Abstract
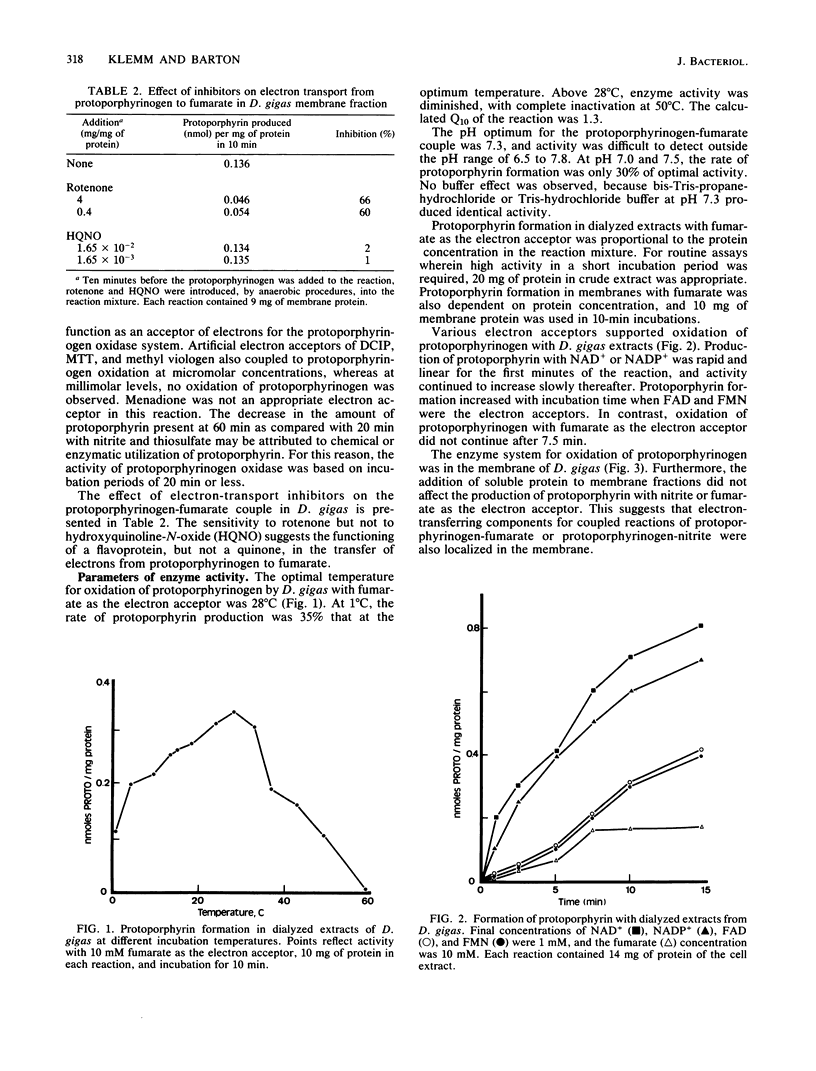
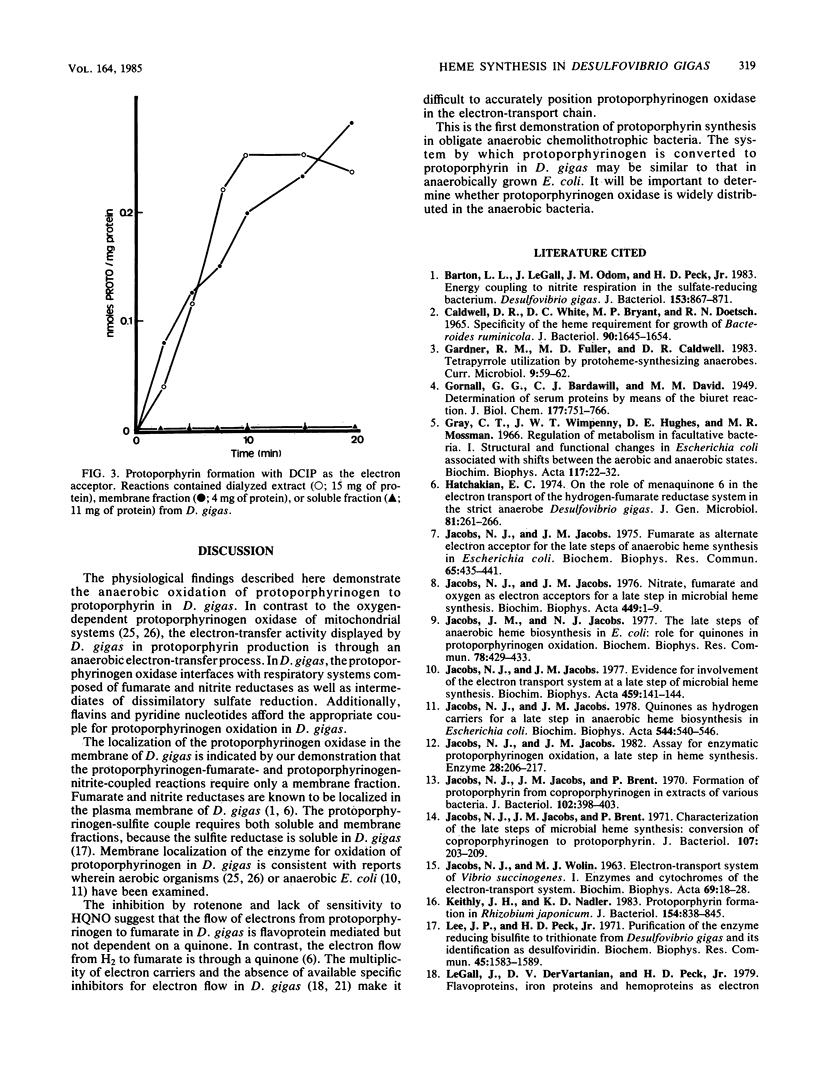
The anaerobic oxidation of protoporphyrinogen to protoporphyrin was demonstrated in extracts of Desulfovibrio gigas. Protoporphyrin formation occurred in the presence of nitrite, hydroxylamine, sulfite, thiosulfate, ATP plus sulfate, NAD+, NADP+, flavin adenine dinucleotide, flavin mononucleotide, fumarate, 2,6-dichlorophenol-indophenol, methyl viologen, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. With dialyzed cell extracts, highest activities were observed with sulfite, NAD+, and NADP+ as electron acceptors. The enzyme for protoporphyrinogen oxidation was localized in the membrane of D. gigas and displayed optimal activity at pH 7.3 and 28 degrees C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton L. L., LeGall J., Odom J. M., Peck H. D., Jr Energy coupling to nitrite respiration in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1983 Feb;153(2):867–871. doi: 10.1128/jb.153.2.867-871.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. I. Enzymes and cytochromes of electron-transport system. Biochim Biophys Acta. 1963 Jan 1;69:18–28. doi: 10.1016/0006-3002(63)91221-6. [DOI] [PubMed] [Google Scholar]
- Jacobs J. M., Jacobs N. J. The late steps of anaerobic heme biosynthesis in E. coli: role for quinones in protoporphyrinogen oxidation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):429–433. doi: 10.1016/0006-291x(77)91272-4. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Assay for enzymatic protoporphyrinogen oxidation, a late step in heme synthesis. Enzyme. 1982;28(2-3):206–219. doi: 10.1159/000459103. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Characterization of the late steps of microbial heme synthesis: conversion of coproporphyrinogen to protoporphyrin. J Bacteriol. 1971 Jul;107(1):203–209. doi: 10.1128/jb.107.1.203-209.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Formation of protoporphyrin from coproporphyrinogen in extracts of various bacteria. J Bacteriol. 1970 May;102(2):398–403. doi: 10.1128/jb.102.2.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Evidence for involvement of the electron transport system at a late step of anaerobic microbial heme synthesis. Biochim Biophys Acta. 1977 Jan 6;459(1):141–144. doi: 10.1016/0005-2728(77)90017-2. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Fumarate as alternate electron acceptor for the late steps of anaerobic heme synthesis in Escherichia coli. Biochem Biophys Res Commun. 1975 Jul 8;65(1):435–441. doi: 10.1016/s0006-291x(75)80112-4. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Nitrate, fumarate, and oxygen as electron acceptors for a late step in microbial heme synthesis. Biochim Biophys Acta. 1976 Oct 13;449(1):1–9. doi: 10.1016/0005-2728(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Quinones as hydrogen carriers for a late step in anaerobic heme biosynthesis in Escherichia coli. Biochim Biophys Acta. 1978 Dec 18;544(3):540–546. doi: 10.1016/0304-4165(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Keithly J. H., Nadler K. D. Protoporphyrin formation in Rhizobium japonicum. J Bacteriol. 1983 May;154(2):838–845. doi: 10.1128/jb.154.2.838-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981 Jul;147(1):161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., LASCELLES J. HAEMOPROTEINS AND HAEM SYNTHESIS IN FACULTATIVE PHOTOSYNTHETIC AND DENITRIFYING BACTERIA. Biochem J. 1965 Jan;94:120–126. doi: 10.1042/bj0940120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R. Cytochrome c3 and desulphoviridin; pigments of the anaerobe Desulphovibrio desulphuricans. J Gen Microbiol. 1956 Jul;14(3):545–572. doi: 10.1099/00221287-14-3-545. [DOI] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]


