Abstract
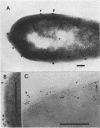
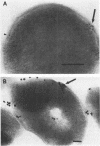
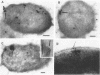
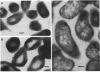
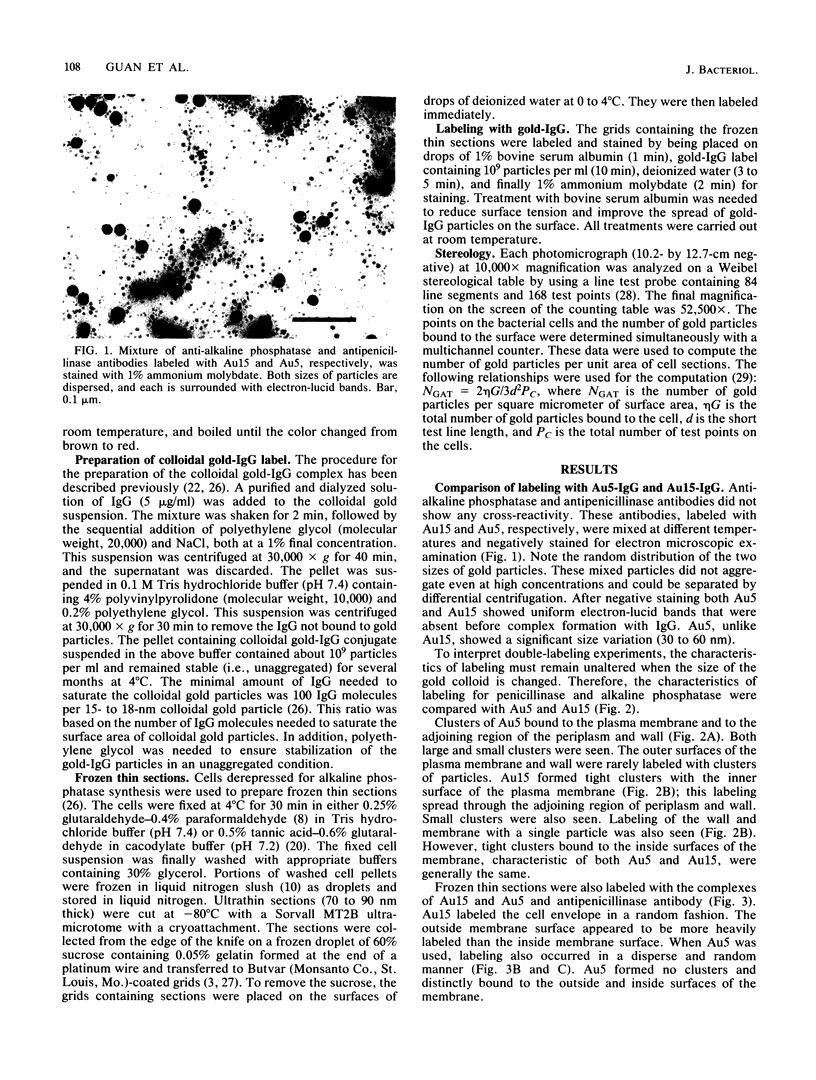
The subcellular distribution of alkaline phosphatase and penicillinase was determined by double labeling frozen thin sections of Bacillus licheniformis 749/C with colloidal gold-immunoglobulin G (IgG). Antipenicillinase and anti-alkaline phosphatase antibodies were used to prepare complexes with 5- and 15-nm colloidal gold particles, respectively. The character of the labeling of membrane-bound alkaline phosphatase and penicillinase was different: the immunolabels for alkaline phosphatase (15-nm particles) were bound to a few sites at the inner surface of the plasma membrane, and the gold particles formed clusters of various sizes at the binding sites; the immunolabels for penicillinase (5-nm particles), on the other hand, were bound to the plasma membrane in a dispersed and random fashion. In the cytoplasm, immunolabels for both proteins were distributed randomly, and the character of their binding was similar. The labeling was specific: pretreating the frozen thin sections with different concentrations of anti-alkaline phosphatase or penicillinase blocked the binding of the immunolabel prepared with the same antibody. Binding could be fully blocked by pretreatment with 800 micrograms of either antibody per ml.
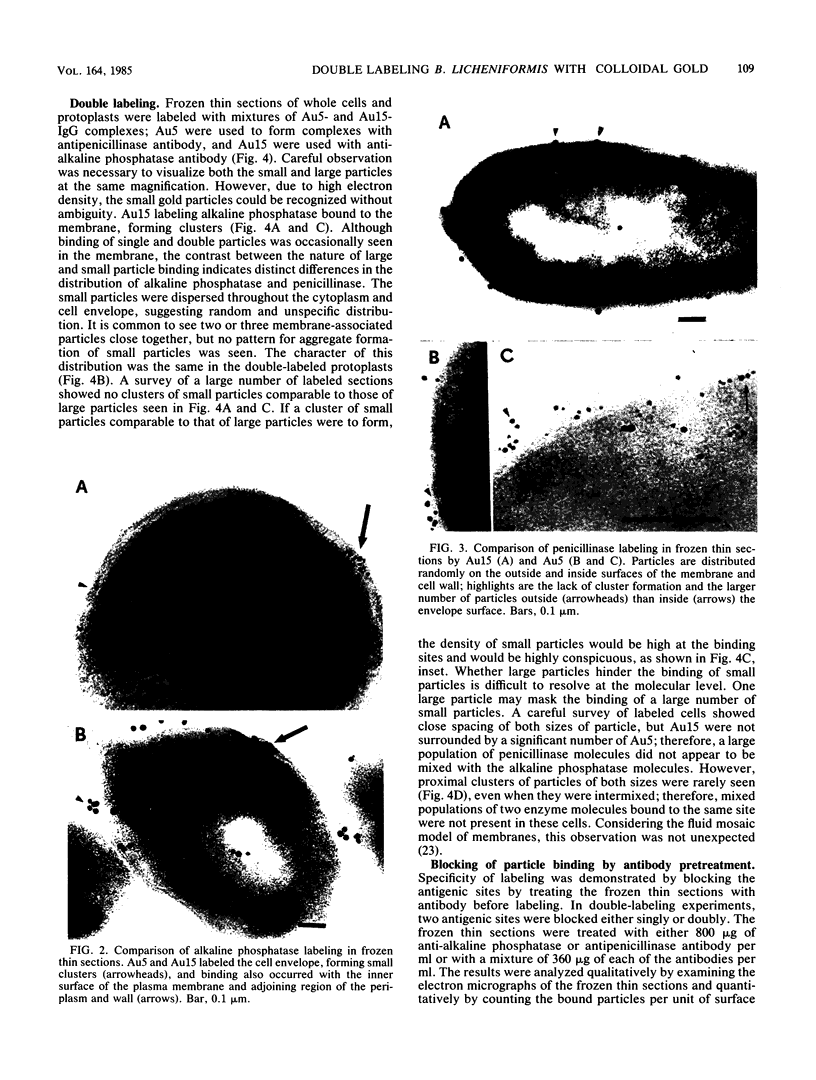
Full text
PDF
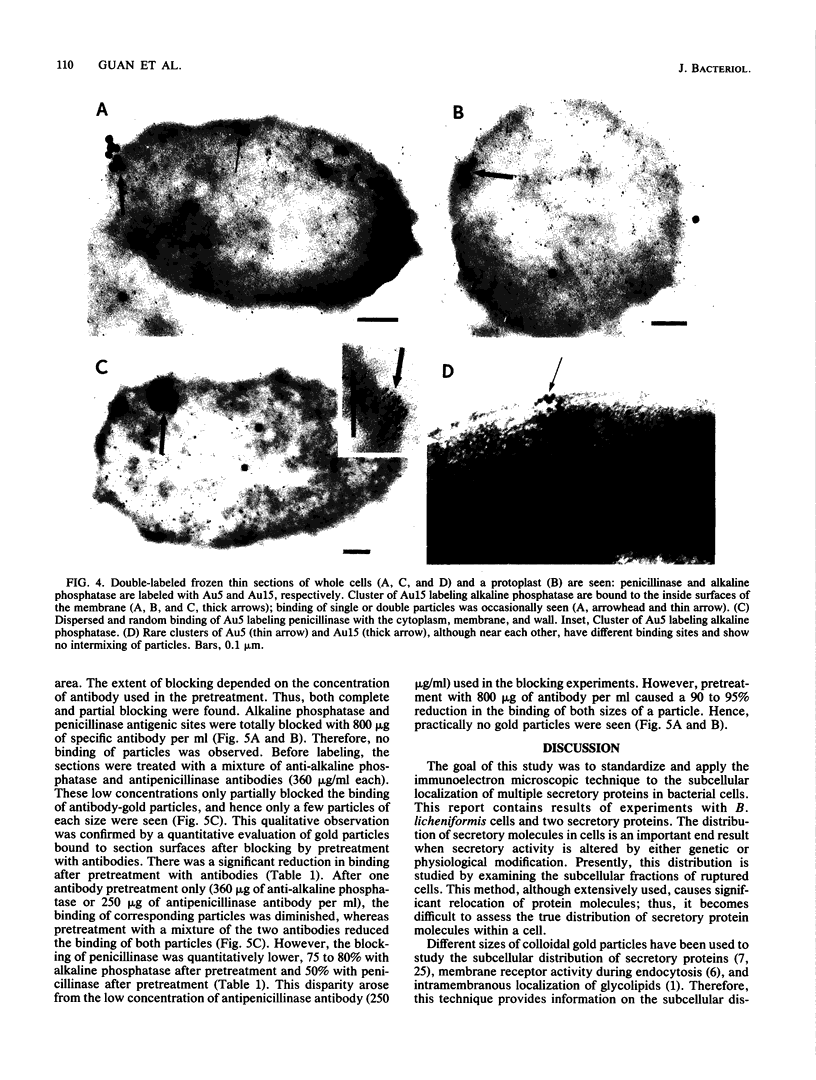


Images in this article
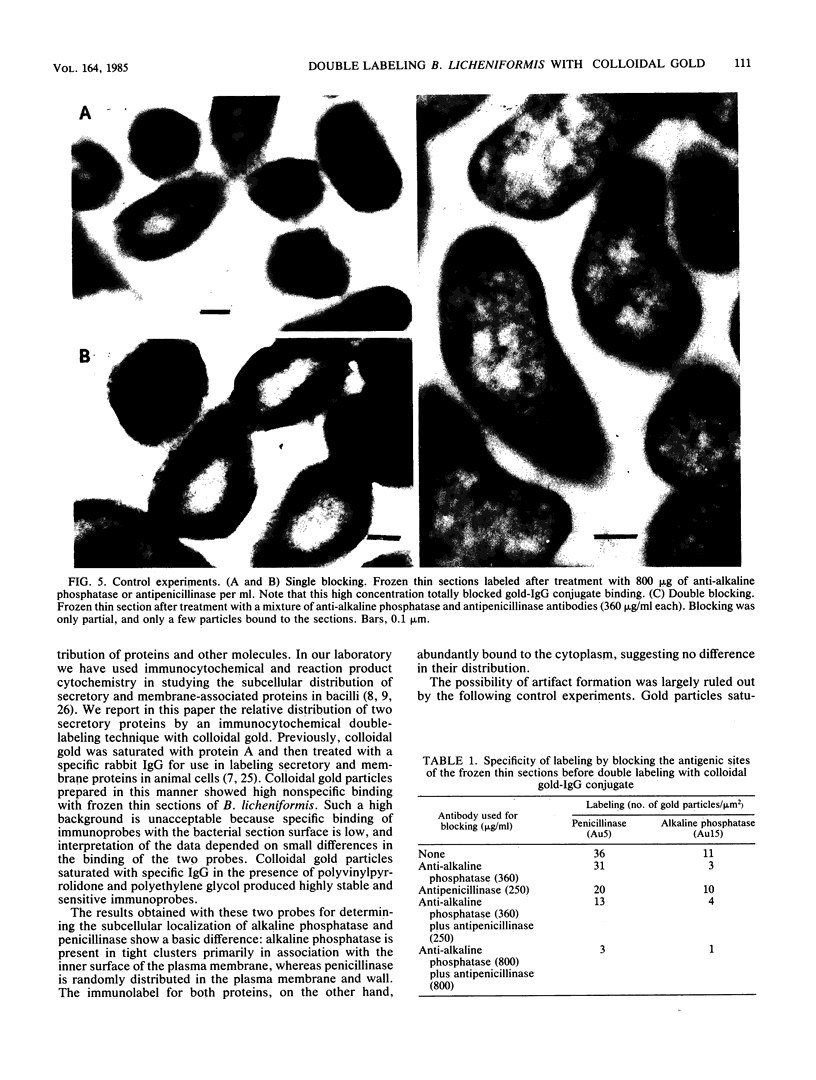
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. L., Pinto da Silva P. Restriction of glycolipids to the outer half of a plasma membrane: concanavalin A labeling of membrane halves in Acanthamoeba castellanii. Cell. 1983 Jul;33(3):959–966. doi: 10.1016/0092-8674(83)90039-9. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. K. Frozen thin sections of fresh tissue for electron microscopy, with a description of pancreas and liver. J Cell Biol. 1971 Dec;51(3):772–804. doi: 10.1083/jcb.51.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Tai P. C. The mechanism of protein secretion across membranes. Nature. 1980 Jan 31;283(5746):433–438. doi: 10.1038/283433a0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Ghosh B. K. Immunoelectron microscopic localization of penicillinase in Bacillus licheniformis. J Bacteriol. 1979 Mar;137(3):1374–1385. doi: 10.1128/jb.137.3.1374-1385.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Vallespir S., Ghosh B. K. Specificity of subcellular distribution of alkaline phosphatase in Bacillus licheniformis 749/C. Can J Microbiol. 1984 Jan;30(1):113–125. doi: 10.1139/m84-019. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Nanninga N. Polymorphism of the mesosome in Bacillus licheniformis (749/C and 749). Influence of chemical fixation monitored by freeze-etching. J Ultrastruct Res. 1976 Jul;56(1):107–120. doi: 10.1016/s0022-5320(76)80144-x. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Wouters J. T., Lampen J. O. Distribution of the sites of alkaline phosphatase(s) activity in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):928–937. doi: 10.1128/jb.108.2.928-937.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Ghosh A., Ghosh B. K. Properties of the membrane-bound alkaline phosphatase from glucose- and lactate-grown cells of Bacillus subtilis SB 15. J Biol Chem. 1977 Oct 10;252(19):6813–6822. [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Sato M., Itakura K., Inouye M. Prolipoprotein signal peptidase of Escherichia coli requires a cysteine residue at the cleavage site. EMBO J. 1983;2(1):87–91. doi: 10.1002/j.1460-2075.1983.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Hsu C. P., Itakura K., Inouye M. Requirement for signal peptide cleavage of Escherichia coli prolipoprotein. Science. 1983 Jul 1;221(4605):59–61. doi: 10.1126/science.6344218. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Kumar R., Ghosh A., Ghosh B. K. Alkaline phosphatase secretion-negative mutant of Bacillus licheniformis 749/C. J Bacteriol. 1983 May;154(2):946–954. doi: 10.1128/jb.154.2.946-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Spencer D. B., Hansa J. G., Stuckmann K. V., Hulett F. M. Membrane-associated alkaline phosphatase from Bacillus licheniformis that requires detergent for solubilization: lactoperoxidase 125I localization and molecular weight determination. J Bacteriol. 1982 May;150(2):826–834. doi: 10.1128/jb.150.2.826-834.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Willemsen R., van Kerkhof P., Slot J. W., Geuze H. J., Lodish H. F. Vesicular stomatitis virus glycoprotein, albumin, and transferrin are transported to the cell surface via the same Golgi vesicles. J Cell Biol. 1983 Dec;97(6):1815–1822. doi: 10.1083/jcb.97.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinglu G., Ghosh A., Ghosh B. K. Subcellular localization of alkaline phosphatase in Bacillus licheniformis 749/C by immunoelectron microscopy with colloidal gold. J Bacteriol. 1984 Aug;159(2):668–677. doi: 10.1128/jb.159.2.668-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]