Abstract
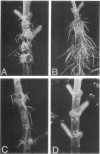
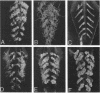
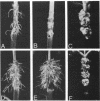
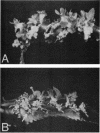
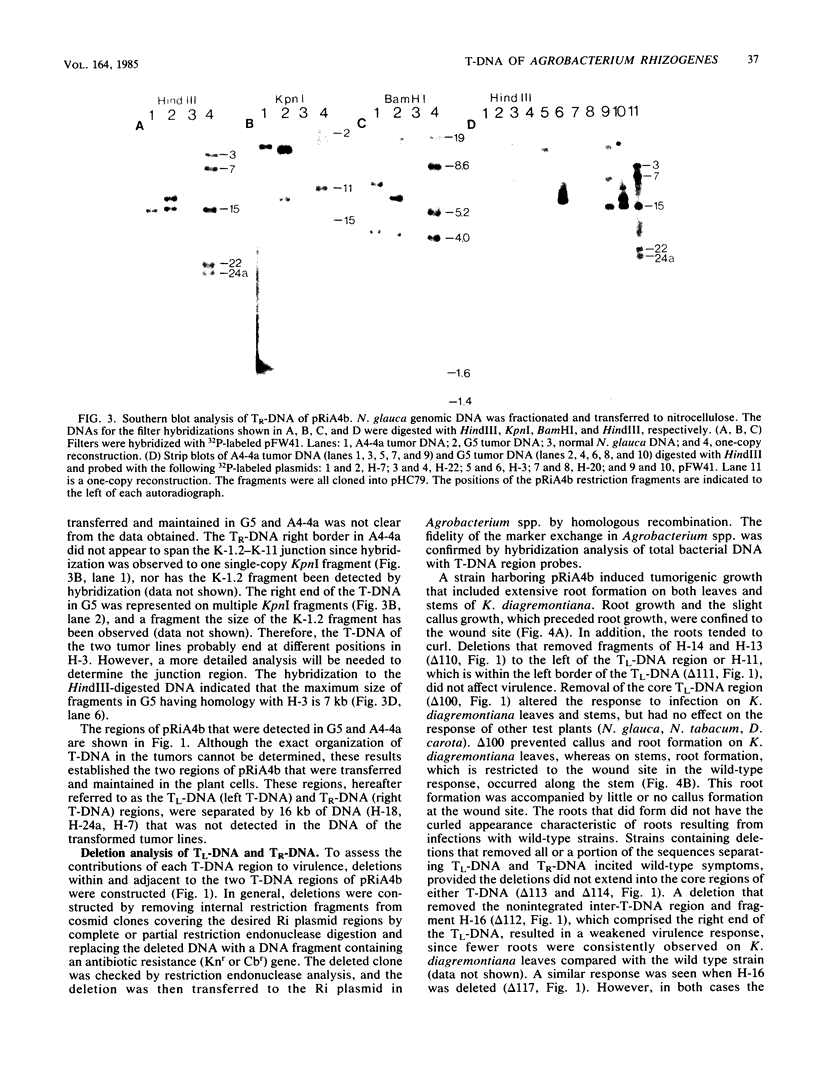
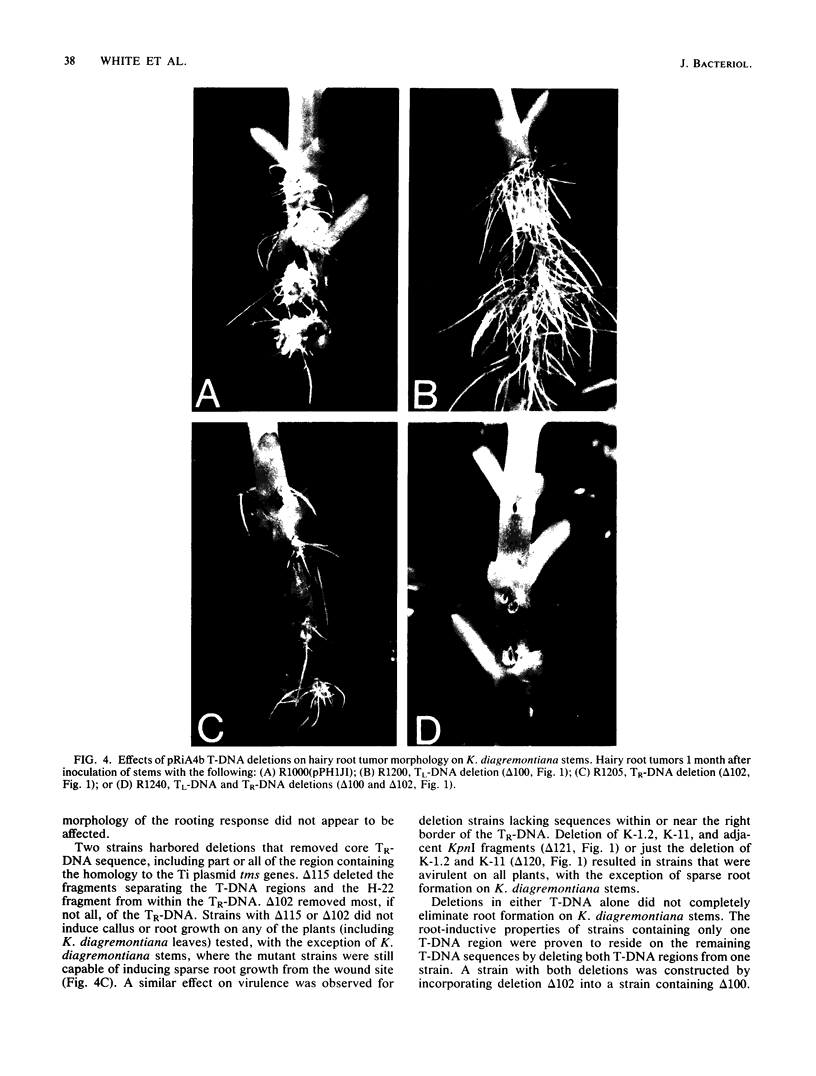
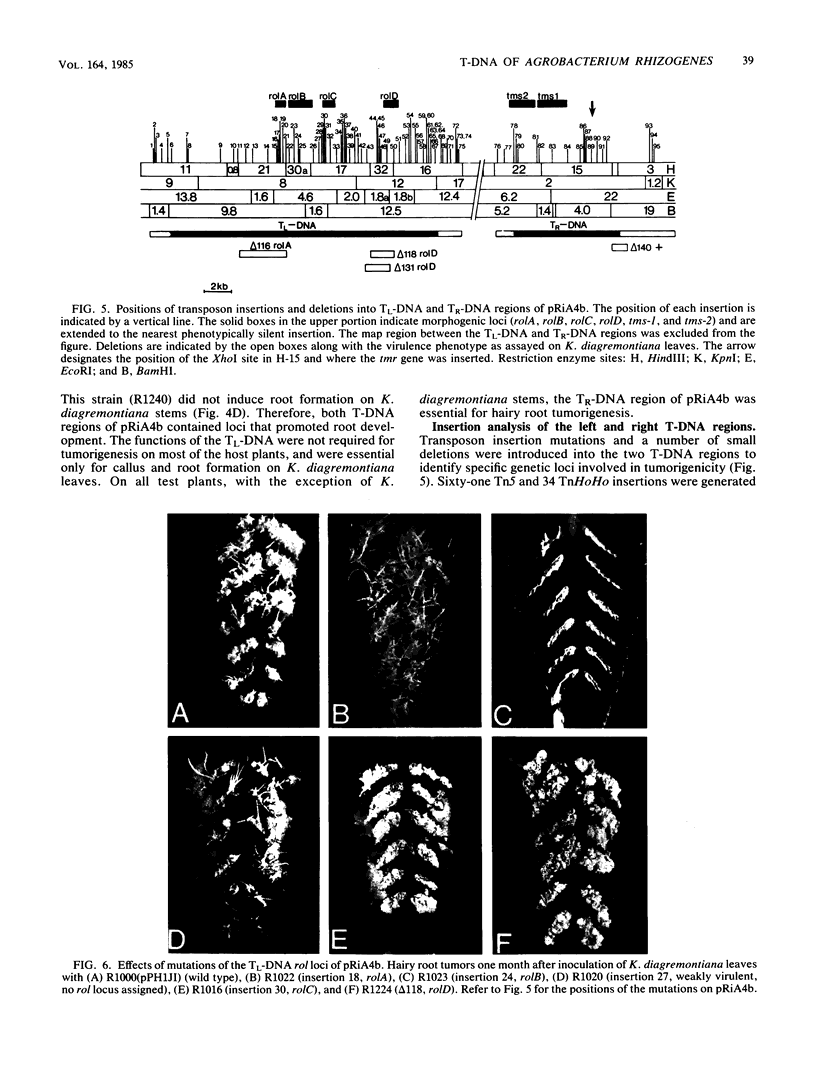
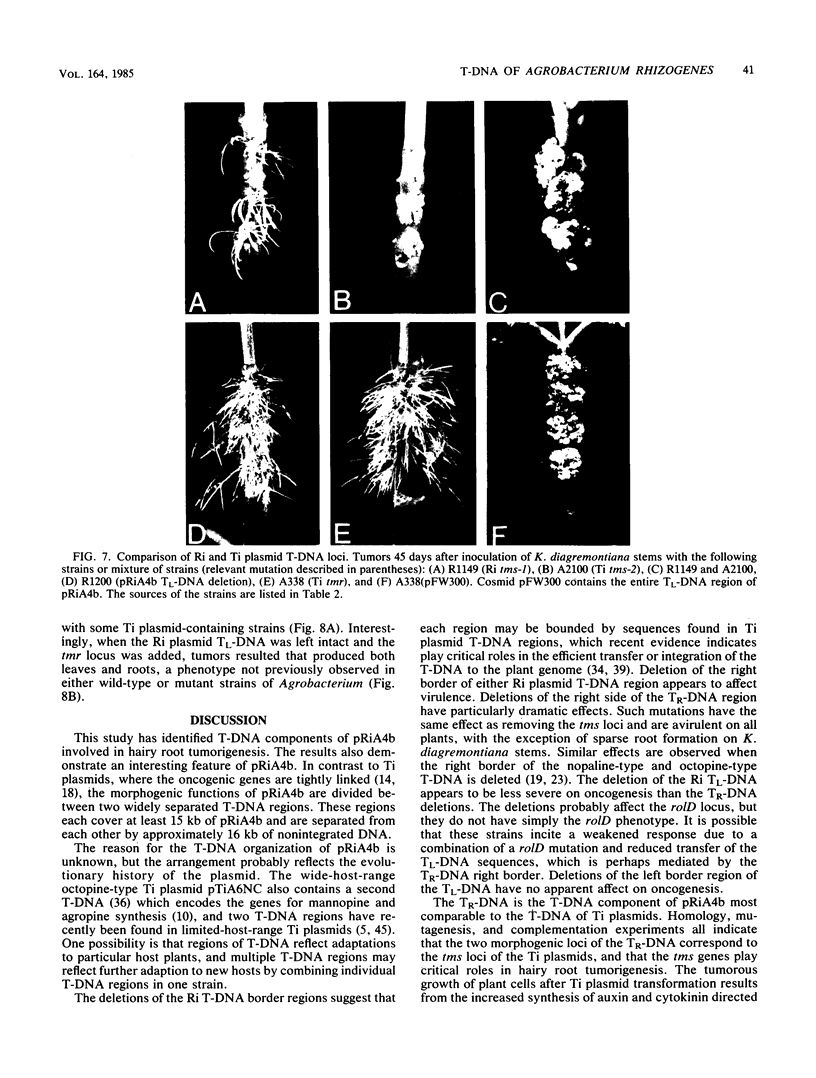
The T-DNA regions of the root-inducing (Ri) plasmid pRiA4b of Agrobacterium rhizogenes were characterized. Two regions, designated TL-DNA and TR-DNA, were found to be integrated and stably maintained in the plant genome. The TL-DNA spanned a 15- to 20-kilobase region of pRiA4b and was separated from the TR-DNA region by at least 15 kilobases of nonintegrated plasmid DNA. The TR-DNA region also spanned a 15- to 20-kilobase region of pRiA4b and included a region of homology to the tms morphogenic loci of the tumor-inducing (Ti) plasmid of Agrobacterium tumefaciens. Eighteen deletions and 95 transposon insertions were generated in the T-DNA regions and tested for alterations in virulence. Insertions into four loci in the TL-DNA affected the morphology of root formation of Kalanchoë diagremontiana leaves and stems, but had no visible effects on other host plants. Insertions into two loci (tms-1 and tms-2) in the TR-DNA eliminated virulence symptoms on all plants tested, with the exception of K. diagremontiana stems, where sparse root formation occurred. Complementation experiments with Ri and Ti plasmid T-DNA mutations indicate that the tms genes of the two plasmids serve similar functions and suggest a functional relationship between one or more genes of the TL-DNA and the cytokinin synthesis locus tmr of the Ti plasmid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi D. E., Morris R. O., Hinz R., Mischke B. S., Kosuge T., Garfinkel D. J., Gordon M. P., Nester E. W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci U S A. 1983 Jan;80(2):407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz W. G., Thomashow M. F. Comparison of T-DNA oncogene complements of Agrobacterium tumefaciens tumor-inducing plasmids with limited and wide host ranges. J Bacteriol. 1984 Oct;160(1):319–326. doi: 10.1128/jb.160.1.319-326.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. C., Koplow J., David C., Tempé J., Chilton M. D. Structure of T-DNA in roots transformed by Agrobacterium rhizogenes. J Mol Appl Genet. 1983;2(2):201–209. [PubMed] [Google Scholar]
- Chilton M. D., Currier T. C., Farrand S. K., Bendich A. J., Gordon M. P., Nester E. W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino P., Mauro M. L., Micheli G., Risuleo G., Hooykaas P. J., Schilperoort R. Fingerprinting and sequence homology of plasmids from different virulent strains of Agrobacterium rhizogenes. Plasmid. 1981 Mar;5(2):170–182. doi: 10.1016/0147-619x(81)90018-4. [DOI] [PubMed] [Google Scholar]
- Costantino P., Spanò L., Pomponi M., Benvenuto E., Ancora G. The T-DNA of Agrobacterium rhizogenes is transmitted through meiosis to the progeny of hairy root plants. J Mol Appl Genet. 1984;2(5):465–470. [PubMed] [Google Scholar]
- De Paolis A., Mauro M. L., Pomponi M., Cardarelli M., Spanò L., Costantino P. Localization of agropine-synthesizing functions in the TR region of the root-inducing plasmid of Agrobacterium rhizogenes 1855. Plasmid. 1985 Jan;13(1):1–7. doi: 10.1016/0147-619x(85)90050-2. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983 Jun 3;220(4601):1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Holsters M., de Waele D., Depicker A., Messens E., van Montagu M., Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978 Jul 11;163(2):181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- Huffman G. A., White F. F., Gordon M. P., Nester E. W. Hairy-root-inducing plasmid: physical map and homology to tumor-inducing plasmids. J Bacteriol. 1984 Jan;157(1):269–276. doi: 10.1128/jb.157.1.269-276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Klee H., Montoya A., Horodyski F., Lichtenstein C., Garfinkel D., Fuller S., Flores C., Peschon J., Nester E., Gordon M. Nucleotide sequence of the tms genes of the pTiA6NC octopine Ti plasmid: two gene products involved in plant tumorigenesis. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1728–1732. doi: 10.1073/pnas.81.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Koekman B. P., Ooms G., Klapwijk P. M., Schilperoort R. A. Genetic map of an octopine TI-plasmid. Plasmid. 1979 Jul;2(3):347–357. doi: 10.1016/0147-619x(79)90018-0. [DOI] [PubMed] [Google Scholar]
- Lahners K., Byrne M. C., Chilton M. D. T-DNA fragments of hairy root plasmid pRi8196 are distantly related to octopine and nopaline Ti plasmid T-DNA. Plasmid. 1984 Mar;11(2):130–140. doi: 10.1016/0147-619x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Moore L., Warren G., Strobel G. Involvement of a plasmid in the hairy root disease of plants caused by Agrobacterium rhizogenes. Plasmid. 1979 Oct;2(4):617–626. doi: 10.1016/0147-619x(79)90059-3. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Moolenaar G., Schilperoort R. A. Grown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. Gene. 1981 Jun-Jul;14(1-2):33–50. doi: 10.1016/0378-1119(81)90146-3. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Shaw C. H., Watson M. D., Carter G. H., Shaw C. H. The right hand copy of the nopaline Ti-plasmid 25 bp repeat is required for tumour formation. Nucleic Acids Res. 1984 Aug 10;12(15):6031–6041. doi: 10.1093/nar/12.15.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell. 1984 Jul;37(3):959–967. doi: 10.1016/0092-8674(84)90430-6. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang K., Herrera-Estrella L., Van Montagu M., Zambryski P. Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from agrobacterium to the plant genome. Cell. 1984 Sep;38(2):455–462. doi: 10.1016/0092-8674(84)90500-2. [DOI] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol. 1980 Mar;141(3):1134–1141. doi: 10.1128/jb.141.3.1134-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Relationship of plasmids responsible for hairy root and crown gall tumorigenicity. J Bacteriol. 1980 Nov;144(2):710–720. doi: 10.1128/jb.144.2.710-720.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M., Montoya A., Knauf V., Lowe B., Gordon M., Nester E. Limited-host-range plasmid of Agrobacterium tumefaciens: molecular and genetic analyses of transferred DNA. J Bacteriol. 1985 Jul;163(1):341–348. doi: 10.1128/jb.163.1.341-348.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]