Abstract
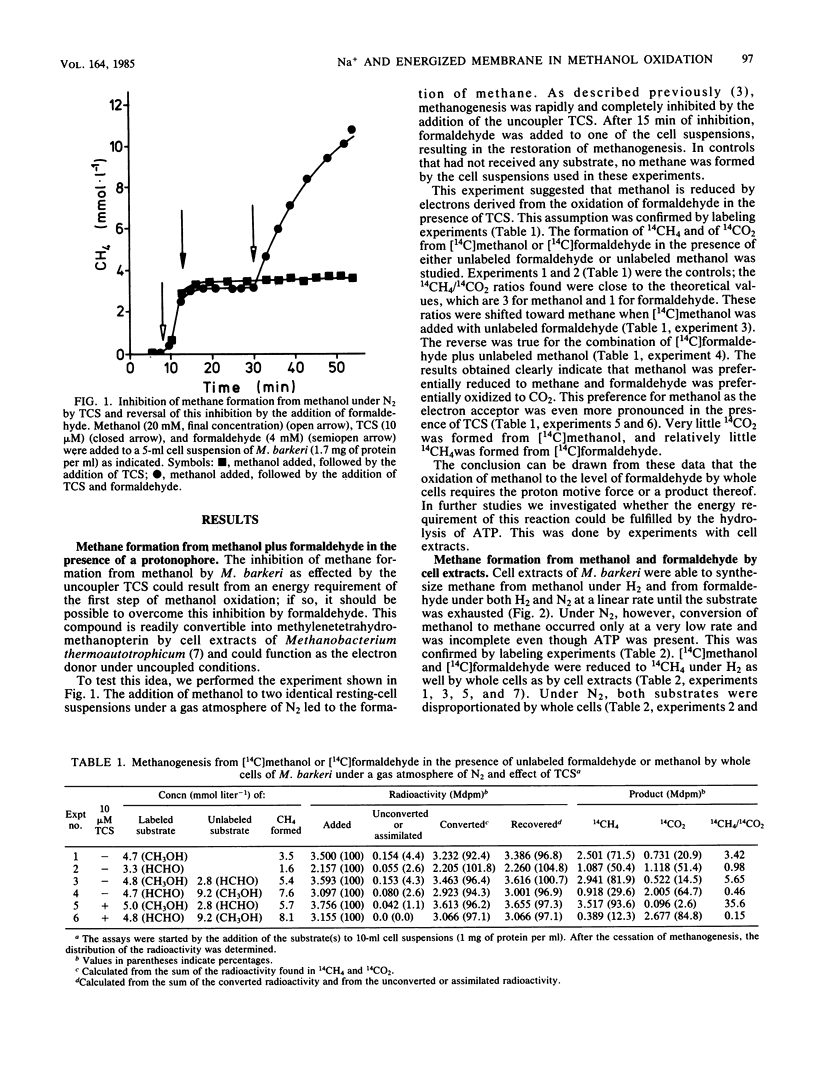
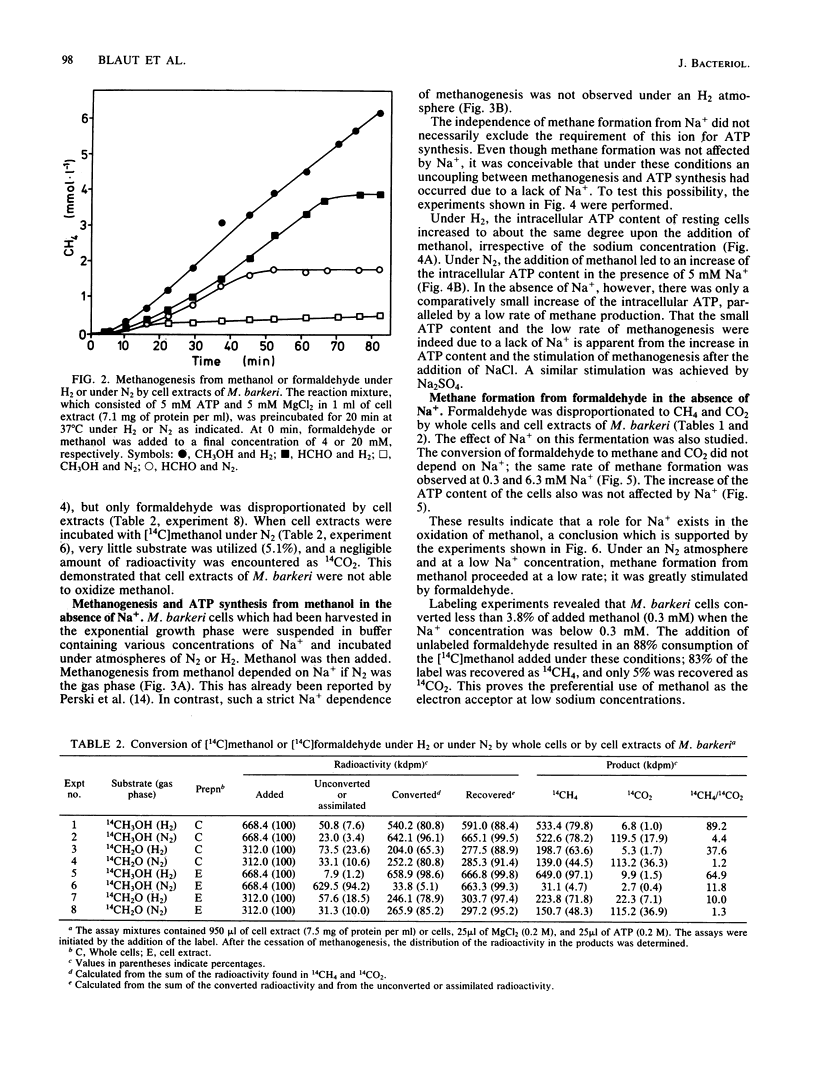
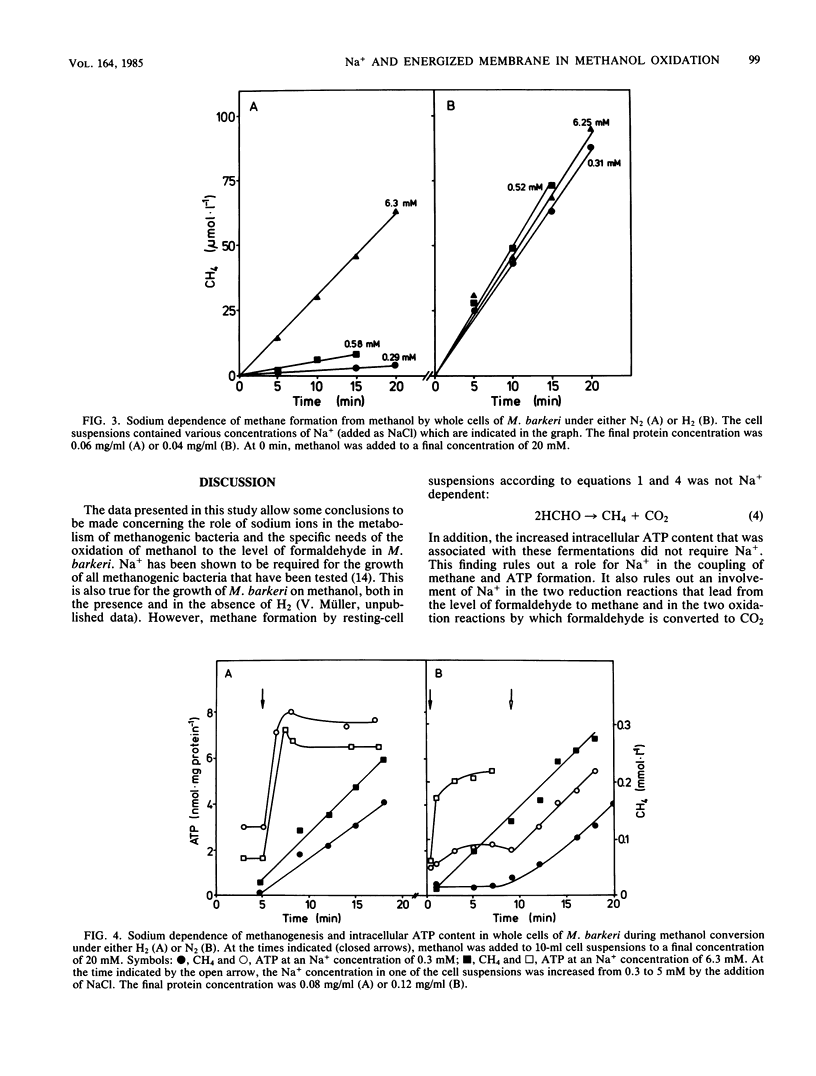
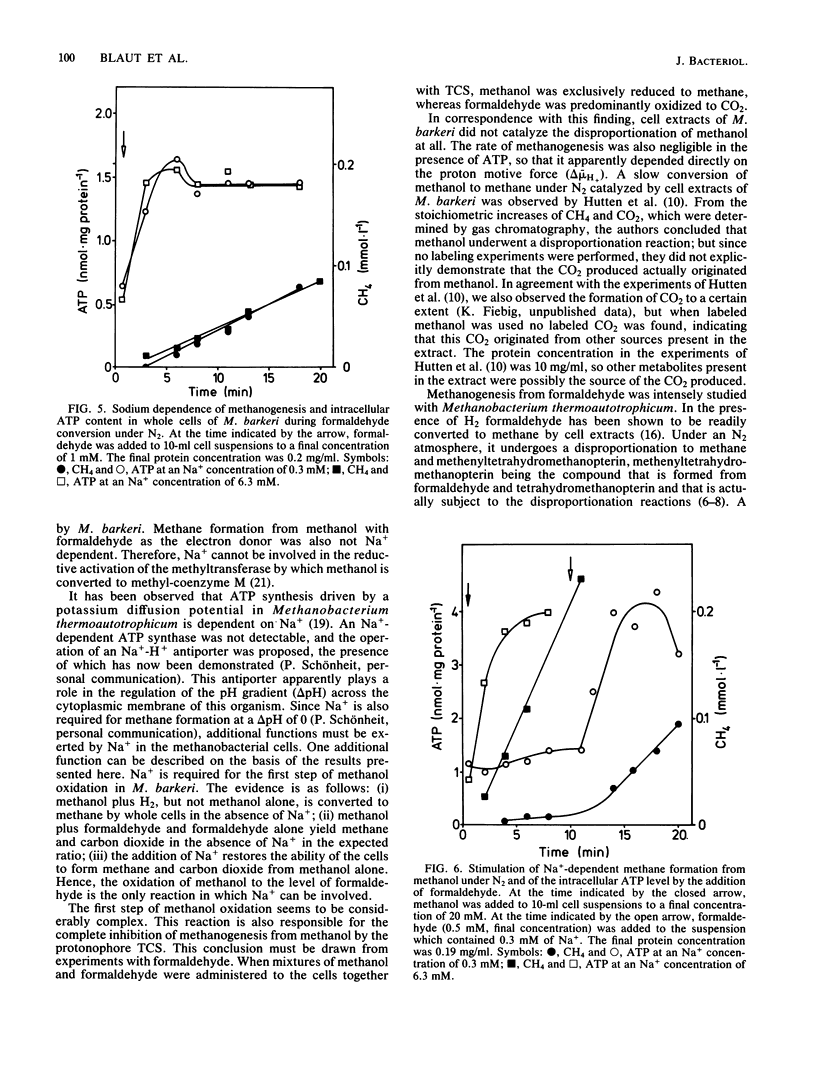
Methanogenesis from methanol by cell suspensions of Methanosarcina barkeri was inhibited by the uncoupler tetrachlorosalicylanilide. This inhibition was reversed by the addition of formaldehyde. 14C labeling experiments revealed that methanol served exclusively as the electron acceptor, whereas formaldehyde was mainly oxidized to CO2 under these conditions. These data support the hypothesis (M. Blaut and G. Gottschalk, Eur. J. Biochem. 141: 217-222, 1984) that the first step in methanol oxidation depends on the proton motive force or a product thereof. Cell extracts of M. barkeri converted methanol and formaldehyde to methane under an H2 atmosphere. Under an N2 atmosphere, however, formaldehyde was disproportionated to CH4 and CO2, whereas methanol was metabolized to a very small extent only, irrespective of the presence of ATP. It was concluded that cell extracts of M. barkeri are not able to oxidize methanol. In further experiments, the sodium dependence of methanogenesis and ATP formation by whole cells was investigated. Methane formation from methanol alone and the corresponding increase in the intracellular ATP content were strictly dependent on Na+. If, in contrast, methanol was utilized together with H2, methane and ATP were synthesized in the absence of Na+. The same is true for the disproportionation of formaldehyde to methane and carbon dioxide. From these experiments, it is concluded that in M. barkeri, Na+ is involved not in the process of ATP synthesis but in the first step of methanol oxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978 Oct 31;17(22):4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Wolfe R. S. Formaldehyde oxidation and methanogenesis. J Bacteriol. 1984 May;158(2):721–726. doi: 10.1128/jb.158.2.721-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Wolfe R. S. Tetrahydromethanopterin-dependent methanogenesis from non-physiological C1 donors in Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Feb;161(2):696–701. doi: 10.1128/jb.161.2.696-701.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten T. J., De Jong M. H., Peeters B. P., van der Drift C., Vogels G. D. Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol. 1981 Jan;145(1):27–34. doi: 10.1128/jb.145.1.27-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn W., Gottschalk G. Characterization of the cytochromes occurring in Methanosarcina species. Eur J Biochem. 1983 Sep 1;135(1):89–94. doi: 10.1111/j.1432-1033.1983.tb07621.x. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Wolfe R. S. Interaction of coenzyme M and formaldehyde in methanogenesis. Biochem J. 1981 Sep 1;197(3):565–571. doi: 10.1042/bj1970565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B., Brock T. D. Measuring radioactive methane with the liquid scintillation counter. Appl Environ Microbiol. 1979 May;37(5):897–899. doi: 10.1128/aem.37.5.897-899.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Sliepenbeek H. T., Houwen F. P., van der Drift C., Vogels G. D. Activation and inactivation of methanol: 2-mercaptoethanesulfonic acid methyltransferase from Methanosarcina barkeri. J Bacteriol. 1983 Jan;153(1):6–11. doi: 10.1128/jb.153.1.6-11.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


