Abstract
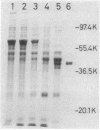
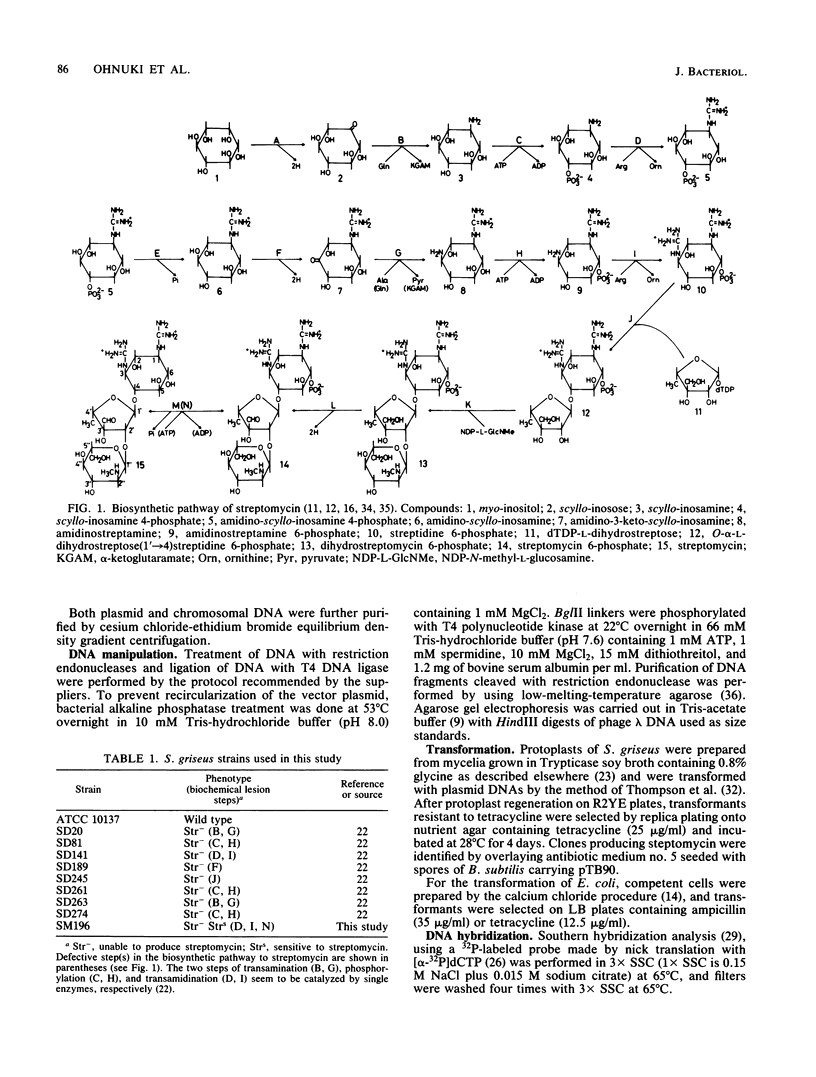
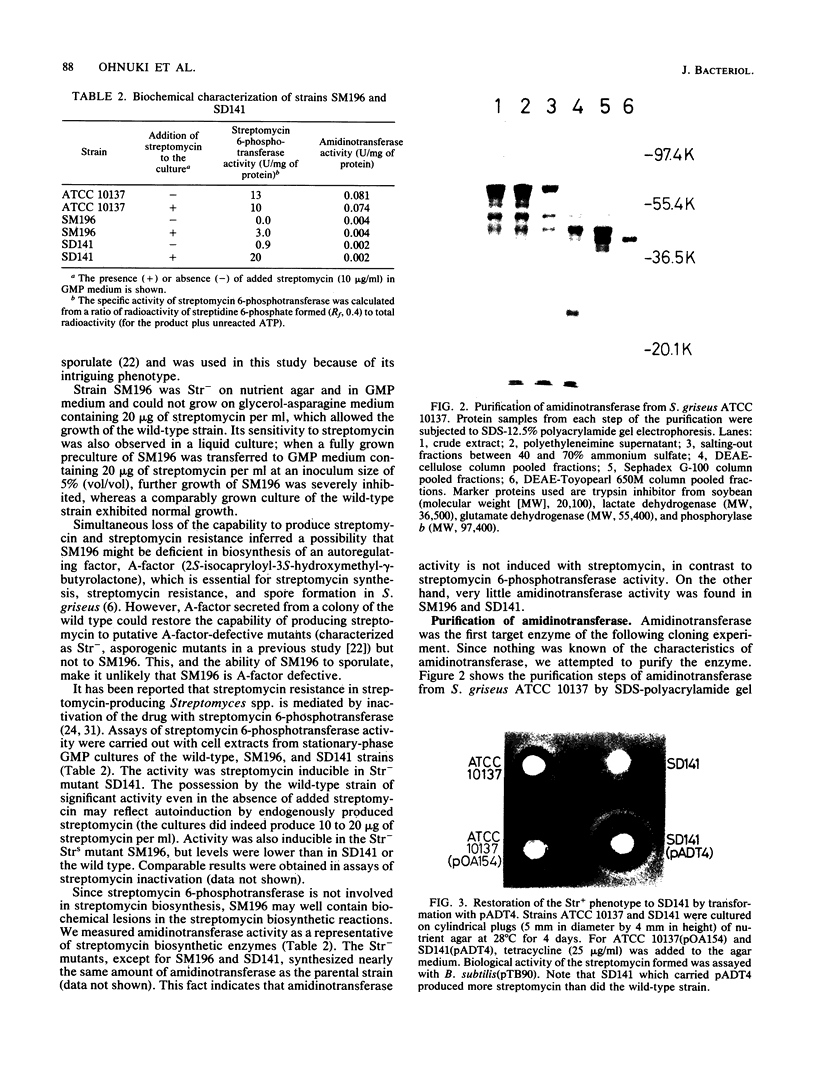
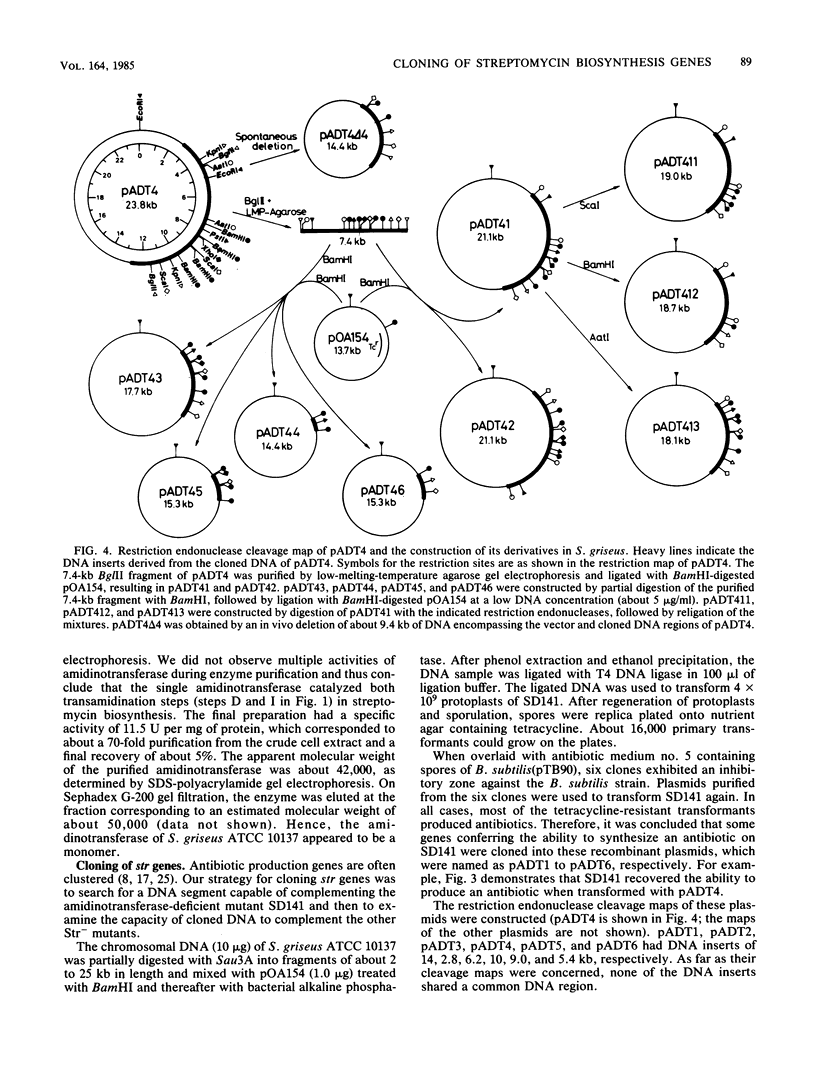
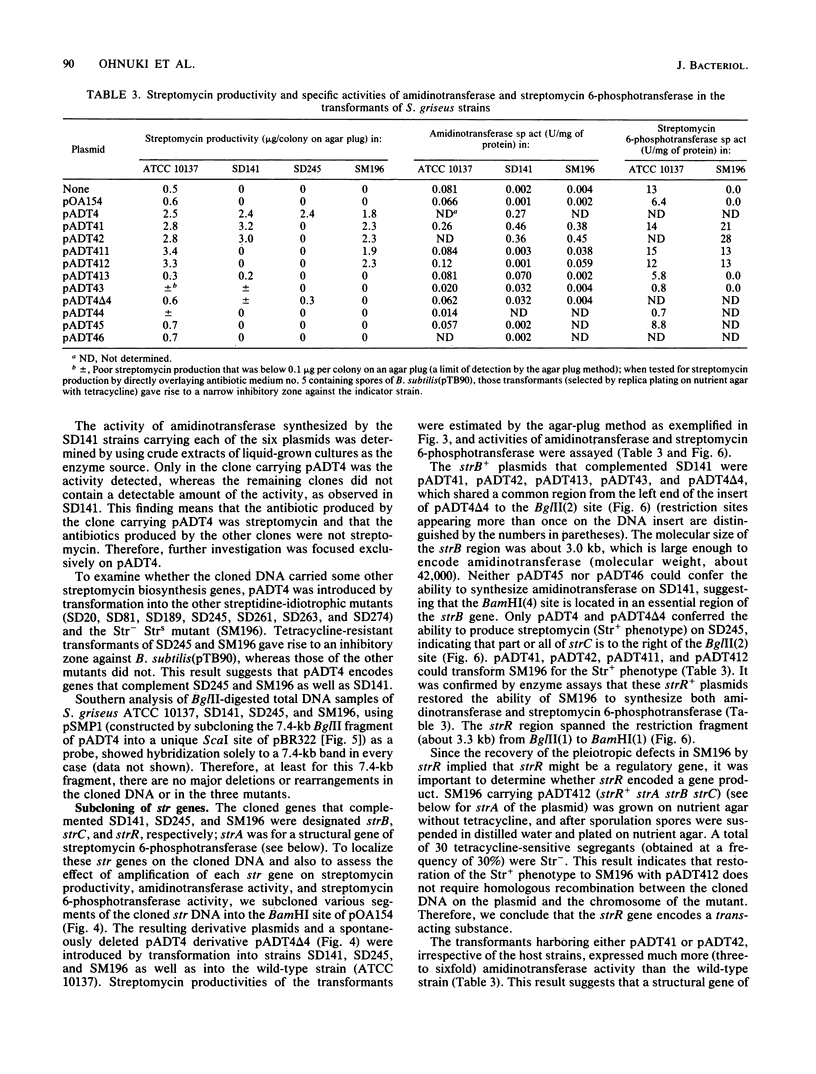
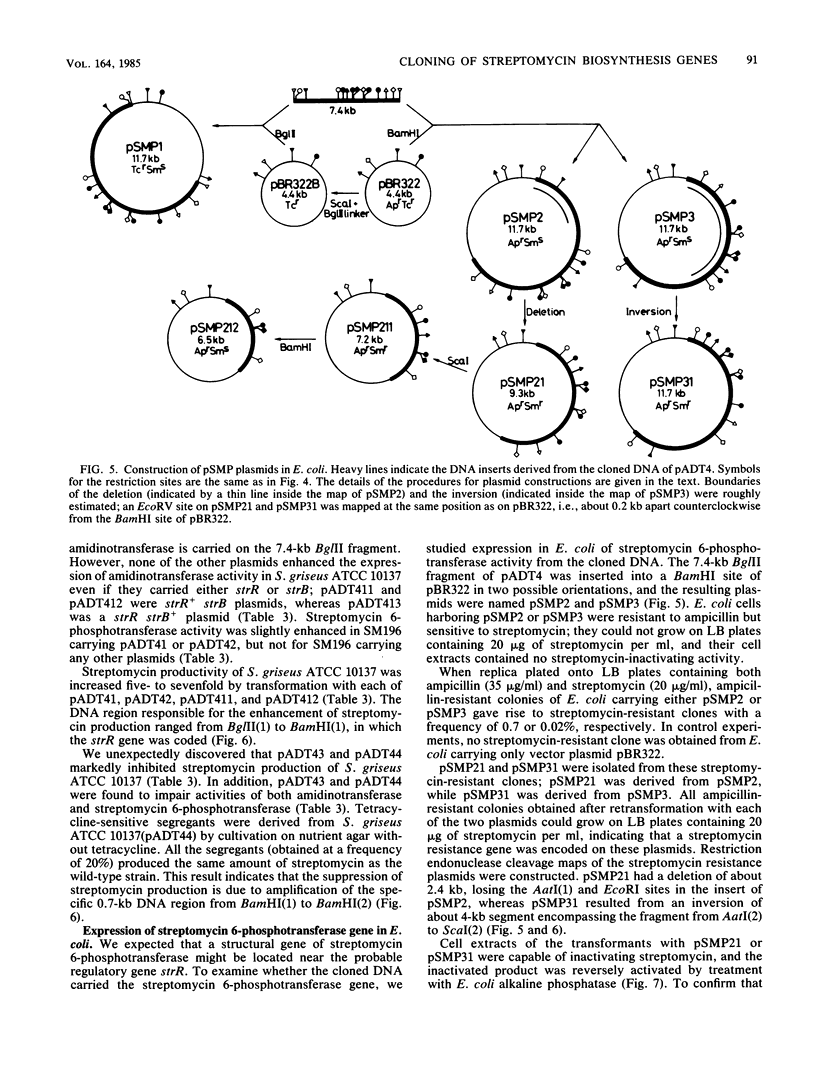
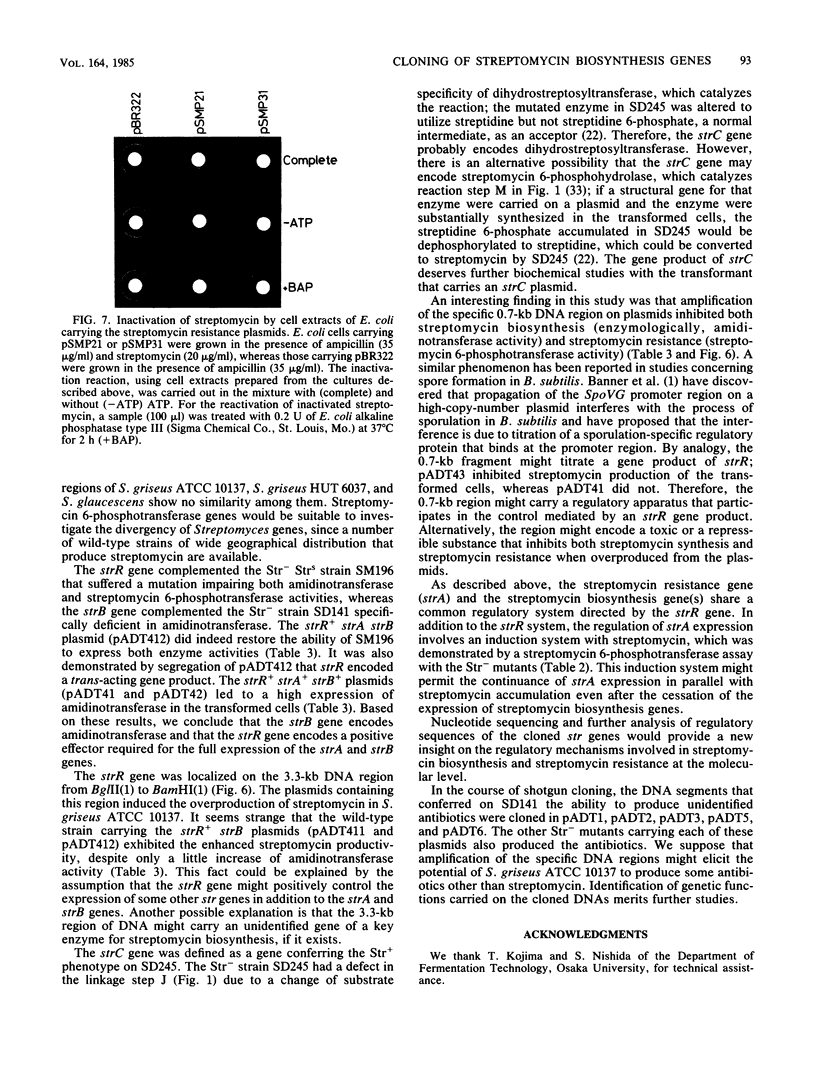
An str gene cluster containing at least four genes (strR, strA, strB, and strC) involved in streptomycin biosynthesis or streptomycin resistance or both was self-cloned in Streptomyces griseus by using plasmid pOA154. The strA gene was verified to encode streptomycin 6-phosphotransferase, a streptomycin resistance factor in S. griseus, by examining the gene product expressed in Escherichia coli. The other three genes were determined by complementation tests with streptomycin-nonproducing mutants whose biochemical lesions were clearly identified. strR complemented streptomycin-sensitive mutant SM196 which exhibited impaired activity of both streptomycin 6-phosphotransferase and amidinotransferase (one of the streptomycin biosynthetic enzymes) due to a regulatory mutation; strB complemented strain SD141, which was specifically deficient in amidinotransferase; and strC complemented strain SD245, which was deficient in linkage between streptidine 6-phosphate and dihydrostreptose. By deletion analysis of plasmids with appropriate restriction endonucleases, the order of the four genes was determined to be strR-strA-strB-strC. Transformation of S. griseus with plasmids carrying both strR and strB genes enhanced amidinotransferase activity in the transformed cells. Based on the gene dosage effect and the biological characteristics of the mutants complemented by strR and strB, it was concluded that strB encodes amidinotransferase and strR encodes a positive effector required for the full expression of strA and strB genes. Furthermore, it was found that amplification of a specific 0.7-kilobase region of the cloned DNA on a plasmid inhibited streptomycin biosynthesis of the transformants. This DNA region might contain a regulatory apparatus that participates in the control of streptomycin biosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner C. D., Moran C. P., Jr, Losick R. Deletion analysis of a complex promoter for a developmentally regulated gene from Bacillus subtilis. J Mol Biol. 1983 Aug 5;168(2):351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Cohen S. N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187(2):265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara O., Beppu T. Induction of streptomycin-inactivating enzyme by A-factor in Streptomyces griseus. J Antibiot (Tokyo) 1982 Sep;35(9):1208–1215. doi: 10.7164/antibiotics.35.1208. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Sharma V. K., Wetherbee J. R., Warren R. H., Banerjee S. P. Adrenergic and cholinergic receptors of cerebral microvessels. J Cereb Blood Flow Metab. 1981;1(3):329–338. doi: 10.1038/jcbfm.1981.36. [DOI] [PubMed] [Google Scholar]
- Hintermann G., Crameri R., Vögtli M., Hütter R. Streptomycin-sensitivity in Streptomyces glaucescens is due to deletions comprising the structural gene coding for a specific phosphotransferase. Mol Gen Genet. 1984;196(3):513–520. doi: 10.1007/BF00436201. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aramori I., Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Tanaka T., Tsunekawa H., Aiba S. Cloning of the genes for penicillinase, penP and penI, of Bacillus licheniformis in some vector plasmids and their expression in Escherichia coli, Bacillus subtilis, and Bacillus licheniformis. J Bacteriol. 1981 Sep;147(3):776–786. doi: 10.1128/jb.147.3.776-786.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniep B., Grisebach H. Biosynthesis of streptomycin. Enzymatic formation of dihydrostreptomycin 6-phosphate from dihydrostreptosyl streptidine 6-phosphate. J Antibiot (Tokyo) 1980 Apr;33(4):416–419. doi: 10.7164/antibiotics.33.416. [DOI] [PubMed] [Google Scholar]
- Kniep B., Grisebach H. Biosynthesis of streptomycin. Purification and properties of a dTDP-L-dihydrostreptose: streptidine-6-phosphate dihydrostreptosyltransferase from Streptomyces griseus. Eur J Biochem. 1980 Mar;105(1):139–144. doi: 10.1111/j.1432-1033.1980.tb04483.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S., Grisebach H. Biosynthesis of streptomycin. Enzymic oxidation of dihydrostreptomycin (6-phosphate) to streptomycin (6-phosphate) with a particulate fraction of Streptomyces griseus. Biochim Biophys Acta. 1979 Aug 22;586(2):231–241. doi: 10.1016/0304-4165(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Isolation and characterization of pock-forming plasmids for Streptomyces griseus from soil actinomycetes. Gene. 1983 Nov;25(1):155–159. doi: 10.1016/0378-1119(83)90178-6. [DOI] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Isolation of streptomycin-nonproducing mutants deficient in biosynthesis of the streptidine moiety or linkage between streptidine 6-phosphate and dihydrostreptose. Antimicrob Agents Chemother. 1985 Mar;27(3):367–374. doi: 10.1128/aac.27.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T., Katoh T., Imanaka T., Aiba S. Molecular cloning of tetracycline resistance genes from Streptomyces rimosus in Streptomyces griseus and characterization of the cloned genes. J Bacteriol. 1985 Mar;161(3):1010–1016. doi: 10.1128/jb.161.3.1010-1016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarski J. M., Shaw P. D. Streptomycin resistance in a streptomycin-producing microorganism. Antimicrob Agents Chemother. 1979 Aug;16(2):176–182. doi: 10.1128/aac.16.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shinkawa H., Sugiyama M., Nimi O., Nomi R. Molecular cloning and expression in Streptomyces lividans of a streptomycin 6-phosphotransferase gene from a streptomycin-producing microorganism. FEBS Lett. 1985 Feb 25;181(2):385–389. doi: 10.1016/0014-5793(85)80298-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M., Mochizuki H., Nimi O., Nomi R. Mechanism of protection of protein synthesis against streptomycin inhibition in a producing strain. J Antibiot (Tokyo) 1981 Sep;34(9):1183–1188. doi: 10.7164/antibiotics.34.1183. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. B. Pathways of biosynthesis of the guanidinated inositol moieties of streptomycin and bluensomycin. Methods Enzymol. 1975;43:429–433. doi: 10.1016/0076-6879(75)43097-x. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Walker M. S. Enzymatic synthesis of streptidine from scyllo-inosamine. Biochemistry. 1967 Dec;6(12):3821–3829. doi: 10.1021/bi00864a028. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]