Abstract
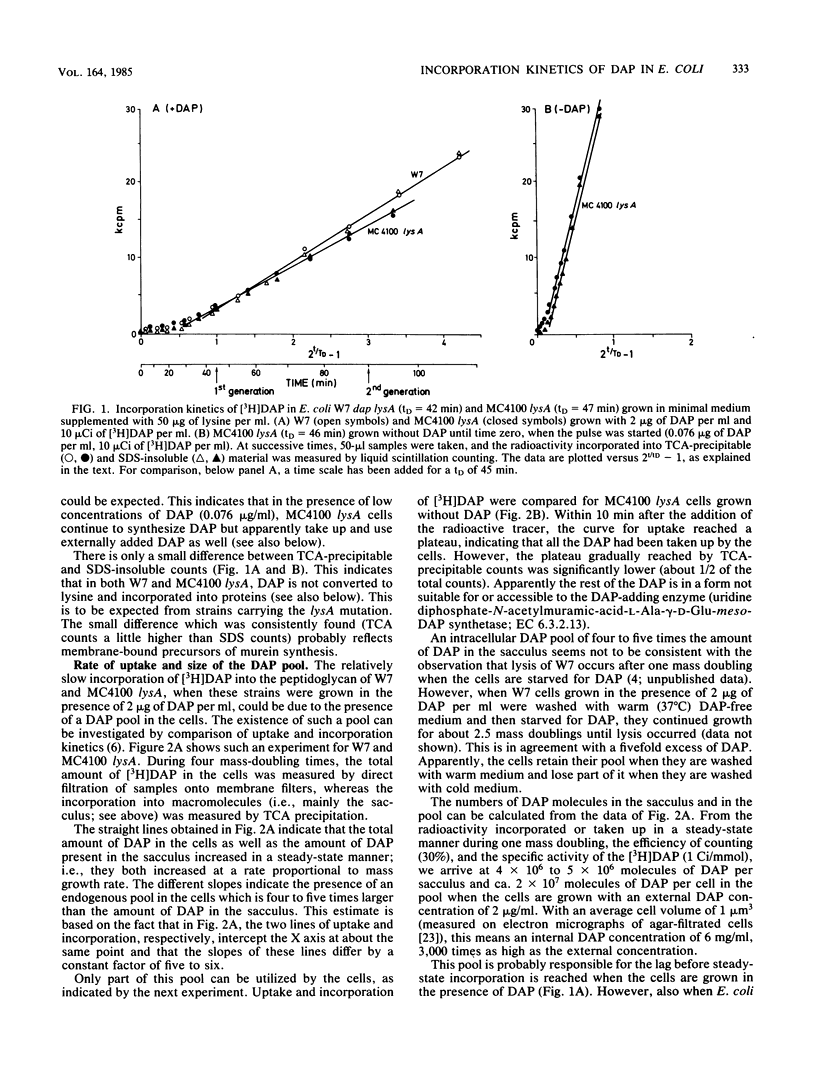
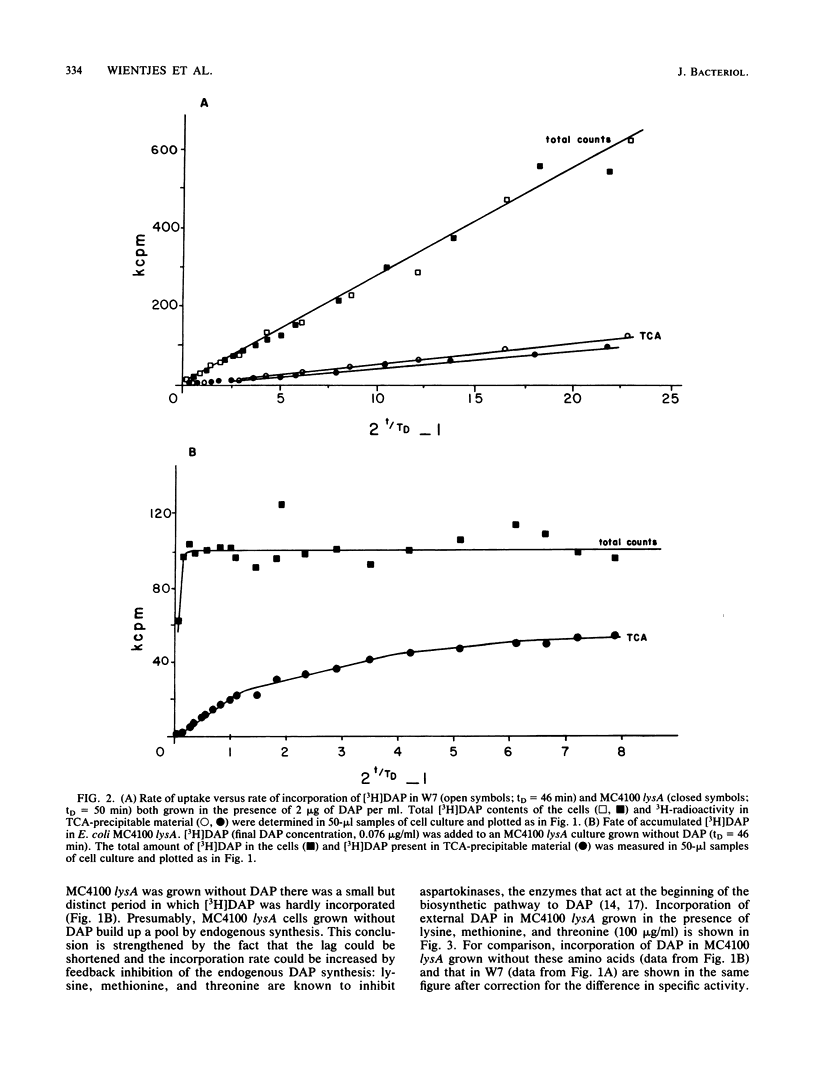
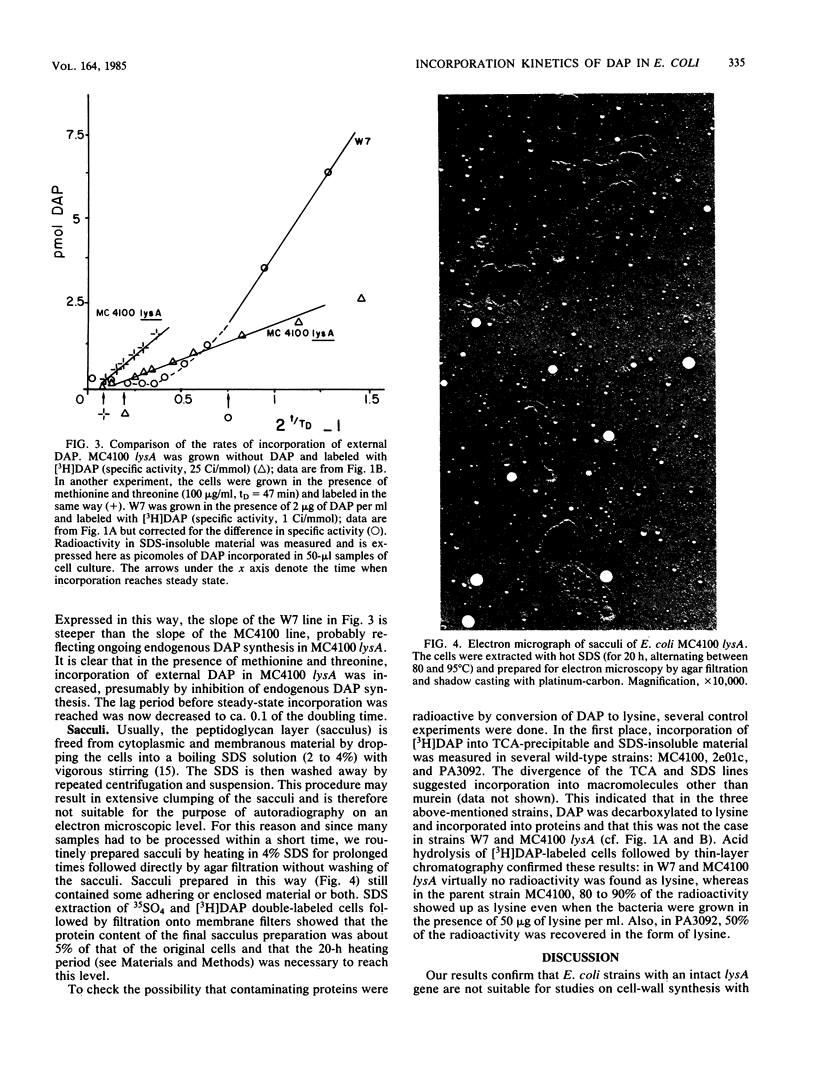
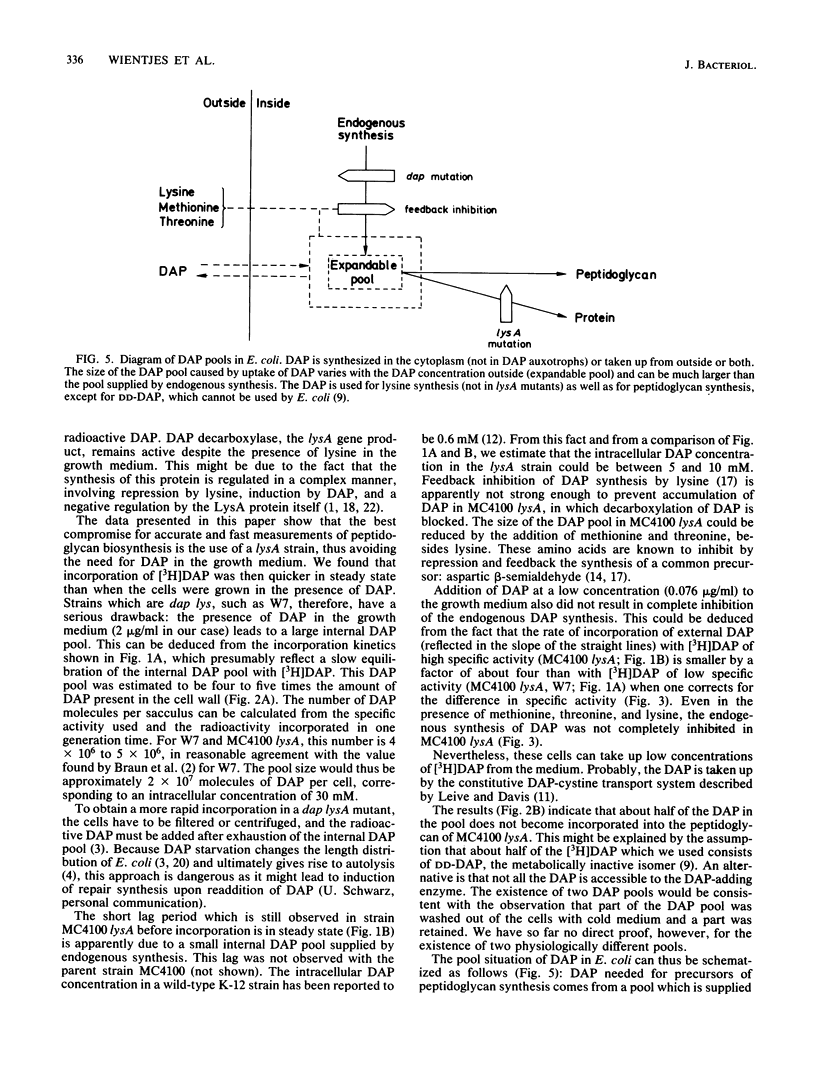
The rate at which the peptidoglycan precursor meso-diaminopimelic acid (DAP) is incorporated into the cell wall of Escherichia coli cells was determined by pulse-label experiments. For different E. coli strains, the incorporation rate was compared with the rate of uptake of DAP into the cell. With E. coli W7, a dap lys mutant generally used in this kind of studies, steady-state incorporation was reached only after about 0.75 of the doubling time. This lag period can be ascribed to the presence of a large internal DAP pool in the cells. An E. coli K-12 lysA strain was constructed which could be grown without DAP in its medium. Consequently, due to the higher specific activity of the added [3H]DAP, faster incorporation and higher levels of radioactivity in the peptidoglycan layer were observed in the K-12 lysA strain than in the W7 strain. In addition, uptake and incorporation were faster in steady state (within about 0.2 of the doubling time), indicating a smaller DAP pool. The lag period could be further diminished and the incorporation rate could be increased by feedback inhibition of the biosynthetic pathway to DAP with threonine and methionine. These results make MC4100 lysA a suitable strain for studies on peptidoglycan synthesis. To explain our observations, we suggest the existence of an expandable pool of DAP in E. coli which varies with the DAP concentration in the growth medium. With 2 microgram of DAP per ml, the size of the pool is severalfold the amount of DAP contained in the cell wall. This pool can be partly washed out of the cells. Grown without DAP, MC4100 lysA still has a small pool caused by endogenous synthesis, which accounts for the fact that steady-state [3H]DAP incorporation in the lysA strain still shows a lag period.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Raichler J., Park J. T. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J Bacteriol. 1983 Sep;155(3):983–988. doi: 10.1128/jb.155.3.983-988.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Reichler J., Park J. T. Evidence for multisite growth of Escherichia coli murein involving concomitant endopeptidase and transpeptidase activities. J Bacteriol. 1983 Oct;156(1):386–392. doi: 10.1128/jb.156.1.386-392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Creanor J., Mitchison J. M. Patterns of protein synthesis during the cell cycle of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1982 Dec;58:263–285. doi: 10.1242/jcs.58.1.263. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of Escherichia coli. Nature. 1952 Mar 29;169(4300):534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid: their distribution in nature and behaviour towards certain enzyme preparations. Biochem J. 1955 Dec;61(4):562–568. doi: 10.1042/bj0610562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Koppes L. J., Overbeeke N., Nanninga N. DNA replication pattern and cell wall growth in Escherichia coli PAT 84. J Bacteriol. 1978 Mar;133(3):1053–1061. doi: 10.1128/jb.133.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Davis B. D. The transport of diaminopimelate and cystine in Escherichia coli. J Biol Chem. 1965 Nov;240(11):4362–4369. [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Stragier P., Borne F., Richaud F., Richaud C., Patte J. C. Regulatory pattern of the Escherichia coli lysA gene: expression of chromosomal lysA-lacZ fusions. J Bacteriol. 1983 Dec;156(3):1198–1203. doi: 10.1128/jb.156.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N. Pattern of meso-dl-2,6-diaminopimelic acid incorporation during the division cycle of Escherichia coli. J Bacteriol. 1980 Oct;144(1):327–336. doi: 10.1128/jb.144.1.327-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J. The regulation of diaminopimelate decarboxylase activity in Escherichia coli strain w. J Gen Microbiol. 1976 Sep;96(1):51–62. doi: 10.1099/00221287-96-1-51. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




