Abstract
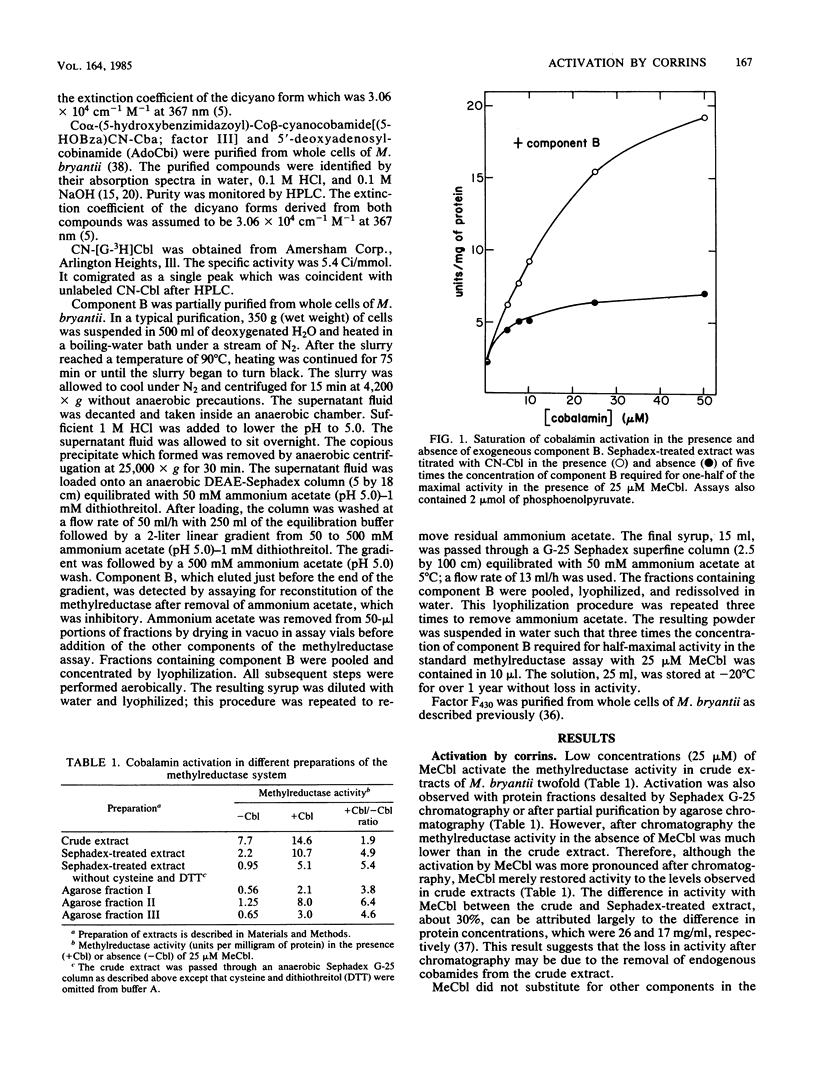
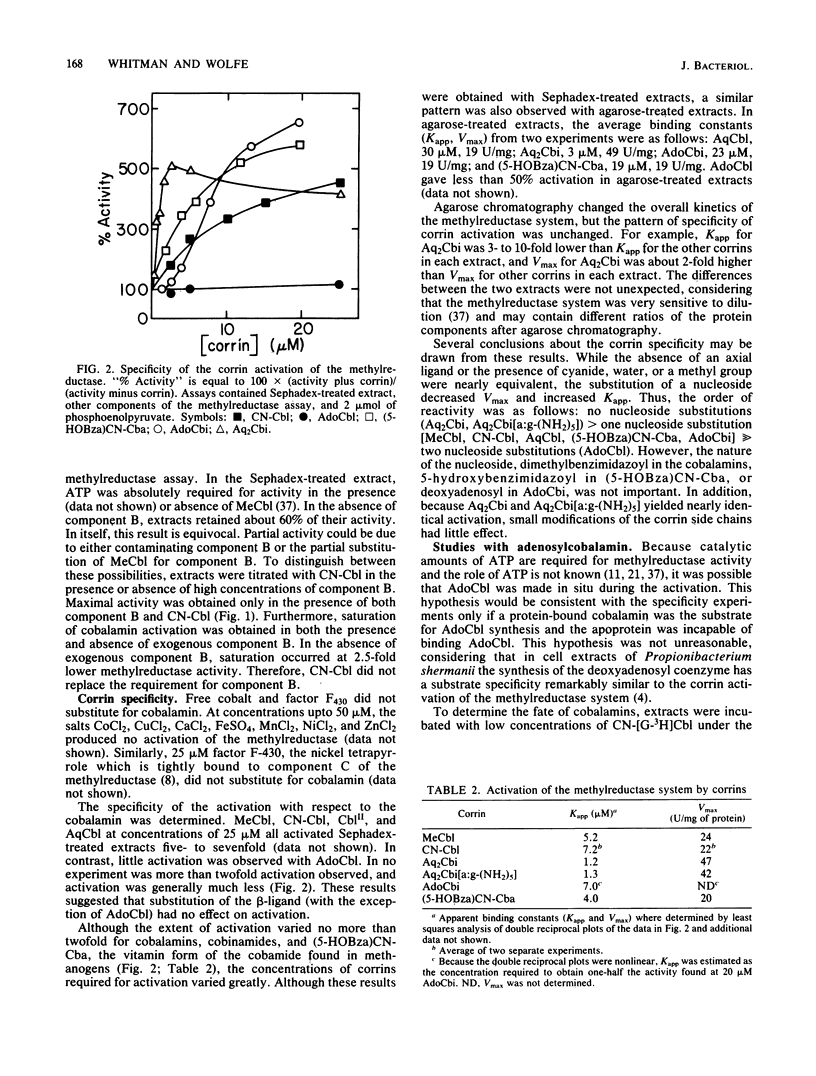
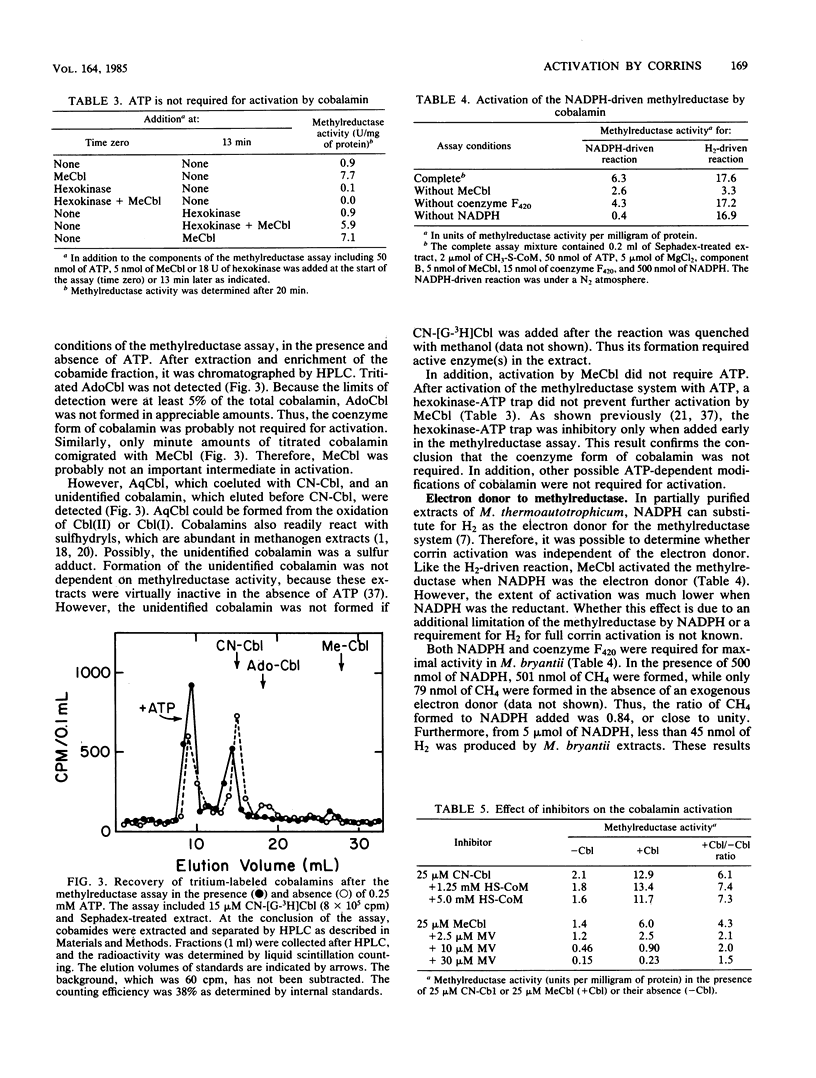
Corrins activated the methylreductase system from Methanobacterium bryantii three- to fivefold in extracts resolved from low-molecular-weight factors. Corrins did not substitute for ATP and component B, which were also required for maximal activity. The concentration of diaquacobinamides required for one-half maximal activity was 1 microM. The concentrations of cyanocobalamin, methylcobalamin, Co alpha-(5-hydroxybenzimidazoyl)-Co beta-cyanocobamide, and 5'-deoxyadenosylcobinamide required for one-half maximal activity were between 4 and 7 microM. Deoxyadenosylcobalamin was nearly inactive. Activation was independent of thiols, coenzyme M, and ATP. Activation was also observed after partial purification of the methylreductase system by agarose column chromatography. Corrins were required in catalytic concentrations, methylcobalamin was not required, and methanogenesis was enzymatic. Corrin activation of the methylreductase is a novel effect on methanogenesis. However, the physiological significance of the corrin activation is uncertain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAYLOCK B. A., STADTMAN T. C. Biosynthesis of methane from the methyl moiety of methylcobalamin. Biochem Biophys Res Commun. 1963 Apr 2;11:34–38. doi: 10.1016/0006-291x(63)90023-8. [DOI] [PubMed] [Google Scholar]
- BRADY R. O., CASTANERA E. G., BARKER H. A. The enzymatic synthesis of cobamide coenzymes. J Biol Chem. 1962 Jul;237:2325–2332. [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. J Bacteriol. 1979 Jan;137(1):264–273. doi: 10.1128/jb.137.1.264-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock B. A. Cobamide-dependent methanol-cyanocob(I)alamin methyltransferase of Methanosarcina barkeri. Arch Biochem Biophys. 1968 Mar 20;124(1):314–324. doi: 10.1016/0003-9861(68)90333-0. [DOI] [PubMed] [Google Scholar]
- DELLWEG H., BERNHAUER K. Zur Chemie und Biochemie der Cobalamine. IV. Aetiocobalamin-phosphoribose. Arch Biochem Biophys. 1957 Jul;69:74–80. doi: 10.1016/0003-9861(57)90474-5. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B., Wolfe R. S. Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3707–3710. doi: 10.1073/pnas.79.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Frick T., Francia M. D., Wood J. M. Mechanism for the interaction of thiols with methylcobalamin. Biochim Biophys Acta. 1976 May 28;428(3):808–817. doi: 10.1016/0304-4165(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- LADD J. N., HOGENKAMP H. P., BARKER H. A. Structure of cobamide coenzymes: influence of pH on absorption spectra and ionophoretic mobilities. J Biol Chem. 1961 Jul;236:2114–2118. [PubMed] [Google Scholar]
- LEZIUS A. G., BARKER H. A. CORRINOID COMPOUNDS OF METHANOBACILLUS OMELIANSKII. I. FRACTIONATION OF THE CORRINOID COMPOUNDS AND IDENTIFICATION OF FACTOR 3 AND FACTOR 3 COENZYME. Biochemistry. 1965 Mar;4:510–518. doi: 10.1021/bi00879a021. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Wolfe R. S. Carbon dioxide reduction factor and methanopterin, two coenzymes required for CO2 reduction to methane by extracts of Methanobacterium. J Biol Chem. 1983 Jun 25;258(12):7536–7540. [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL J. L. THE CATALYSIS OF THE AUTO-OXIDATION OF 2-MERCAPTOETHANOL AND OTHER THIOLS BY VITAMIN B12 DERIVATIVES. POLAROGRAPHIC AND OTHER INVESTIGATIONS. Biochem J. 1963 Aug;88:296–308. doi: 10.1042/bj0880296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL J. L. Vitamin B12 derivatives and the CO2-pyruvate exchange reaction: a reappraisal. J Biol Chem. 1962 Jan;237:PC263–PC265. [PubMed] [Google Scholar]
- Robertson A. M., Wolfe R. S. ATP requirement for methanogenesis in cell extracts of methanobacterium strain M.o.H. Biochim Biophys Acta. 1969 Dec 30;192(3):420–429. doi: 10.1016/0304-4165(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Balch W. E. Coenzyme M: preparation and assay. Methods Enzymol. 1980;67:545–552. doi: 10.1016/s0076-6879(80)67067-0. [DOI] [PubMed] [Google Scholar]
- Romesser J. A., Wolfe R. S. Coupling of methyl coenzyme M reduction with carbon dioxide activation in extracts of Methanobacterium thermoautotrophicum. J Bacteriol. 1982 Nov;152(2):840–847. doi: 10.1128/jb.152.2.840-847.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Escalante-Semerena J. C., Wolfe R. S. Component A2 of the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Apr;162(1):61–66. doi: 10.1128/jb.162.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauzer G. N., Grate J. H., Katz R. N. Coenzyme M and methylcobalamin in methane biosynthesis: results of model studies. Bioinorg Chem. 1978;8(1):1–10. doi: 10.1016/s0006-3061(00)80000-9. [DOI] [PubMed] [Google Scholar]
- TOOHEY J. I., PERLMAN D., BARKER H. A. Purification and properties of cobamide coenzymes obtained from Propionibacterium arabinosum. J Biol Chem. 1961 Jul;236:2119–2127. [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. A simplified assay for coenzyme M (HSCH2CH2SO3). Resolution of methylcobalamin-coenzyme M methyltransferase and use of sodium borohydride. J Biol Chem. 1974 Aug 10;249(15):4886–4890. [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Toraya T., Ushio K., Fukui S., Hogenkamp P. C. Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of the coenzyme. J Biol Chem. 1977 Feb 10;252(3):963–970. [PubMed] [Google Scholar]
- WEISSBACH H., LADD J. N., VOLCANI B. E., SMYTH R. D., BARKER H. A. Structure of the adenylcobamide coenzyme: degradation by cyanide, acid, and light. J Biol Chem. 1960 May;235:1462–1473. [PubMed] [Google Scholar]
- Weissbach H., Brot N., Lovenberg W. Reduction of cobamides by reduced ferredoxin. J Biol Chem. 1966 Jan 25;241(2):317–321. [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by ATP. J Bacteriol. 1983 May;154(2):640–649. doi: 10.1128/jb.154.2.640-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1196–1201. doi: 10.1016/0006-291x(80)90413-1. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Purification and analysis of cobamides of Methanobacterium bryantii by high-performance liquid chromatography. Anal Biochem. 1984 Feb;137(1):261–265. doi: 10.1016/0003-2697(84)90380-4. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Moura I., Moura J. J., Santos M. H., Xavier A. V., LeGall J., Scandellari M. Role of vitamin B12 in methyl transfer for methane biosynthesis by Methanosarcina barkeri. Science. 1982 Apr 16;216(4543):303–305. doi: 10.1126/science.7063887. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Pouwels A., Houwen F., van der Drift C., Vogels G. D. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch Microbiol. 1983 Jun;134(3):238–242. doi: 10.1007/BF00407765. [DOI] [PubMed] [Google Scholar]
- van der Meijden P., Heythuysen H. J., Sliepenbeek H. T., Houwen F. P., van der Drift C., Vogels G. D. Activation and inactivation of methanol: 2-mercaptoethanesulfonic acid methyltransferase from Methanosarcina barkeri. J Bacteriol. 1983 Jan;153(1):6–11. doi: 10.1128/jb.153.1.6-11.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden P., te Brömmelstroet B. W., Poirot C. M., van der Drift C., Vogels G. D. Purification and properties of methanol:5-hydroxybenzimidazolylcobamide methyltransferase from Methanosarcina barkeri. J Bacteriol. 1984 Nov;160(2):629–635. doi: 10.1128/jb.160.2.629-635.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


