body sizes, at least in mammals. However, in our focal species there was no evidence for such a positive scaling, and in fact paracellular absorption was negatively related to body size. The analysis indicated that fractional absorption is greatest in small-sized birds. The pattern is consistent with the hypothesis that paracellular absorption is elevated in birds, relative to nonflying mammals, as compensation for smaller intestines. The differential between birds and nonflying mammals in both intestinal length and fractional absorption declines with increasing body size (compare Figs. 1A and 2A).
Table 1.
Fractional absorption (f), or bioavailability, of metabolically inert carbohydrate probe molecules in intact birds and mammals
| Species |
Body mass, g | Probe | f | Source | |
|---|---|---|---|---|---|
| Common name | Scientific name | ||||
| Broad-tailed hummingbird | Selasphorus platycercus | 3.5 | l-glucose | 0.59 | 51 |
| Yellow-rumped warbler | Dendroica coronata | 12 | l-glucose | 0.91 | 52 |
| House sparrow* | Passer domesticus | 25 | l-rhamnose | 0.64 | 21 |
| House sparrow | Passer domesticus | 25 | l-glucose | 0.603 | 35 |
| House sparrow | Passer domesticus | 25 | Mannitol | 0.63 | 53 |
| American robin | Turdus migratorius | 70 | l-glucose | 0.92 | 36 |
| Rainbow lorikeet | Trichoglossus haematodus | 125 | l-glucose | 0.8 | 54 |
| Northern bobwhite | Colinus virginianus | 150 | l-glucose | 0.7 | 55 |
| Rock dove* | Columba livia | 300 | l-rhamnose | 0.39 | 39 |
| American coot* | Fulica americana | 542 | l-rhamnose | 0.17 | This study |
| Ring-necked pheasant* | Phasianus colchicus | 900 | l-rhamnose | 0.14 | This study |
| Mallard* | Anas platyrhynchos | 1,200 | l-rhamnose | 0.22 | This study |
| Great fruit-eating bat | Artibeus lituratus | 70 | l-rhamnose | 0.90 | 56 |
| Egyptian fruit bat | Rousettus aegyptiacus | 125 | l-rhamnose | 0.62 | 37 |
| White laboratory mouse* | Mus musculus | 30 | l-rhamnose | 0.193 | This study |
| Hamster | Mesocricetus auratus | 156 | l-rhamnose | 0.0235 | 57 |
| Hamster | Mesocricetus auratus | 156 | Mannitol | 0.0117 | 57 |
| Galea* | Galea galea | 247 | l-rhamnose | 0.22 | This study |
| Laboratory rat* | Rattus norvegicus | 300 | l-rhamnose | 0.134 | 39, 57 |
| Laboratory rat | Rattus norvegicus | 300 | Mannitol | 0.056 | This study, 57–62 |
| Common marmoset* | Callithrix jacchus | 370 | l-rhamnose | 0.302 | 63 |
| Guinea pig | Cavia aperea | 980 | l-rhamnose | 0.064 | 57 |
| Guinea pig | Cavia aperea | 980 | Mannitol | 0.054 | 57, 58 |
| Cat | Felis domesticus | 2,800 | Mannitol | 0.285 | 58 |
| Rabbit | Oryctolagus cunciculus | 3,000 | Mannitol | 0.021 | 58 |
| Rhesus macaque* | Macaca mulatta | 8,912 | l-rhamnose | 0.082 | This study |
| Dog | Canis familiaris | 12,875 | l-rhamnose | 0.168 | 64 |
| Human | Homo sapiens | 70,000 | l-rhamnose | 0.118 | 57, 65–76 |
| Human | Homo sapiens | 70,000 | Mannitol | 0.218 | 57, 58, 71, 75, 77–82 |
The 10 focal species of birds and nonflying mammals measured during this study are indicated by *.
Fig. 2.
Fractional absorption (bioavailability) in intact animals of inert, nonactively transported, water-soluble carbohydrates, a measure of paracellular absorption. (A) For all individuals in the 10 focal species (indicated by * in Table 1), absorption of l-rhamnose was measured with a standard protocol. In the comparison of birds and nonflying mammals by ANCOVA, fractional absorption declined with increasing log10body mass (F1,6 = 19.5) and differed significantly between the two taxa (F1,6 = 9.5), although the difference diminished with increasing size (interaction F1,6 = 7.3). Values of l-rhamnose fractional absorption in two species of bats were as high, or higher, than the values of the avian species, and above those for nonflying mammals. (B) The broader survey includes additional measures from the literature (Table 1) of fractional absorption of l-rhamnose or similarly sized water-soluble carbohydrate probes, l- glucose (MM = 180) and mannitol (MM = 182). Each point represents the fractional absorption of a probe by a species. In five of the 29 cases, the value shown is the mean of more than one study. In the comparison of birds, bats, and nonflying mammals by ANCOVA, fractional absorption differed significantly according to taxa (F2,23 = 11.9), although the difference diminished with increasing size (interaction F2,23 = 5.46). None of the nonflying mammals exhibited fractional absorption of these carbohydrates as high as that in small birds or bats.
If there has indeed been natural selection for smaller intestinal size in flying endotherms, and increased paracellular absorption as a compensation, then we might expect to find the same patterns in a comparison within mammals between flyers and nonfliers. Within mammals flight evolved once in the order Chiroptera, which is composed of two major suborders: the Microchiroptera and Megachiroptera (27). Several biologists noted that bats tend to have less intestinal tissue than comparably sized nonflying mammals (28, 29), a pattern confirmed with our dataset. Bats had significantly shorter intestines (data not shown) and less small intestinal nominal surface area than nonflying mammals, although the difference diminished as body size increased (Fig. 1C). We measured fractional absorption of l-rhamnose in Egyptian fruit bats (Rousettus aegyptiacus; Megachiroptera) and in the great fruit-eating bat (Artibeus lituratus, Microchiroptera) with our standard pharmacokinetic protocol. The mean fractional absorption values for both bat species were as high, or higher, than the values of the avian species, and above those for nonflying mammals (Fig. 2A), indicating relatively higher paracellular absorption.
To broaden our comparison and test the veracity of our finding we surveyed the literature for other studies of fractional absorption of water-soluble carbohydrates (Table 1). Our broader survey includes studies that used similar methodology to ours (either serial blood or urine sampling postinjection and/or postoral administration) to measure fractional absorption of l-rhamnose or the similarly sized water-soluble carbohydrate probes l-glucose [molecular mass (MM) = 180] and mannitol (MM = 182), which are also metabolically inert and lack affinity for mediated transport mechanisms. Fractional absorptions were similar within a species when measured with two or more different compounds. Although paracellular absorption should decline with increasing MM, because of sieving at the tight junction (21, 30), there was no significant difference in the adjusted least-square mean fractional absorption (arcsine square root transformed) according to MM over this small range for either nonflying mammals (F1,12 = 1.07, P > 0.3) or birds (F1,7 = 1.18, P > 0.3). Fractional absorptions were similar within a species when measured with two or more different compounds (Table 1). For three species (guinea pigs, laboratory rats, and humans) of a total of 23 species, we calculated a single species mean value for fractional absorption of l-rhamnose and/or mannitol because there were data available from several studies. In the comparison of taxa we did not distinguish between measurements of fractional absorption that were made in the presence of luminal nutrients vs. their absence (i.e., in fasted animals). Although permeability of the paracellular pathway is increased when Na+-coupled glucose and amino acid transport occurs (17, 31, 32), the increase in fractional absorption in rats and house sparrows (+0.06 ± 0.03, n = 4 studies with l-rhamnose, l- glucose, and mannitol; refs. 21, 33, and 34) was small relative to the large difference in fractional absorption between birds and nonflying mammals. The patterns in fractional absorption in our focal species held up when we included other species surveyed (Fig. 2B). None of the nonflying mammals exhibit fractional absorption of these carbohydrates as high as occurs in small birds or bats. High paracellular absorption in small birds from seven different avian families with varied diets (omnivores, nectarivores, and granivores) suggests that it is an important pathway of absorption of water-soluble compounds in small avian species generally, rather than being associated with a specific diet type. Thus, the data from uniform methods on our focal species and the data from less uniform methods in the broader species survey are consistent with the hypothesis that paracellular absorption is elevated in flying birds and mammals, relative to nonflying mammals, as compensation for smaller intestines.
It appears that reduced intestinal size and elevated intestinal paracellular absorption can be added to the suite of adaptations (1) that have evolved in actively flying vertebrates. Our demonstration of a difference in digestive physiology between flyers and nonfliers begs many questions in physiology and ecology.
The differences in paracellular absorption between fliers and nonfliers do not extend to absorption generally. For example, whereas the nonflying mammals had relatively low fractional absorption of l-rhamnose, their absorption (measured by our standard protocol) of 3-O-methyl-d-glucose, a nonmetabolizable analogue of d-glucose that is actively as well as passively absorbed, was universally high (0.76 ± 0.06, n = 5 species) and not significantly different from that of flying species (0.90 ± 0.04, n = 4, 2 bird and 2 bat species, t7 = 2.02, P > 0.08). Paracellular absorption can account for the majority of glucose absorption in at least two avian species (35, 36) and at least two bat species (37, 38) according to our comparison of simultaneous apparent rates of absorption of paracellular probes (such as l-rhamnose or l-glucose) and 3-O-methyl-d-glucose or d-glucose. In accordance with August Krogh's dictum (83), the study of the cellular and subcellular details of paracellular absorption might be advanced by study of such species with relatively high paracellular absorption.
The difference in paracellular absorption between fliers and nonfliers is not simply explained by mediated absorption in birds of the carbohydrate probes that are presumed to be absorbed passively or by longer retention of digesta in fliers than in nonfliers. In studies using radiolabeled l-glucose and l-rhamnose, we have failed to find evidence that their uptake by intestine in vitro is inhibited by high concentrations (50–100 mM) of unlabeled l-glucose, l- rhamnose, or d-glucose (24, 33, 39). Nor is the difference in paracellular absorption between fliers and nonfliers explained by longer retention of digesta in the gut of the former relative to the latter. Avian species typically have shorter mean retention time of digesta than do similarly sized nonflying mammalian species (24), and a shorter retention time probably occurs in bats relative to nonflying mammals (28). Because small vertebrate flyers typically achieve higher paracellular absorption with less intestinal length and surface area than do similarly sized nonflying mammals, there apparently are differences in intestinal permeability per unit intestinal tissue. We have confirmed this elsewhere in a comparison of pigeons and laboratory rats (39). Under similar recirculating duodenal perfusion conditions, we found that anesthetized rats and pigeons absorbed d-glucose at a comparable rate, but that pigeons had significantly greater (>2× higher) absorption of inert carbohydrate probes (24, 34).
The mechanisms responsible for relatively higher paracellular absorption in some species are unknown, but could include the following: (i) Greater villous area per unit intestinal nominal surface area might be associated with more cell junctions across which paracellular transport occurs, if villous area is increased mainly by increase in number of similar-sized enterocytes. Barry (40) and Mayhew and coworkers (41, 42) have made measurements on a bat species and a similar-sized nonflying mammal species by using uniform methodology, and in all cases the ratio of villous area relative to nominal surface area in the bat species exceeded that in the nonflying mammal by ≥59%. We have found this same pattern in our comparisons of small birds with relatively high paracellular absorption and nonflying mammals with relatively lower paracellular absorption (24). (ii) Larger effective pore radius in the junction, caused by differences in claudins and other proteins that create the sieving effect, will increase paracellular permeation over certain size ranges of molecules (21, 30). (iii) Greater water flux across the tight junction will increase solute permeation by increased solvent drag (17).
From an evolutionary perspective, one can argue that there are both benefits and costs to high intestinal permeability to hydrosoluble biochemicals, which would explain why it is present in some, but not all, vertebrates. We have suggested that a selective advantage in the case of active fliers is that it can compensate for smaller intestines, and Pappenheimer (43) suggested that passive absorption may confer a selective advantage because it requires little energy and provides a mechanism whereby rate of absorption is matched to rate of substrate hydrolysis in the intestine. The significant negative correlation of paracellular absorption with body mass revealed by our analysis, opposite to Pappenheimer's prediction (43), supports the notion of adaptive value for nutrient absorption in active fliers with reduced guts. Although carbohydrate probes have primarily been used to measure paracellular absorption, other hydrosoluble molecules, including peptides (23) and dyes (19), also show high fractional absorption in small omnivorous birds. Taken in the context of the present analysis, it is reasonable to predict that paracellular absorption of peptides and amino acids might be similarly important in small volant vertebrates consuming diets primarily composed of protein and fat. A possible cost is that a high intestinal permeability that permits passive absorption is less selective than a carrier-mediated system for nutrient absorption and might permit water soluble toxins made by humans (e.g., carbamate insecticides, glyphosate herbicide) and naturally occurring in foods (e.g., caffeine, nicotine, some flavonoids) to be absorbed to a greater extent in the intestine (44). Opposing costs and benefits can lead to variation among species in intestinal permeability to hydrosoluble biochemicals. For vertebrates with high intestinal permeability, vulnerability to hydrophilic toxins could be an important ecological driving force, constraining food exploratory behavior, limiting the breadth of the dietary niche, and selecting for compensatory behaviors such as searching for and ingesting specific substances that inhibit hydrophilic toxin absorption (45).
Most of the physiological and ecological issues suggested by our findings remain to be studied in vertebrate, and possibly invertebrate, fliers. Also, we could not consider diet as a factor in our analysis of paracellular absorption, so we do not know whether the difference between fliers and nonfliers occurs for all types of feeders (as it does for intestinal surface area) or mainly those that eat carbohydrate-rich foods. An enlarged comparative dataset (e.g., including insectivorous/carnivorous birds varying in body size and insectivorous bats) will permit the most robust, phylogenetically informed test of the hypothesis that increased intestinal paracellular absorption has evolved as a compensation for smaller intestinal size in flying vertebrates.
Materials and Methods
As a measure of passive, paracellular absorption, we used standard methods from pharmacokinetics to measure the whole-organism fractional absorption of water-soluble compounds that are similar in molecular size to water-soluble nutrients such as amino acids and glucose (which range in MM from 75 to 204) but are metabolically inert and lack affinity for intestinal-mediated uptake mechanisms. We applied the standard methods to five each of avian and nonflying mammalian species chosen to cover a wide range in body size (focal species) and two bat species (Table 1). For all individuals in our focal species, l-rhamnose (MM = 164) was injected, and also administered orally (typically 30–40 mM, volume ≈1% of body mass) to intact animals in separate experiments, and blood and/or urine samples were serially collected and analyzed for probe molecules. Probes were analyzed by HPLC (37). Fractional absorption (f) was calculated as [AUC postgavage]/[AUC postinjection] (AUC = dose-corrected area under the curve of plasma or urine probe concentration vs. time). This simple pharmacokinetic method does not require assumptions about pool sizes (e.g., one or two pools) or kinetics (e.g., first order) (46). In mammals, the probes can be recovered in urine, and estimates of oral absorption take account of possible differential recovery of probes when injected. In our studies with laboratory mice, rats, and marmosets, recoveries of carbohydrate probes were uniformly high, which is in agreement with measures by others in rats and humans (47). Fractional absorption of l-rhamnose in laboratory rats measured by serially sampling blood (0.24 ± 0.03) did not differ significantly from that measured by urine recovery (0.22 ± 0.03, n = 4, by repeated measures F1,3 = 1.14; P > 0.3). Here, and elsewhere, statistical analyses of fractional absorption were made on arcsin-square-root transformed values (48). In some cases test solutions included 50 mM 3-O-methyl d-glucose. l- rhamnose is commonly used in humans in tests of passive (noncarrier-mediated) intestinal permeability (49), and 3-O-methyl d-glucose is an actively transported, nonmetabolizable d-glucose analogue.
Supplementary Material
Acknowledgments
C. Korine, B. Pinshow, F. Sanchez, and M. S. Wojciechowski assisted in the studies with Egyptian fruit bats. A. Pereira Cruz-Neto, F. D. Cid, L. Otani, V. Fasulo, and R.I. Antón helped with the great fruit-eating bats. This research was primarily funded by National Science Foundation Grants IBN-0216709 and ISO-0615678 (to W.H.K.) and Agencia Nacional de Promoción Científica y Tecnológica–Fondo Nacional para Ciencia y Técnica Grant 25561 (to E.C.-V.). It was also supported in part by the Blaustein Center for Scientific Cooperation, National Institutes of Health Grant P51 RR000167 (to the Wisconsin National Primate Research Center), and Universidad Nacional de San Luis CyT Grant 22Q/751. This research was conducted in part at the Wisconsin National Primate Research Center, which was constructed with support from National Institutes of Health Research Facilities Improvement Program Grants RR15459-01 and RR020141-01. T.J.M. was supported by Australian Research Council Grant DP0665730 during revisions of the manuscript. This is paper no. 586 of the Mitrani Department of Desert Ecology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703159104/DC1.
References
- 1.Maina JN. J Exp Biol. 2000;203:3045–3064. doi: 10.1242/jeb.203.20.3045. [DOI] [PubMed] [Google Scholar]
- 2.Nagy KA. Nutr Abstr Rev B. 2001;71:21R–31R. [Google Scholar]
- 3.McLelland J. In: Form and Function in Birds. King AS, McLelland J, editors. London: Academic; 1979. pp. 69–181. [Google Scholar]
- 4.Karasov WH, McWilliams SR. In: Physiological and Ecological Adaptations to Feeding in Vertebrates. Starck JM, Wang T, editors. Enfield, NH: Science Publishers; 2005. pp. 87–112. [Google Scholar]
- 5.Nudds RL, Bryant DM. Am J Physiol. 2002;283:R249–R256. doi: 10.1152/ajpregu.00409.2001. [DOI] [PubMed] [Google Scholar]
- 6.Norberg UM. Funct Ecol. 1995;9:48–54. [Google Scholar]
- 7.Guillemette M. Auk. 1994;111:900–909. [Google Scholar]
- 8.Piersma T. J Avian Biol. 1998;29:511–520. [Google Scholar]
- 9.Piersma T, Gill REJ. Auk. 1998;115:196–203. [Google Scholar]
- 10.Karasov WH, Pinshow B. Physiol Zool. 1998;71:435–448. doi: 10.1086/515428. [DOI] [PubMed] [Google Scholar]
- 11.Piersma T, Gudmundsson GA, Lilliendahl K. Physiol Biochem Zool. 1999;72:405–415. doi: 10.1086/316680. [DOI] [PubMed] [Google Scholar]
- 12.Landys-Ciannelli MM, Piersma T, Jukema J. Funct Ecol. 2003;17:151–179. [Google Scholar]
- 13.Battley PF, Piersma T. In: Physiological and Ecological Adaptations to Feeding in Vertebrates. Starck JM, Wang T, editors. Enfield, NH: Science Publishers; 2005. pp. 201–228. [Google Scholar]
- 14.Chivers DJ, Hladik CM. J Morphol. 1980;166:337–386. doi: 10.1002/jmor.1051660306. [DOI] [PubMed] [Google Scholar]
- 15.Ricklefs RE. Condor. 1996;98:279–292. [Google Scholar]
- 16.Karasov WH, Hume ID. In: Handbook of Comparative Physiology. Dantzler W, editor. Bethesda: Am Physiol Soc; 1997. pp. 409–480. [Google Scholar]
- 17.Pappenheimer JR, Reiss KZ. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 18.Ma TY, Hollander D, Riga R, Bhalla D. J Lab Clin Med. 1993;122:590–600. [PubMed] [Google Scholar]
- 19.Chang MH, Karasov WH. Zoology. 2004;107:121–133. doi: 10.1016/j.zool.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton I, Rothwell J, Archer D, Axon TR. Clin Sci. 1987;73:189–196. doi: 10.1042/cs0730189. [DOI] [PubMed] [Google Scholar]
- 21.Chediack JG, Caviedes-Vidal E, Fasulo V, Yamin LJ, Karasov WH. J Comp Physiol B. 2003;173:187–197. doi: 10.1007/s00360-002-0314-8. [DOI] [PubMed] [Google Scholar]
- 22.He YL, Murby S, Warhurst G, Gifford L, Walker D, Ayrton J, Eastmond R, Rowland M. J Pharmacol Sci. 1998;87:626–633. doi: 10.1021/js970120d. [DOI] [PubMed] [Google Scholar]
- 23.Chediack JG, Caviedes-Vidal E, Karasov WH. J Comp Physiol B. 2006;176:303–309. doi: 10.1007/s00360-005-0052-9. [DOI] [PubMed] [Google Scholar]
- 24.Lavin SR. Madison: University of Wisconsin; 2007. PhD thesis. [Google Scholar]
- 25.Felsenstein J. Am Nat. 1985;125:1–15. [Google Scholar]
- 26.Pappenheimer JR. Comp Biochem Physiol. 1998;121:45–58. doi: 10.1016/s1095-6433(98)10100-9. [DOI] [PubMed] [Google Scholar]
- 27.Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ, Murphy WJ. Science. 2005;307:580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- 28.Klite PD. J Bacteriol. 1965;90:375–379. doi: 10.1128/jb.90.2.375-379.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keegan DJ, Mödinger R. S Afr J Zool. 1979;14:220–223. [Google Scholar]
- 30.Chang RLS, Robertson CR, Deen WM, Brenner BM. Biophys J. 1975;15:861–886. doi: 10.1016/S0006-3495(75)85862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner JR, Madara JL. Gastroenterology. 1995;109:1391–1396. doi: 10.1016/0016-5085(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 32.Sadowski DC, Meddings JB. Can J Physiol Pharmacol. 1993;71:835–839. doi: 10.1139/y93-125. [DOI] [PubMed] [Google Scholar]
- 33.Chang MH, Chediack JG, Caviedes-Vidal E, Karasov WH. J Comp Physiol B. 2004;174:181–188. doi: 10.1007/s00360-003-0403-3. [DOI] [PubMed] [Google Scholar]
- 34.Lavin SR, McWhorter TJ, Karasov WH. Integr Comp Biol. 2004;44:717. [Google Scholar]
- 35.Chang MH, Karasov WH. J Exp Biol. 2004;207:3109–3121. doi: 10.1242/jeb.01154. [DOI] [PubMed] [Google Scholar]
- 36.McWhorter TJ, Karasov WH, Green AK. Integr Comp Biol. 2004;44:602. [Google Scholar]
- 37.Tracy CR, McWhorter TJ, Korine C, Wojciechowski MS, Pinshow B, Karasov WH. J Exp Biol. 2007;210:1726–1734. doi: 10.1242/jeb.02766. [DOI] [PubMed] [Google Scholar]
- 38.Caviedes-Vidal E, Chediack JG, Cruz-Neto AP, Karasov WH. Integr Comp Biol. 2004;44:534. [Google Scholar]
- 39.Lavin SR, McWhorter TJ, Karasov WH. J Exp Biol. 2007;210:2754–2764. doi: 10.1242/jeb.006114. [DOI] [PubMed] [Google Scholar]
- 40.Barry RE., Jr J Mammal. 1976;57:273–289. [PubMed] [Google Scholar]
- 41.Mayhew TM, Middleton C. J Anat. 1985;141:1–17. [PMC free article] [PubMed] [Google Scholar]
- 42.Makanya AN, Maina JN, Mayhew TM, Tschanz SA, Burri PH. J Exp Biol. 1997;200:2415–2423. doi: 10.1242/jeb.200.18.2415. [DOI] [PubMed] [Google Scholar]
- 43.Pappenheimer JR. Am J Physiol. 1993;265:G409–G417. doi: 10.1152/ajpgi.1993.265.3.G409. [DOI] [PubMed] [Google Scholar]
- 44.Diamond J. News Physiol Sci. 1991;6:92–96. [Google Scholar]
- 45.Diamond J, Bishop KD, Gilardi JD. Ibis. 1999;141:181–193. [Google Scholar]
- 46.Welling PG. Pharmacokinetics: Processes and Mathematics. Washington, DC: Am Chem Soc; 1986. [Google Scholar]
- 47.Riviere JE. Comparative Pharmacokinetics: Principles, Techniques and Applications. Ames: Iowa State Univ Press; 1999. [Google Scholar]
- 48.Wilkinson L. Systat for Windows: Statistics. Evanston, IL: Systat; 1992. version 5. [Google Scholar]
- 49.Travis S, Menzies I. Clin Sci. 1992;82:471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]
- 50.White CR. Physiol Biochem Zool. 2003;76:135–140. doi: 10.1086/367939. [DOI] [PubMed] [Google Scholar]
- 51.McWhorter TJ, Hartman, Bakken B, Karasov WH, Martínez del Rio C. Biol Lett. 2006;2:131–134. doi: 10.1098/rsbl.2005.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Afik D, McWilliams SR, Karasov WH. Physiol Zool. 1997;70:370–377. doi: 10.1086/639618. [DOI] [PubMed] [Google Scholar]
- 53.Chediack JG, Caviedes-Vidal E, Karasov WH, Pestchanker M. J Exp Biol. 2001;204:723–731. doi: 10.1242/jeb.204.4.723. [DOI] [PubMed] [Google Scholar]
- 54.Karasov WH, Cork SJ. Am J Physiol. 1994;267:G16–G26. doi: 10.1152/ajpgi.1994.267.1.G18. [DOI] [PubMed] [Google Scholar]
- 55.Levey DJ, Cipollini ML. Comp Biochem Physiol. 1996;113:225–231. [Google Scholar]
- 56.Caviedes-Vidal E, Karasov WH, Chediack JG, Fasulo V, Cruz-Neto AP, Otani L. PLoS. 2007 doi: 10.1371/journal.pone.0001425. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delahunty T, Hollander D. Comp Biochem Physiol. 1987;86:565–567. doi: 10.1016/0300-9629(87)90542-1. [DOI] [PubMed] [Google Scholar]
- 58.Bijlsma PB, Peeters RA, Groot JA, Dekker PR, Taminiau JAJM, Meer RvD. Gastroenterology. 1995;108:687–696. doi: 10.1016/0016-5085(95)90440-9. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz RM, Furne JK, Levitt MD. Gastroenterology. 1995;109:1206–1213. doi: 10.1016/0016-5085(95)90580-4. [DOI] [PubMed] [Google Scholar]
- 60.Sigalet DL, Kneteman NN, Fedorak RN, Kizilisik T, Madsen KE, Thomson ABR. J Surg Res. 1996;61:379–384. doi: 10.1006/jsre.1996.0133. [DOI] [PubMed] [Google Scholar]
- 61.Sigalet DL, Martin GR, Poole A. J Ped Surg. 2000;35:661–664. doi: 10.1053/jpsu.2000.5937. [DOI] [PubMed] [Google Scholar]
- 62.Martin GR, Meddings JB, Sigalet DL. J Parenter Enter Nutr. 2003;27:65–70. doi: 10.1177/014860710302700165. [DOI] [PubMed] [Google Scholar]
- 63.McWhorter TJ, Karasov WH. Am J Primatol. 2007;69:1399–1411. doi: 10.1002/ajp.20443. [DOI] [PubMed] [Google Scholar]
- 64.Sørensen SH, Proud FJ, Adam A, Rutgers HC, Batt RM. Clin Chim Acta. 1993;221:115–125. doi: 10.1016/0009-8981(93)90026-z. [DOI] [PubMed] [Google Scholar]
- 65.Menzies IS, Pounder R, Heyer S, Laker MF, Bull J, Wheeler PG, Creamer B. Lancet. 1979:1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- 66.Menzies IS, Noone C, Bull J, Mount JN. Gut. 1983;24:A488. [Google Scholar]
- 67.Menzies IS. In: Intestinal Absorption and Secretion. Skadhauge E, Heintze K, editors. Lancaster, UK: MTP Press; 1984. pp. 527–543. [Google Scholar]
- 68.Saweirs WM, Andrews DJ, Low-Beer TS. Age Ageing. 1985;14:312–315. doi: 10.1093/ageing/14.5.312. [DOI] [PubMed] [Google Scholar]
- 69.Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Clin Sci. 1986;71:71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- 70.Noone C, Menzies IS, Banatvala JE, Scopes JW. Eur J Clin Invest. 1986;16:217–225. doi: 10.1111/j.1365-2362.1986.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 71.Erikson RA, Epsten RM. Am J Gastroenterol. 1988;83:541–544. [PubMed] [Google Scholar]
- 72.Menzies IS, Jenkins AP, Heduan E, Catt SD, Segal MB, Creamer B. Scand J Gastroenterol. 1990;25:1257–1264. doi: 10.3109/00365529008998562. [DOI] [PubMed] [Google Scholar]
- 73.Bjarnason I, Maxton D, Reynolds AP, Catt S, Peters TJ, Menzies IS. Scand J Gastroenterol. 1994;29:630–639. doi: 10.3109/00365529409092484. [DOI] [PubMed] [Google Scholar]
- 74.Dinmore AJ, Edwards JSA, Menzies IS, Travis SPL. J Appl Physiol. 1994;76:1903–1907. doi: 10.1152/jappl.1994.76.5.1903. [DOI] [PubMed] [Google Scholar]
- 75.Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menzies IS, Zuckerman MJ, Nukajam WS, Somasundaram SG, Murphy B, Jenkins AP, Crane RS, Gregory GG. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elia M, Behrnes R, Northrop C, Wraight P, Neale G. Clin Sci. 1987;73:197–204. doi: 10.1042/cs0730197. [DOI] [PubMed] [Google Scholar]
- 78.Cobden I, Hamilton I, Rothwell J, Axon ATR. Clin Chim Acta. 1985;148:53–62. doi: 10.1016/0009-8981(85)90300-6. [DOI] [PubMed] [Google Scholar]
- 79.Brunetto AL, Pearson ADJ, Gibson R, Bateman DN, Rashid MU. Eur J Clin Invest. 1990;20:279–284. doi: 10.1111/j.1365-2362.1990.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 80.Fleming SC, Kapembwa MS, Laker MF, Levin GE, Griffin GE. Clin Chem. 1990;36:797–799. [PubMed] [Google Scholar]
- 81.Fleming SC, Kynaston JA, Laker MF, Pearson ADJ, Kapembwa MS, Griffin GE. J Chromatogr. 1993;640:293–297. doi: 10.1016/0021-9673(93)80193-c. [DOI] [PubMed] [Google Scholar]
- 82.Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. J Chromatogr B. 2003;784:145–154. doi: 10.1016/s1570-0232(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 83.Krogh A. Am J Physiol. 1929;90:243–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.