Abstract
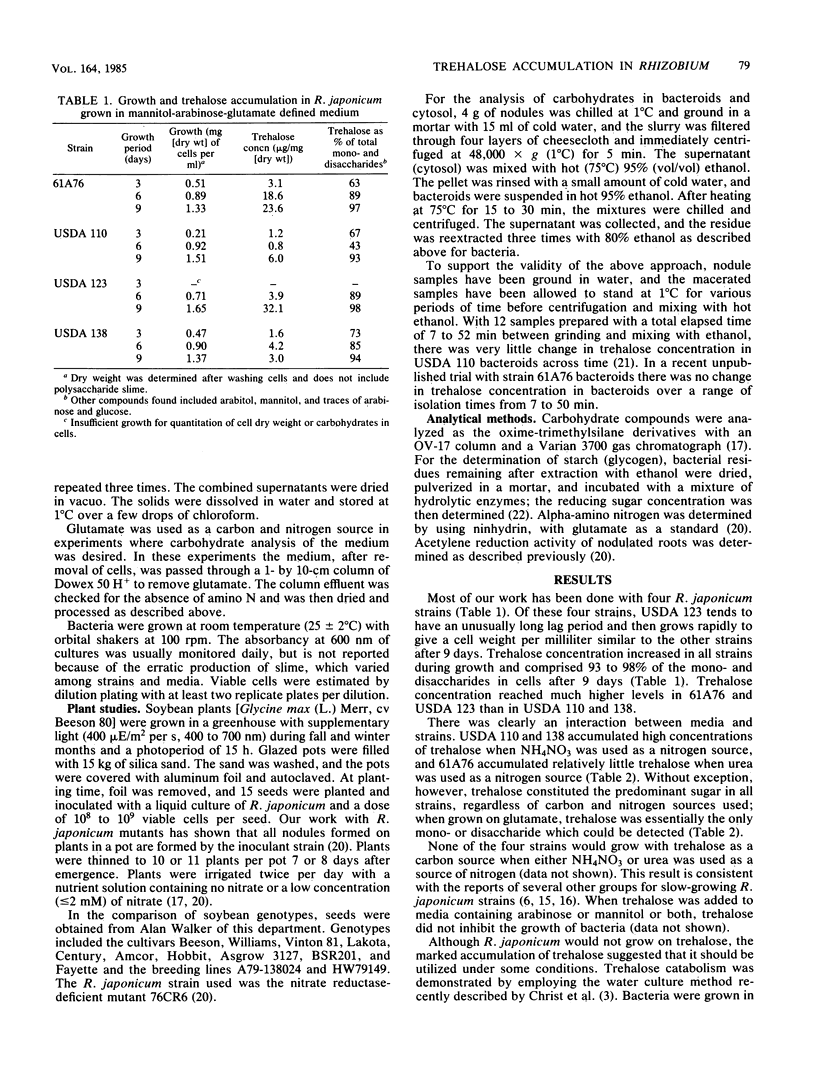
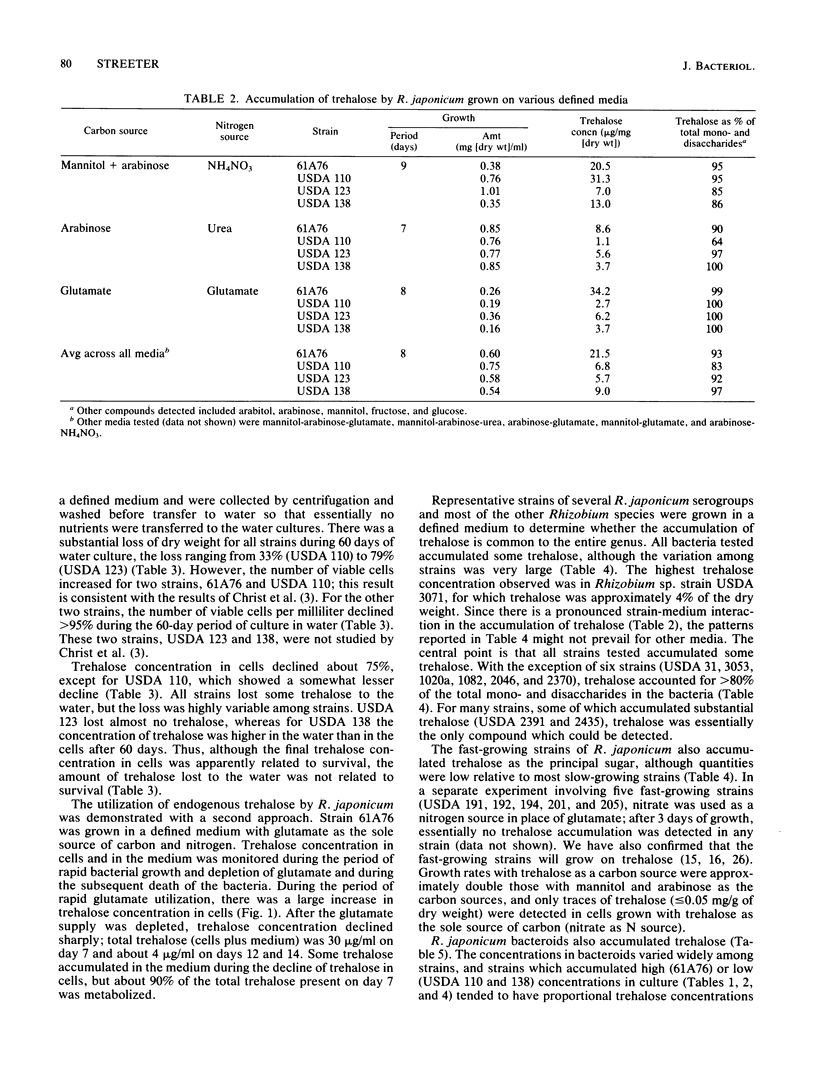
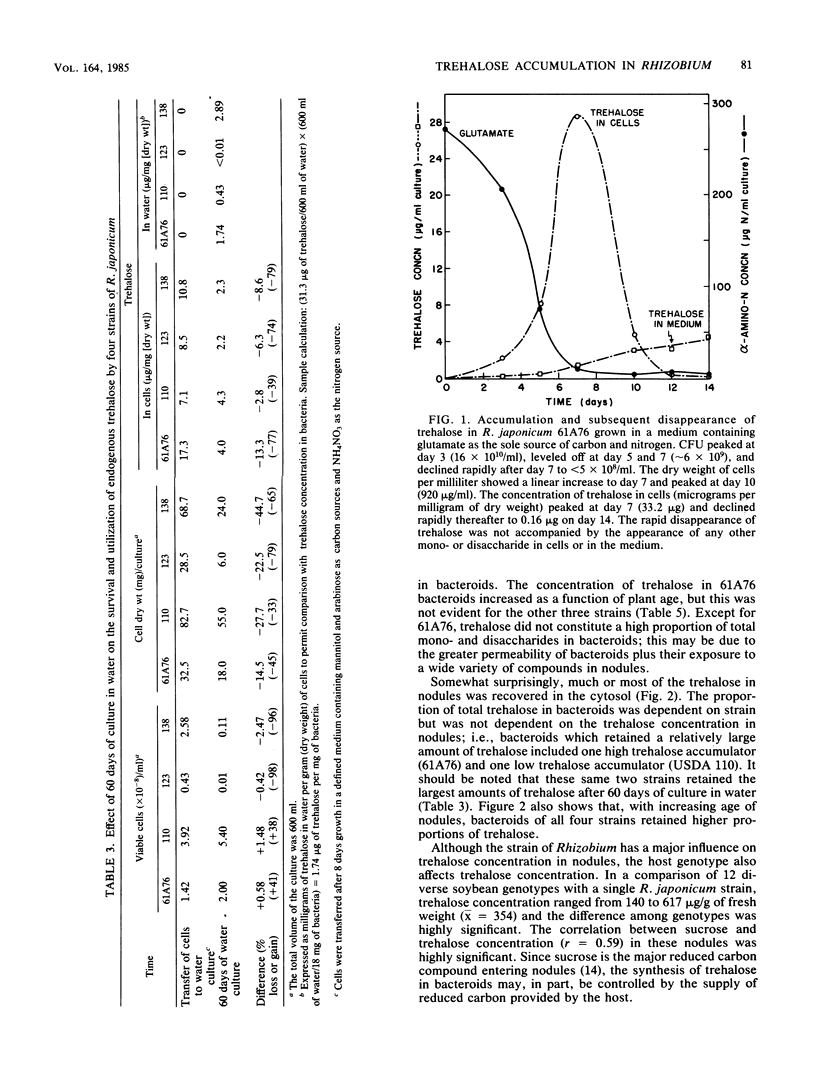
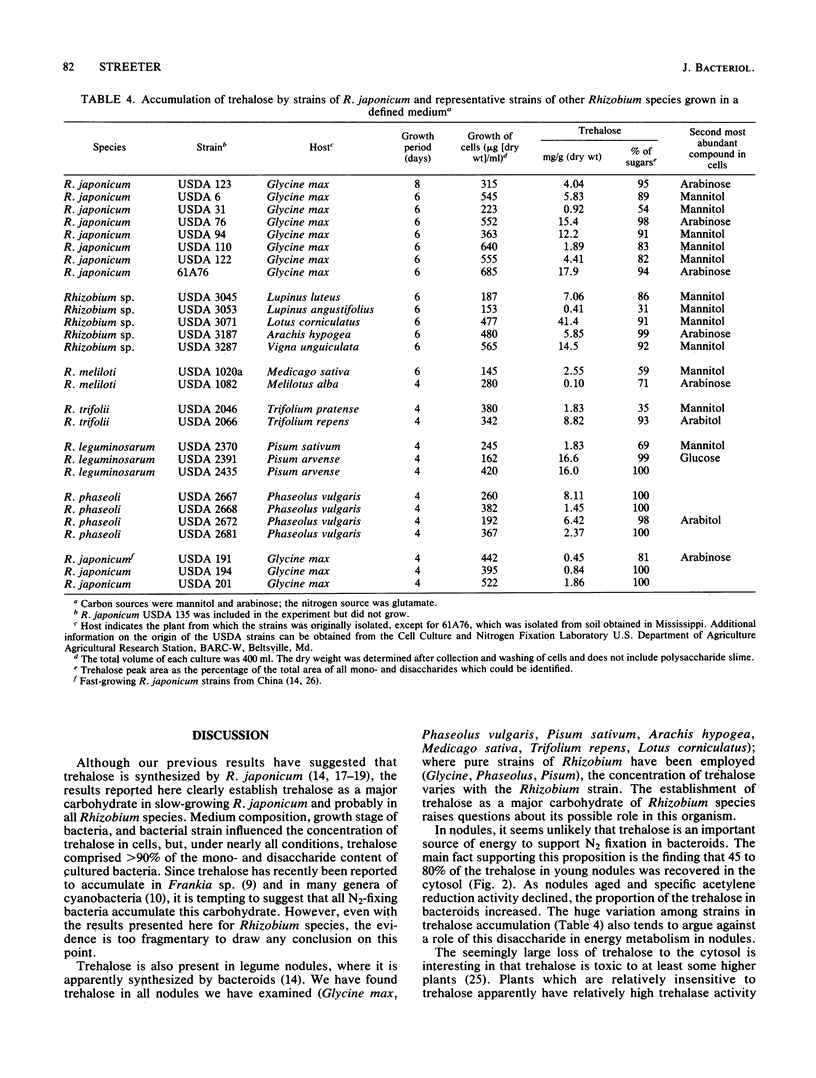
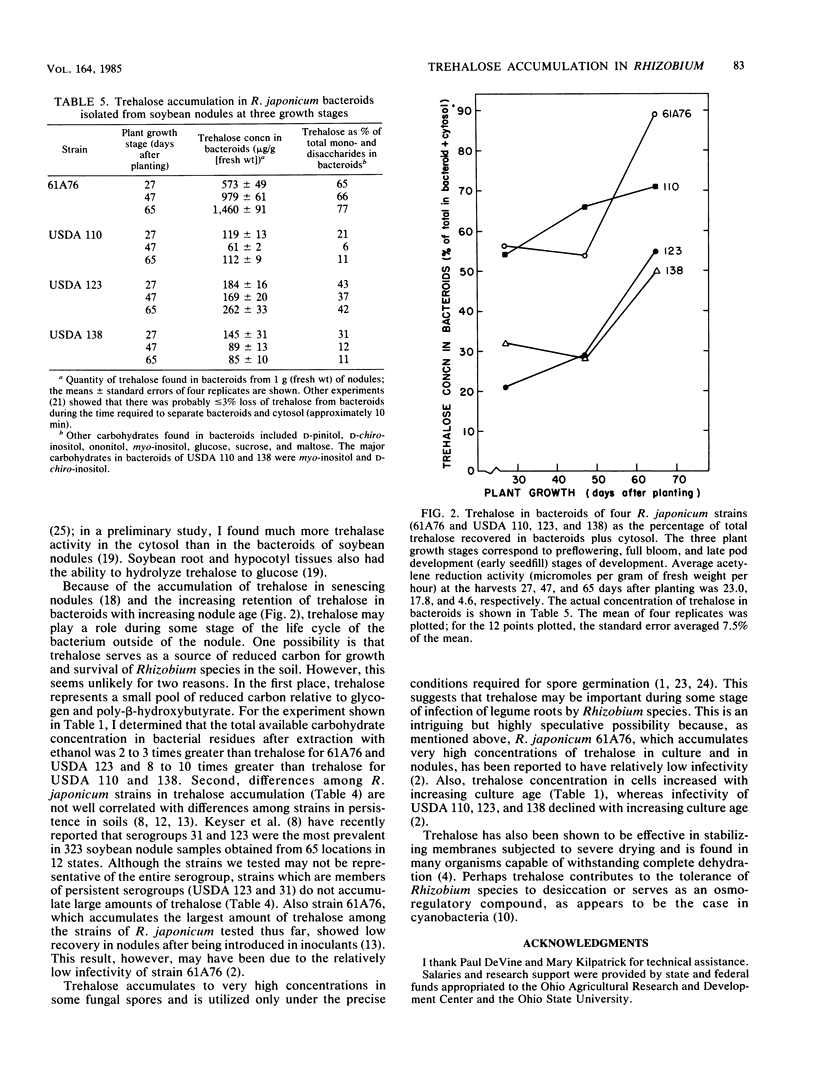
Four strains of Rhizobium japonicum (61A76 and USDA 110, 123, and 138) were grown in eight different defined media. Regardless of the carbon or nitrogen source supplied, alpha, alpha-trehalose was the major carbohydrate (among mono- and disaccharides) accumulated by all four strains. After 7 to 9 days of growth, trehalose generally accounted for 90 to 100% of the mono- and disaccharides detected. None of the four strains would grow with trehalose as a carbon source, but the utilization of endogenous trehalose was demonstrated under carbon starvation conditions in water culture or when the carbon supply in a defined medium was exhausted. Under these conditions, a small amount of trehalose was lost from cells to the medium. In a survey of most of the serogroups of R. japonicum and several strains of other Rhizobium species, all strains tested were found to accumulate some trehalose. Trehalose concentrations varied widely; the highest concentration recorded was 41 micrograms/mg of dry weight. In all but six strains trehalose accounted for greater than 80% of the mono- and disaccharides in cells. Fast-growing strains of R. japonicum also accumulated small amounts trehalose. R. japonicum bacteroids also synthesized trehalose; the quantity in nodules varied in approximate correspondence to accumulation of trehalose by cultured bacteria. In young soybean nodules (29 days after planting), 45 to 80% of the trehalose was recovered in the cytosol. There were differences among R. japonicum strains in the retention of trehalose, and the proportion of trehalose retained by bacteroids increased with increasing plant age for all strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., Den Hollander J. A., Hopfield J. J., Shulman R. G. 13C nuclear magnetic resonance study of trehalose mobilization in yeast spores. J Bacteriol. 1982 Jul;151(1):177–185. doi: 10.1128/jb.151.1.177-185.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist D. K., Wyza R. E., Mills K. K., Bauer W. D., Evans W. R. Preservation of Rhizobium viability and symbiotic infectivity by suspension in water. Appl Environ Microbiol. 1984 May;47(5):895–900. doi: 10.1128/aem.47.5.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. The metabolism of alpha,alpha-trehalose. Adv Carbohydr Chem Biochem. 1974;30:227–256. doi: 10.1016/s0065-2318(08)60266-8. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., Weber D. F., Uratsu S. L. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 1984 Apr;47(4):613–615. doi: 10.1128/aem.47.4.613-615.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhart J. R., Wong P. P. Nitrate reductase activities of rhizobia and the correlation between nitrate reduction and nitrogen fixation. Can J Microbiol. 1979 Oct;25(10):1169–1174. doi: 10.1139/m79-181. [DOI] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Metabolism of C-labeled photosynthate and distribution of enzymes of glucose metabolism in soybean nodules. Plant Physiol. 1983 Jul;72(3):634–640. doi: 10.1104/pp.72.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Carbohydrates in Soybean Nodules: II. DISTRIBUTION OF COMPOUNDS IN SEEDLINGS DURING THE ONSET OF NITROGEN FIXATION. Plant Physiol. 1980 Sep;66(3):471–476. doi: 10.1104/pp.66.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Nitrate inhibition of legume nodule growth and activity : I. Long term studies with a continuous supply of nitrate. Plant Physiol. 1985 Feb;77(2):321–324. doi: 10.1104/pp.77.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Mahadevan S., Maheshwari R. Trehalose Toxicity in Cuscuta reflexa: CORRELATION WITH LOW TREHALASE ACTIVITY. Plant Physiol. 1981 Dec;68(6):1369–1374. doi: 10.1104/pp.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]


