Abstract
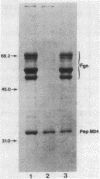
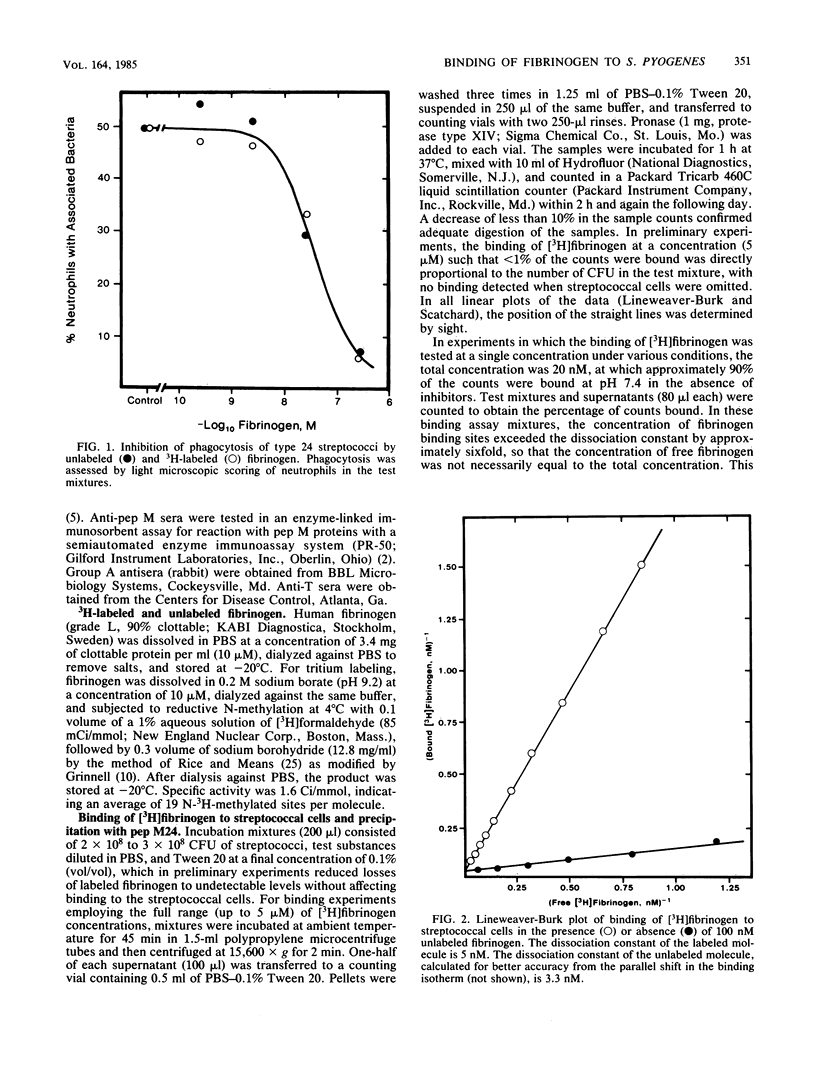
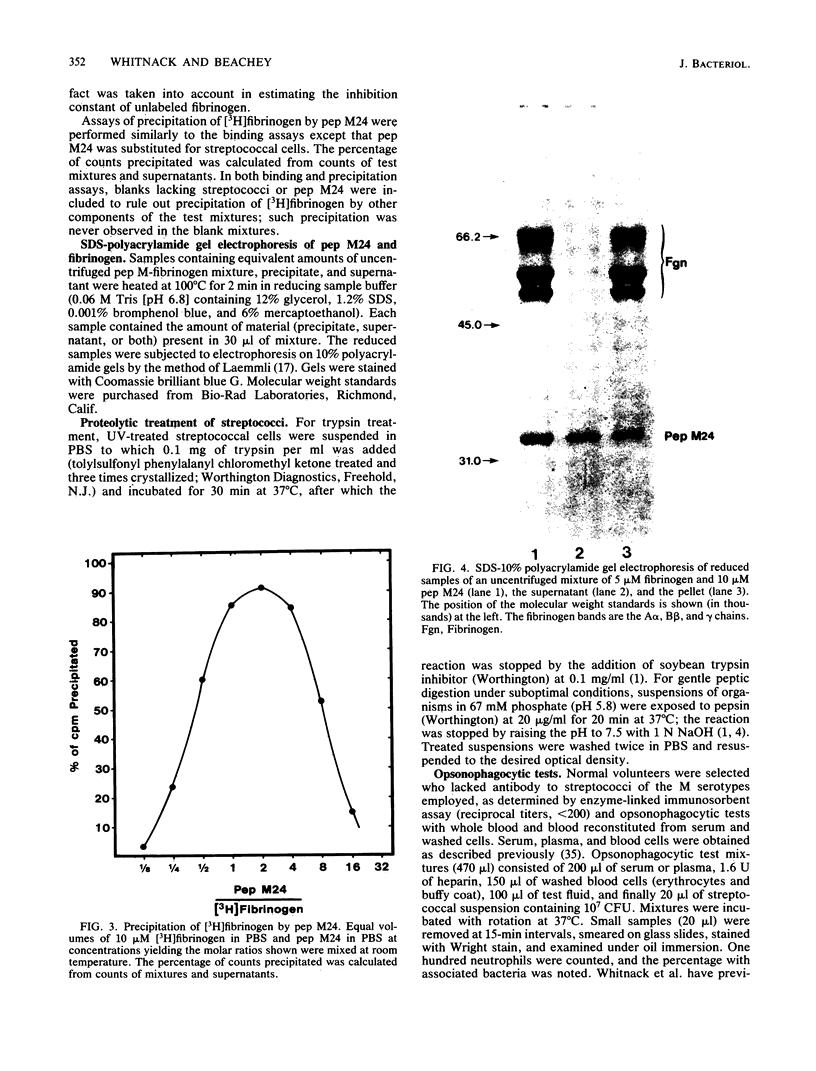
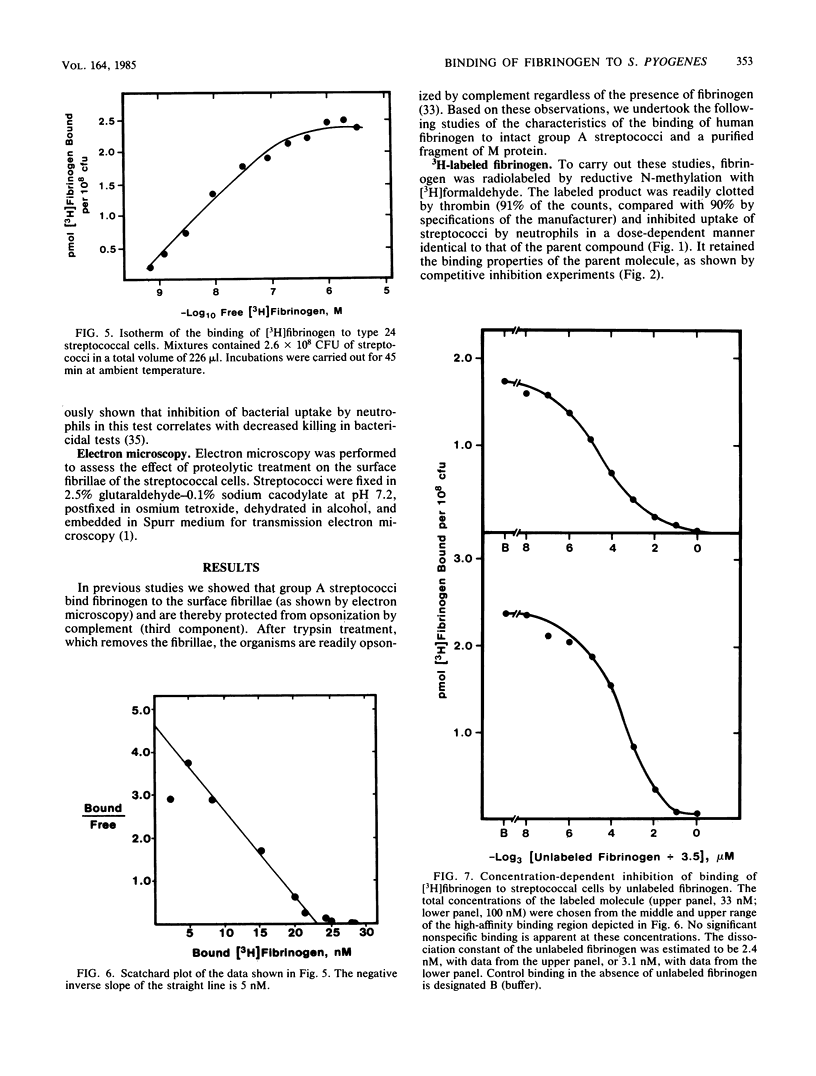
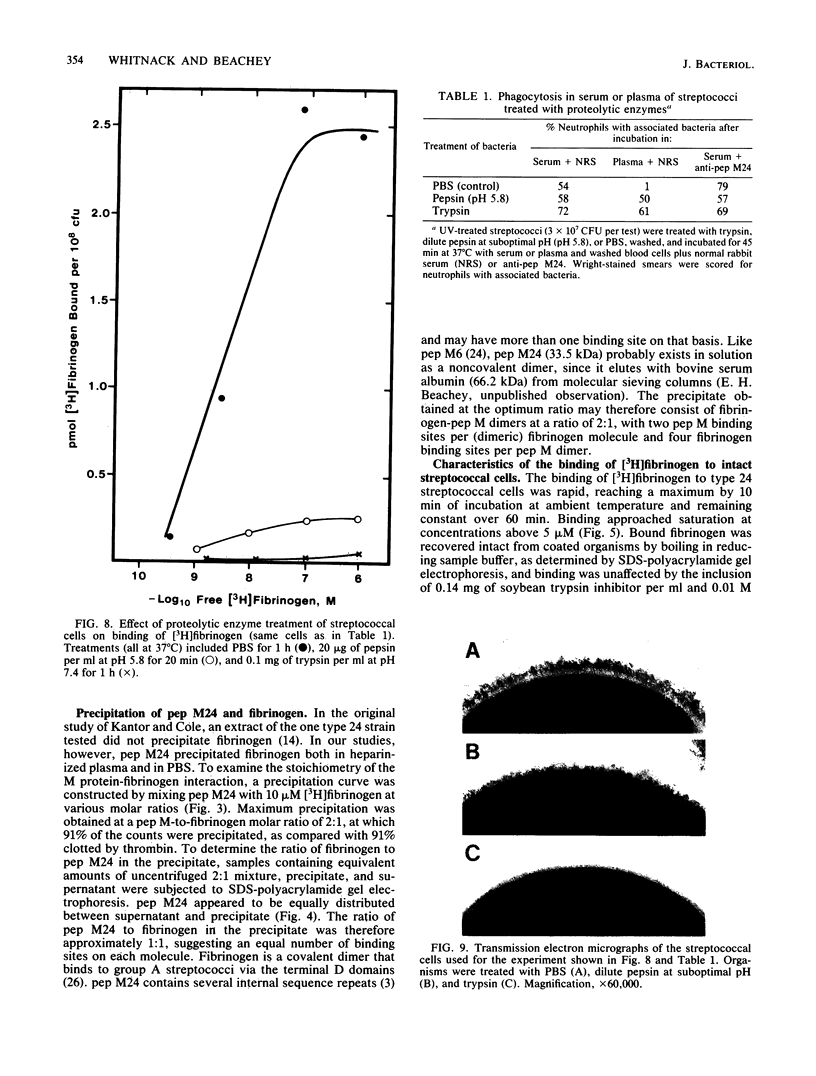
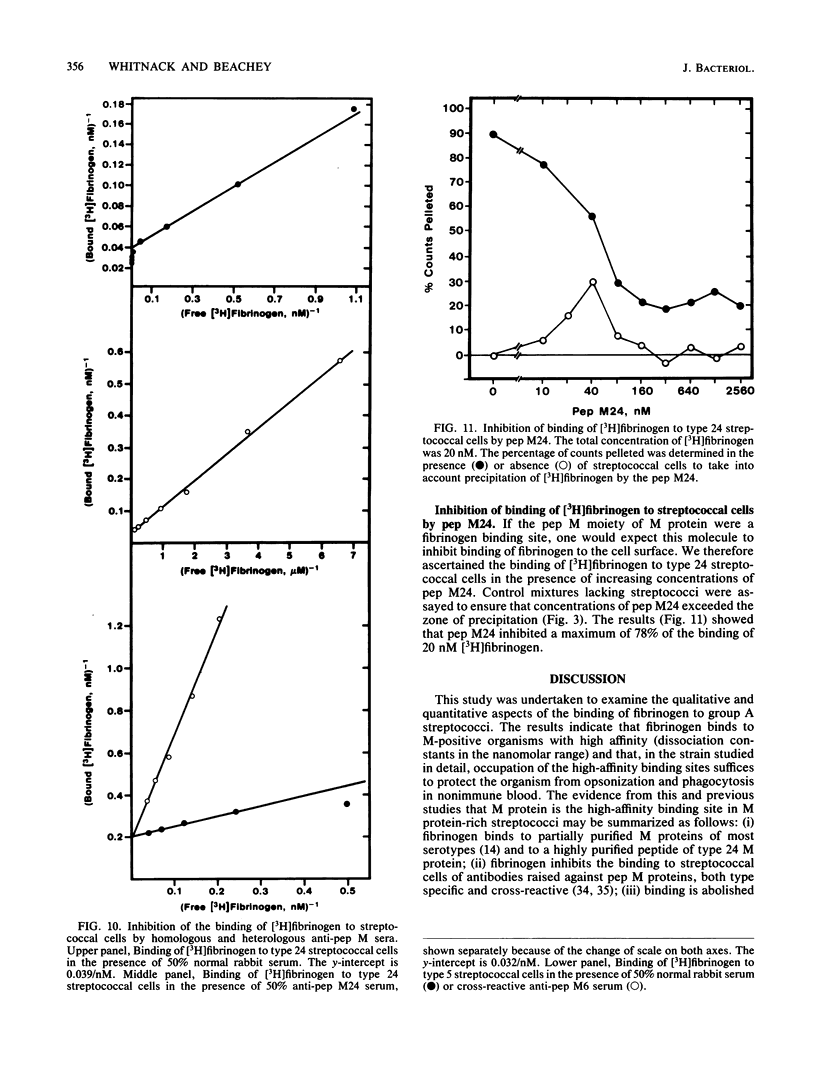
Fibrinogen is known to bind to group A streptococci and precipitate with extracts containing streptococcal M protein. We have previously shown that the binding of fibrinogen to M-positive streptococci prevents opsonization by complement and protects that organism from phagocytosis in nonimmune blood. In the present study, we used 3H-labeled fibrinogen, a highly purified peptide fragment of type 24 M protein (pep M24), and anti-pep M sera to show that fibrinogen binds to M-positive streptococci with high affinity (dissociation constants, 1 to 5 nM); occupation of the high-affinity binding sites suffices to protect the organism from phagocytosis; proteolytic treatments that remove M protein from streptococcal cells abolish binding; binding is competitively inhibited by anti-pep M sera; pep M24 precipitates fibrinogen; and binding to type 24 cells is inhibited by pep M24. We conclude that M protein is the cell surface structure principally responsible for binding fibrinogen on the surface of M-positive streptococci and that this binding contributes to the known antiopsonic property of M proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Dale J. B., Simpson W. A., Kang A. H. Type-specific protective immunity evoked by synthetic peptide of Streptococcus pyogenes M protein. Nature. 1981 Jul 30;292(5822):457–459. doi: 10.1038/292457a0. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Repeating covalent structure of streptococcal M protein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3163–3167. doi: 10.1073/pnas.75.7.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Johnson R. H., Ofek I., Bisno A. L. Human immune response to immunization with a structurally defined polypeptide fragment of streptococcal M protein. J Exp Med. 1979 Oct 1;150(4):862–877. doi: 10.1084/jem.150.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck L., Tylewska S. K., Wadström T., Kronvall G. beta 2-Microglobulin is bound to streptococcal M protein. Scand J Immunol. 1981;13(4):391–394. doi: 10.1111/j.1365-3083.1981.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Ofek I., Simpson W. A., Whitnack E., Beachey E. H. Human plasma fibronectin inhibits adherence of Streptococcus pyogenes to hexadecane. Infect Immun. 1985 Jan;47(1):341–343. doi: 10.1128/iai.47.1.341-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983 Feb;153(2):763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. D. A PROTEOLYTIC ENZYME PRODUCED BY GROUP A STREPTOCOCCI WITH SPECIAL REFERENCE TO ITS EFFECT ON THE TYPE-SPECIFIC M ANTIGEN. J Exp Med. 1945 Jun 1;81(6):573–592. doi: 10.1084/jem.81.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN J. J., COLE R. M. STREPTOCOCCAL M ANTIGEN LOCATION AND SYNTHESIS, STUDIED BY IMMUNOFLUORESCENCE. J Exp Med. 1963 Nov 1;118:659–666. doi: 10.1084/jem.118.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H., Beachey E. H. Purification and immunobiological properties of R antigen and its relation to M protein of type 3 group A Streptococcus. Infect Immun. 1979 Sep;25(3):1051–1059. doi: 10.1128/iai.25.3.1051-1059.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOR F. S., COLE R. M. A fibrinogen precipitating factor (FPF) of group A streptococci. Proc Soc Exp Biol Med. 1959 Oct;102:146–150. doi: 10.3181/00379727-102-25172. [DOI] [PubMed] [Google Scholar]
- KANTOR F. S. FIBRINOGEN PRECIPITATION BY STREPTOCOCCAL M PROTEIN. I. IDENTITY OF THE REACTANTS, AND STOICHIOMETRY OF THE REACTION. J Exp Med. 1965 May 1;121:849–859. doi: 10.1084/jem.121.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., Dole V. P. THE PROPERTIES OF T ANTIGENS EXTRACTED FROM GROUP A HEMOLYTIC STREPTOCOCCI. J Exp Med. 1946 Oct 31;84(5):449–471. doi: 10.1084/jem.84.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C. STUDIES ON THE ANTIGENIC COMPOSITION OF GROUP A HEMOLYTIC STREPTOCOCCI : I. EFFECTS OF PROTEOLYTIC ENZYMES ON STREPTOCOCCAL CELLS. J Exp Med. 1943 Dec 1;78(6):465–476. doi: 10.1084/jem.78.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light K. E. Analyzing nonlinear scatchard plots. Science. 1984 Jan 6;223(4631):76–78. doi: 10.1126/science.6546323. [DOI] [PubMed] [Google Scholar]
- Ludwicka A., Jeljaszewicz J. Paracoagulation of fibrinogen in vitro and in vivo by protein T of Steptococcus pyogenes. Zentralbl Bakteriol Orig A. 1978 Sep;241(3):301–307. [PubMed] [Google Scholar]
- Ofek I., Whitnack E., Beachey E. H. Hydrophobic interactions of group A streptococci with hexadecane droplets. J Bacteriol. 1983 Apr;154(1):139–145. doi: 10.1128/jb.154.1.139-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Runehagen A., Schönbeck C., Hedner U., Hessel B., Kronvall G. Binding of fibrinogen degradation products to S. aureus and to beta-hemolytic streptococci group A, C and G. Acta Pathol Microbiol Scand B. 1981 Apr;89(2):49–55. doi: 10.1111/j.1699-0463.1981.tb00151_89b.x. [DOI] [PubMed] [Google Scholar]
- Schmidt K. H., Köhler W. Interaction of streptococcal cell wall components with fibrinogen. I. adsorption of fibrinogen by immobilized T-proteins of streptococcus pyogenes. Immunobiology. 1981;158(4):330–337. doi: 10.1016/S0171-2985(81)80004-6. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift H. F., Wilson A. T., Lancefield R. C. TYPING GROUP A HEMOLYTIC STREPTOCOCCI BY M PRECIPITIN REACTIONS IN CAPILLARY PIPETTES. J Exp Med. 1943 Aug 1;78(2):127–133. doi: 10.1084/jem.78.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd E. W., Lancefield R. C. VARIANTS OF HEMOLYTIC STREPTOCOCCI; THEIR RELATION TO TYPE-SPECIFIC SUBSTANCE, VIRULENCE, AND TOXIN. J Exp Med. 1928 Nov 30;48(6):751–767. doi: 10.1084/jem.48.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Dale J. B., Beachey E. H. Common protective antigens of group A streptococcal M proteins masked by fibrinogen. J Exp Med. 1984 Apr 1;159(4):1201–1212. doi: 10.1084/jem.159.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Dale J. B., Beachey E. H. Streptococcal defenses against host immune attack: the M protein-fibrinogen interaction. Trans Assoc Am Physicians. 1983;96:197–202. [PubMed] [Google Scholar]