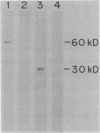
Abstract
Eight strains of Rhizobium lacking hydrogenase uptake (Hup) activity and 17 transconjugant strains carrying the hup cosmids pHU1, pHU52, or pHU53 (G. R. Lambert, M. A. Cantrell, F. J. Hanus, S. A. Russell, K. R. Haddad, and H. J. Evans, Proc. Natl. Acad. Sci. USA, 82:3232-3236, 1985) were screened for Hup activity and the presence of immunologically detectable hydrogenase polypeptides. Crude extracts of these strains were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis with affinity-purified antibodies against the two subunits of purified hydrogenase (Mr 60,000 and 30,000). Derepressed transconjugants carrying the cosmid pHU52 were Hup+ and contained detectable levels of both hydrogenase subunit polypeptides. Non-derepressed strains, Hup- parent strains, and strains carrying cosmids other than pHU52 did not express Hup activity and contained no immunologically detectable protein. These data provide further evidence for the essential involvement of the smaller (Mr 30,000) subunit in the expression of hydrogenase activity in Rhizobium japonicum and suggest that the determinants for hydrogenase subunit synthesis are present on pHU52.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Arp D. J. Rhizobium japonicum hydrogenase: purification to homogeneity from soybean nodules, and molecular characterization. Arch Biochem Biophys. 1985 Mar;237(2):504–512. doi: 10.1016/0003-9861(85)90303-0. [DOI] [PubMed] [Google Scholar]
- Cantrell M. A., Haugland R. A., Evans H. J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Xu L. S., Hanus F. J., Evans H. J. Some properties of the nickel-containing hydrogenase of chemolithotrophically grown Rhizobium japonicum. J Bacteriol. 1984 Sep;159(3):850–856. doi: 10.1128/jb.159.3.850-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Cantrell M. A., Beaty J. S., Hanus F. J., Russell S. A., Evans H. J. Characterization of Rhizobium japonicum hydrogen uptake genes. J Bacteriol. 1984 Sep;159(3):1006–1012. doi: 10.1128/jb.159.3.1006-1012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Hanus F. J., Cantrell M. A., Evans H. J. Rapid Colony Screening Method for Identifying Hydrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 Mar;45(3):892–897. doi: 10.1128/aem.45.3.892-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert G. R., Cantrell M. A., Hanus F. J., Russell S. A., Haddad K. R., Evans H. J. Intra- and interspecies transfer and expression of Rhizobium japonicum hydrogen uptake genes and autotrophic growth capability. Proc Natl Acad Sci U S A. 1985 May;82(10):3232–3236. doi: 10.1073/pnas.82.10.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Hanus F. J., Russell S. A., Evans H. J. Determination of the hydrogenase status of individual legume nodules by a methylene blue reduction assay. Appl Environ Microbiol. 1985 Aug;50(2):537–539. doi: 10.1128/aem.50.2.537-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ueda T. Affinity-purified anti-protein I antibody. Specific inhibitor of phosphorylation of protein I, a synaptic protein. J Biol Chem. 1981 Oct 25;256(20):10657–10663. [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Argüeso T., Maier R. J., Evans H. J. Hydrogen Evolution from Alfalfa and Clover Nodules and Hydrogen Uptake by Free-Living Rhizobium meliloti. Appl Environ Microbiol. 1979 Mar;37(3):582–587. doi: 10.1128/aem.37.3.582-587.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]