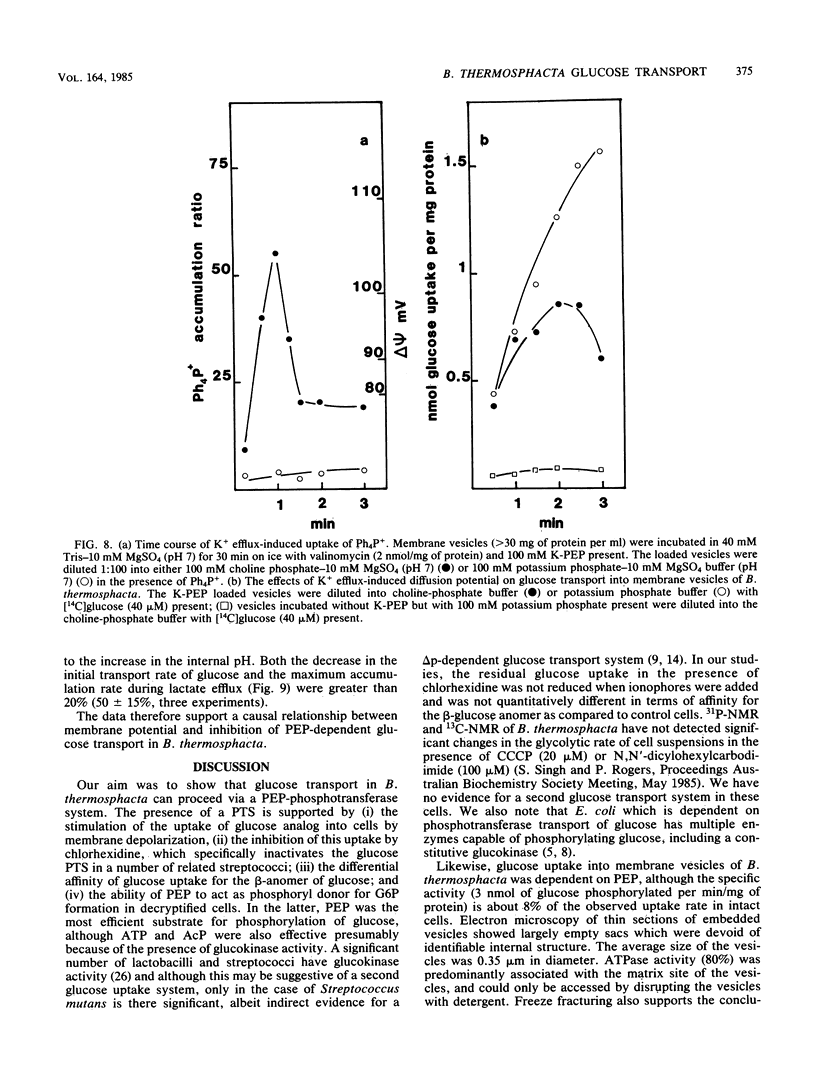
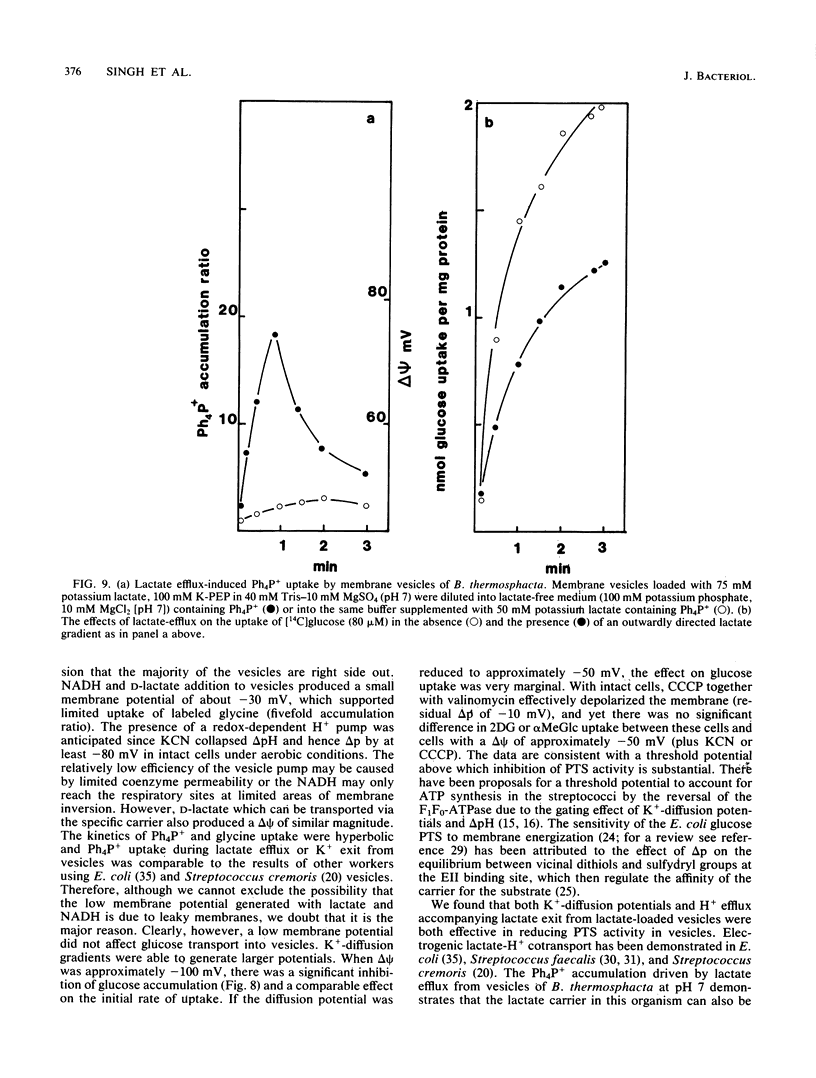
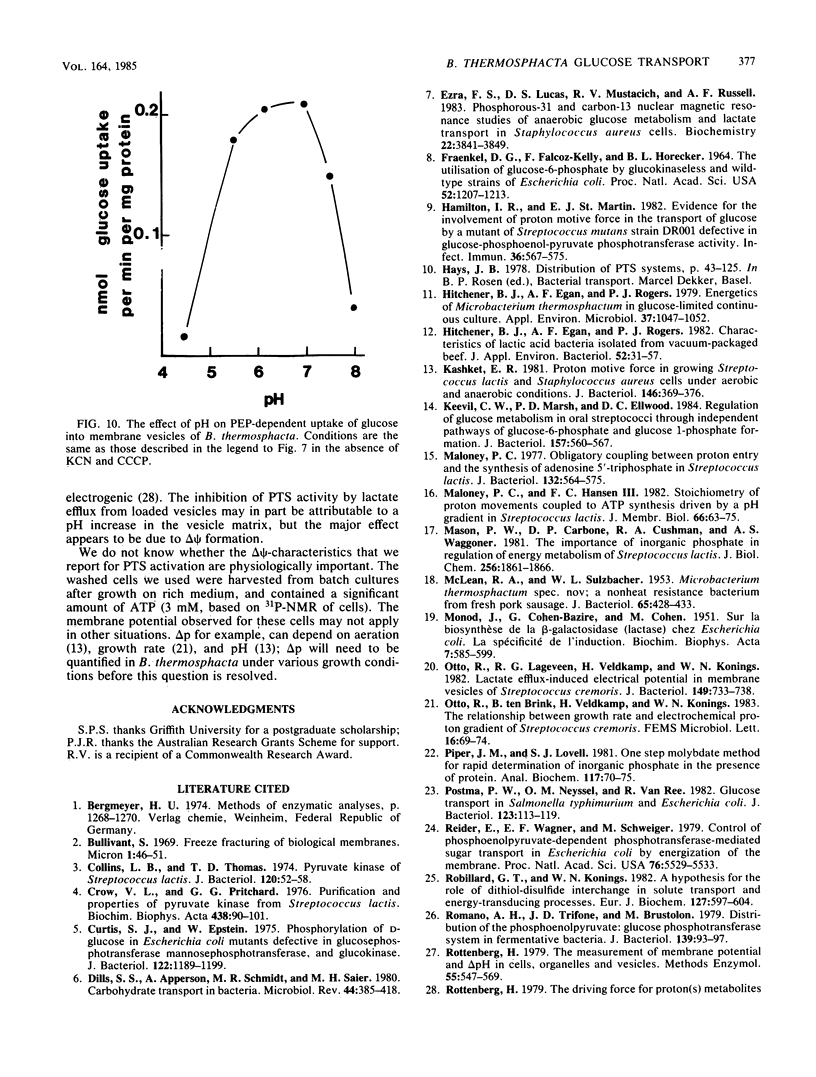
Abstract
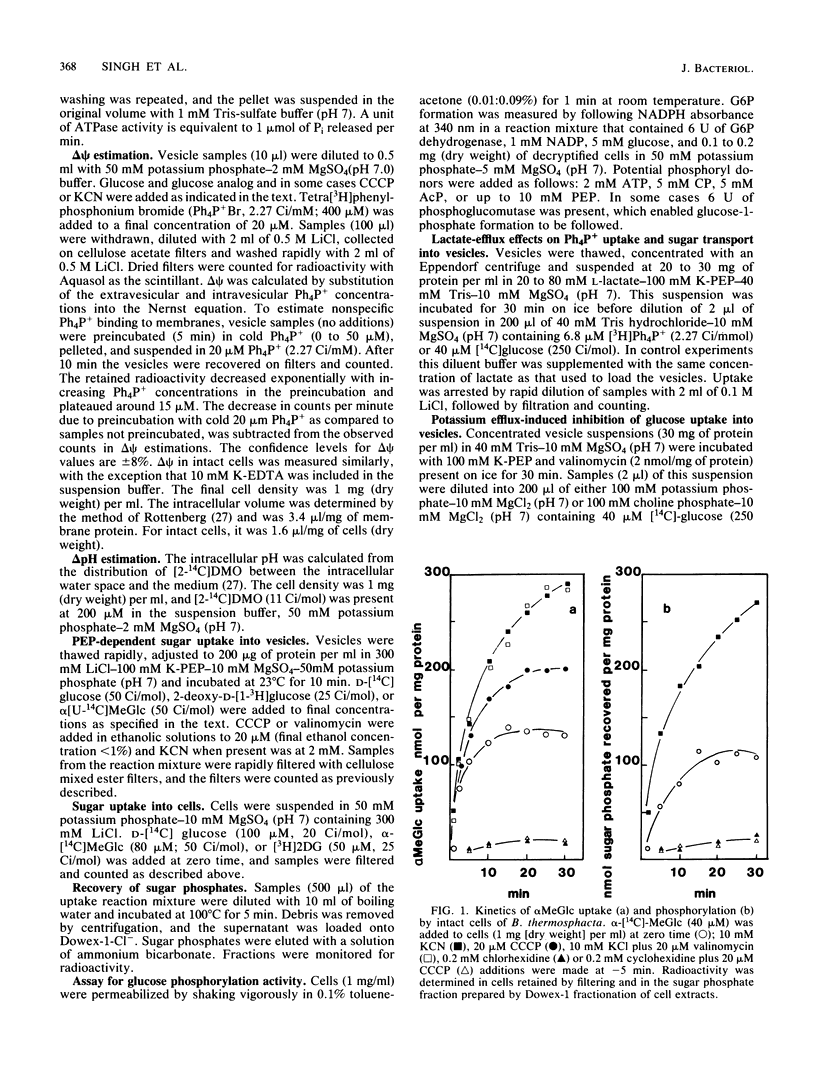
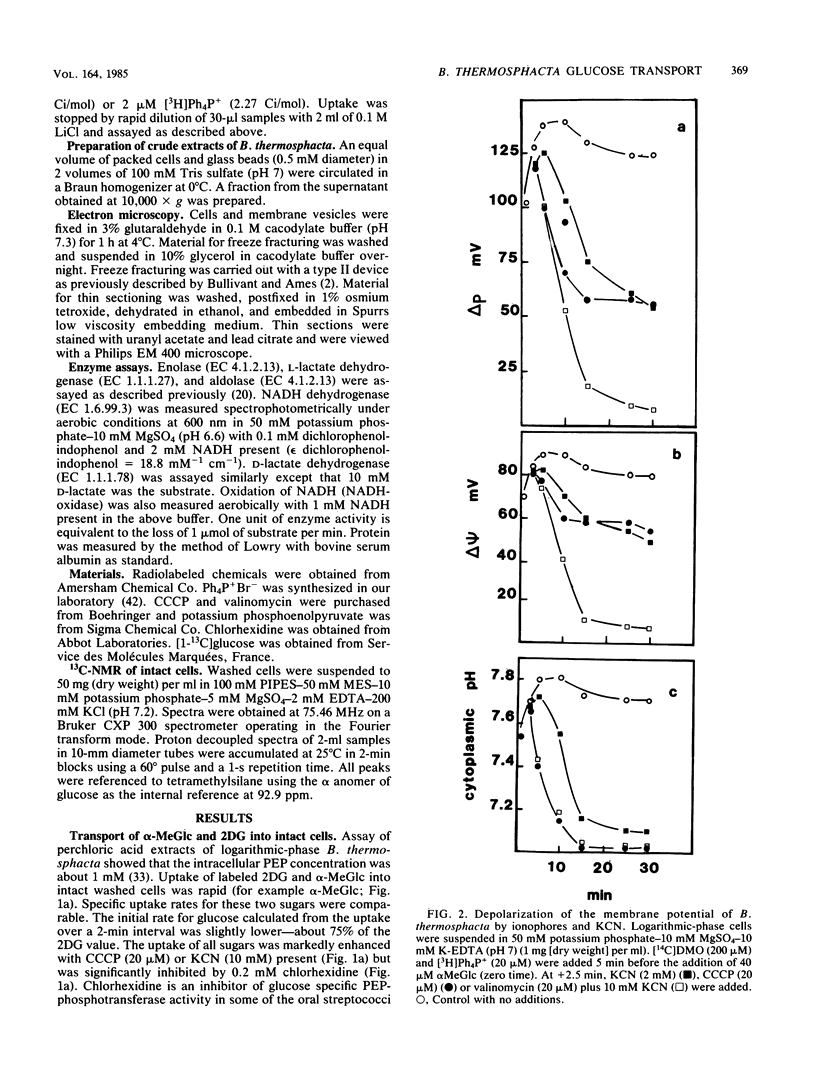
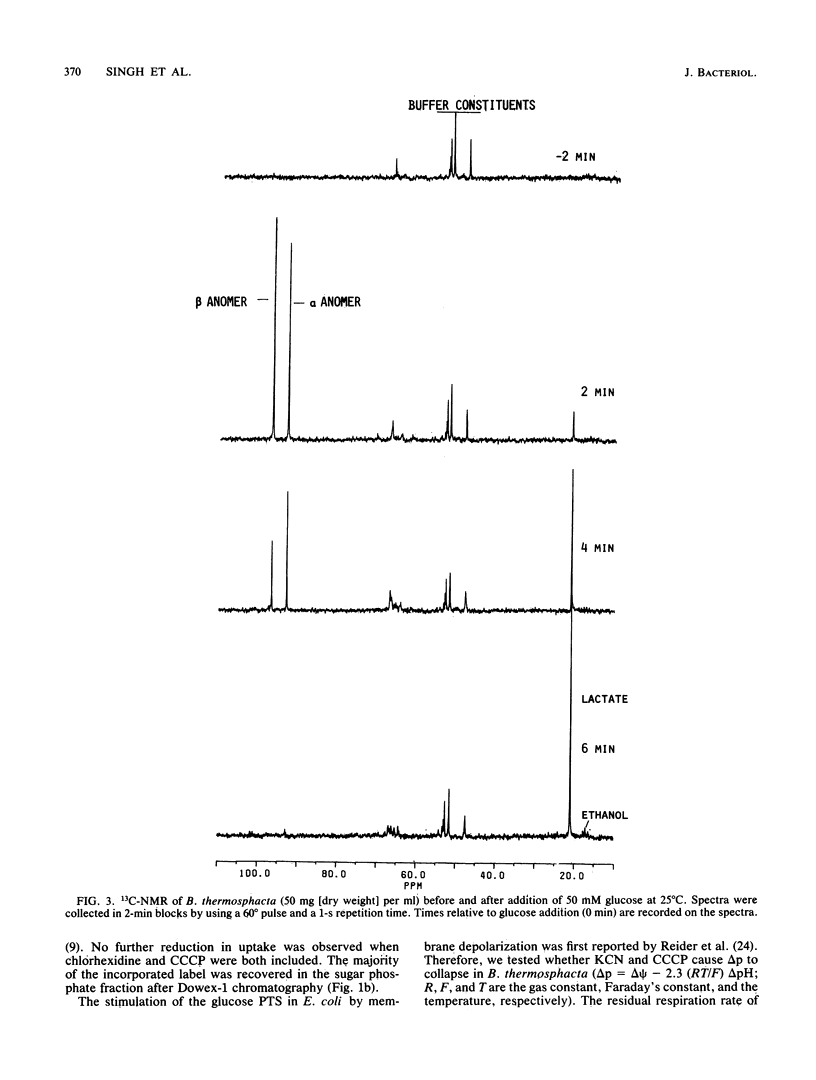
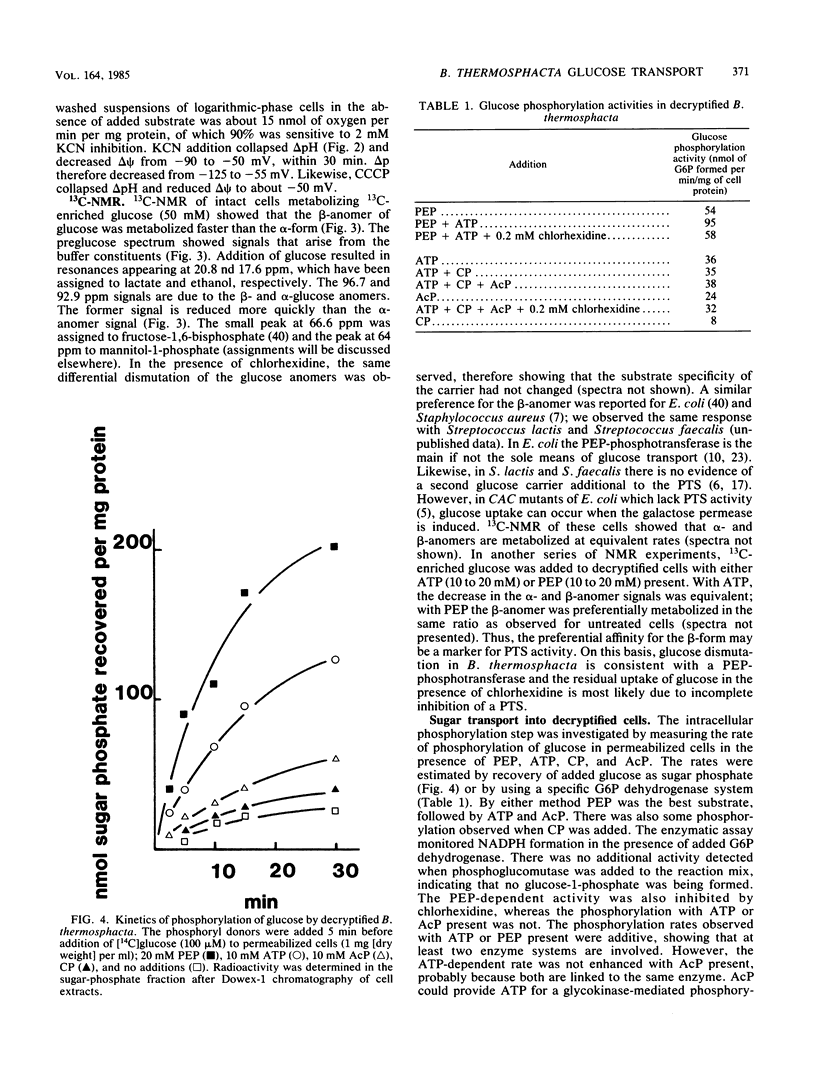
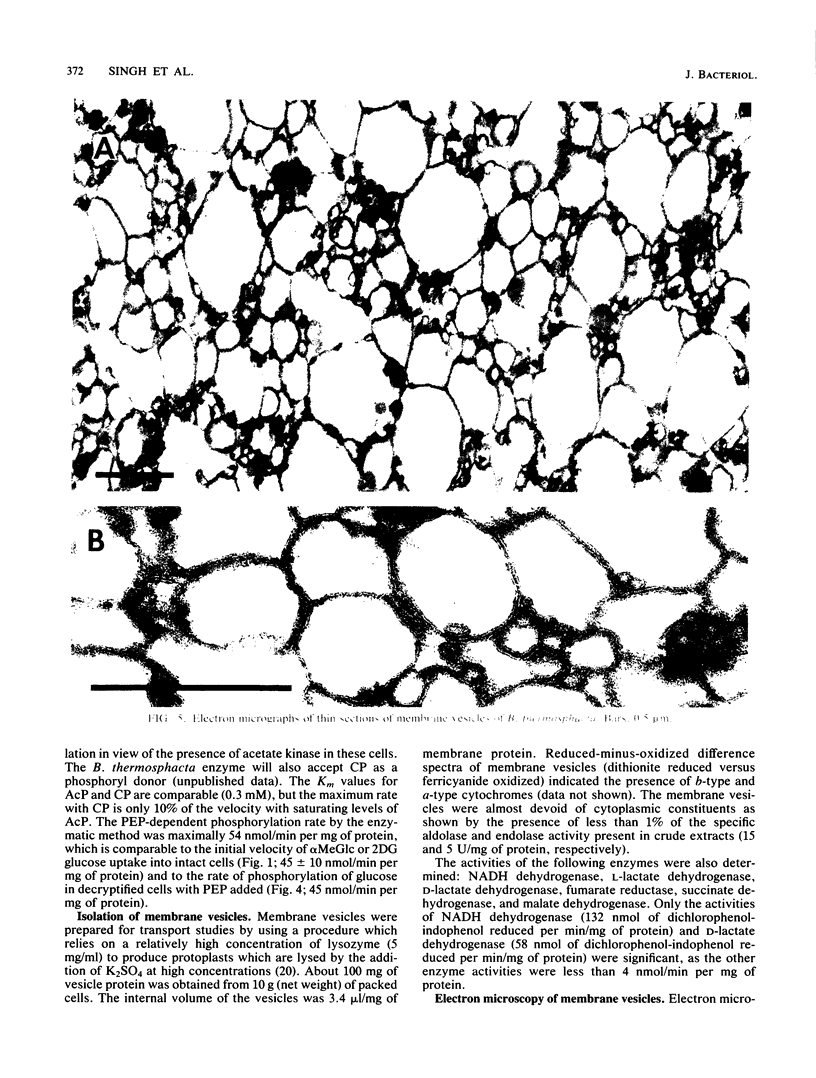
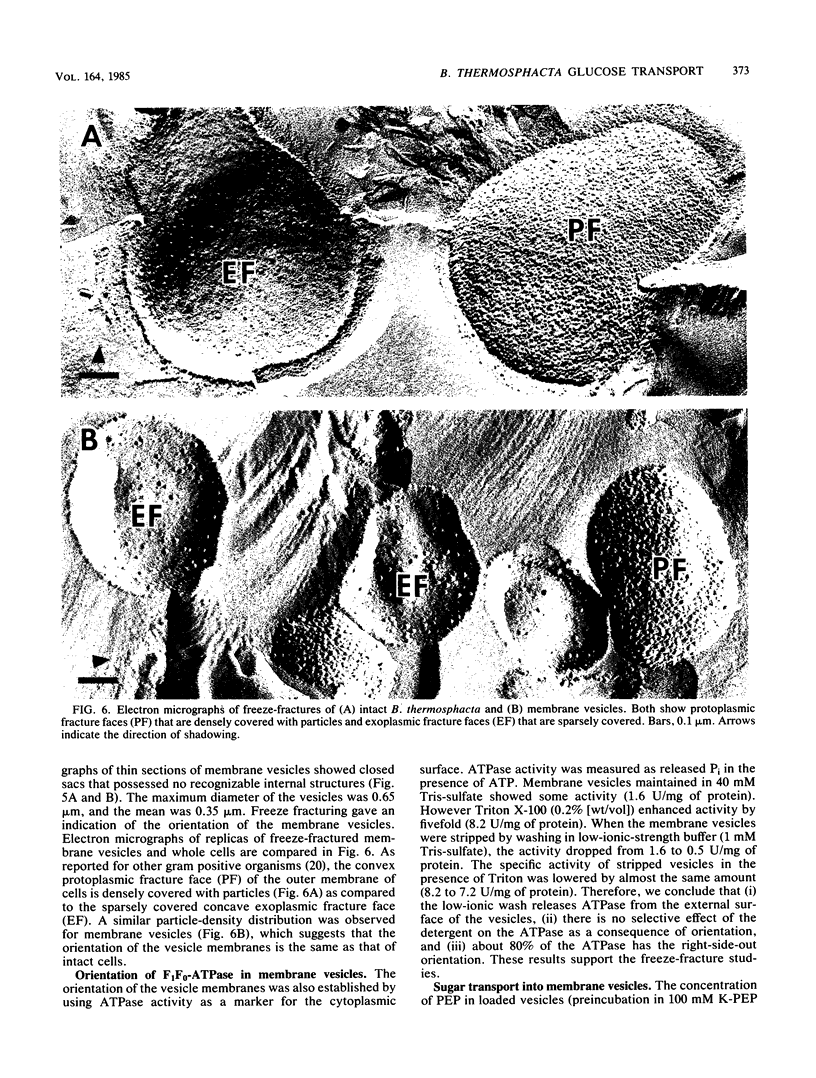
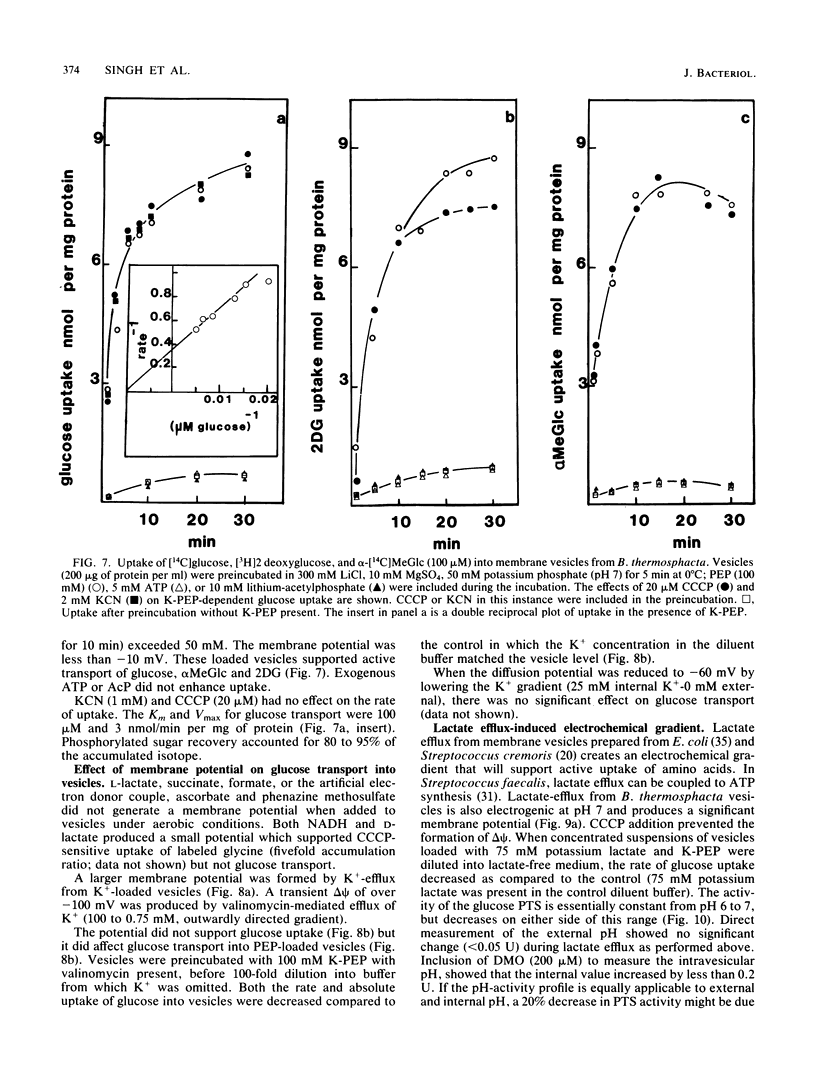
Uptake of 2-deoxyglucose, alpha-methylglucopyranoside, and glucose into intact cells of Brochothrix thermosphacta (formerly Microbacterium thermosphactum, ATCC 11509) was stimulated by KCN or CCCP. The glucose analogs were recovered almost totally as the sugar phosphates. Membrane vesicles were isolated from protoplasts and shown to be right side out by freeze fracturing and by using ATPase as a marker for the cytoplasmic membrane surface. Uptake of glucose into vesicles was dependent on the presence of phosphoenolpyruvate. NADH oxidation, K+ -diffusion gradients, and externally directed lactate gradients (pH greater than 7 initially) were used to generate transmembrane potentials across membrane vesicles. Above a threshold value of about -50 mV, uptake of glucose into membrane vesicles was reduced. Likewise, the maximum uptake of glucose and its two analogs into cells occurred when the protonmotive force was less than about -50 mV.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra F. S., Lucas D. S., Mustacich R. V., Russell A. F. Phosphorus-31 and carbon-13 nuclear magnetic resonance studies of anaerobic glucose metabolism and lactate transport in Staphylococcus aureus cells. Biochemistry. 1983 Aug 2;22(16):3841–3849. doi: 10.1021/bi00285a020. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchener B. J., Egan A. F., Rogers P. J. Characteristics of lactic acid bacteria isolated from vacuum-packaged beef. J Appl Bacteriol. 1982 Feb;52(1):31–37. doi: 10.1111/j.1365-2672.1982.tb04369.x. [DOI] [PubMed] [Google Scholar]
- Hitchener B. J., Egan A. F., Rogers P. J. Energetics of Microbacterium thermosphactum in glucose-limited continuous culture. Appl Environ Microbiol. 1979 Jun;37(6):1047–1052. doi: 10.1128/aem.37.6.1047-1052.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., Marsh P. D., Ellwood D. C. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J Bacteriol. 1984 Feb;157(2):560–567. doi: 10.1128/jb.157.2.560-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN R. A., SULZBACHER W. L. Microbacterium thermosphactum, spec: nov.; a nonheat resistant bacterium from fresh pork sausage. J Bacteriol. 1953 Apr;65(4):428–433. doi: 10.1128/jb.65.4.428-433.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Hansen F. C., 3rd Stoichiometry of proton movements coupled to ATP synthesis driven by a pH gradient in Streptococcus lactis. J Membr Biol. 1982;66(1):63–75. doi: 10.1007/BF01868482. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. Obligatory coupling between proton entry and the synthesis of adenosine 5'-triphosphate in Streptococcus lactis. J Bacteriol. 1977 Nov;132(2):564–575. doi: 10.1128/jb.132.2.564-575.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper J. M., Lovell S. J. One-step molybdate method for rapid determination of inorganic phosphate in the presence of protein. Anal Biochem. 1981 Oct;117(1):70–75. doi: 10.1016/0003-2697(81)90693-x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Neyssel O. M., van Ree R. Glucose transport in Salmonella typhimurium and Escherichia coli. Eur J Biochem. 1982 Mar;123(1):113–119. doi: 10.1111/j.1432-1033.1982.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Reider E., Wagner E. F., Schweiger M. Control of phosphoenolpyruvate-dependent phosphotransferase-mediated sugar transport in Escherichia coli by energization of the cell membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5529–5533. doi: 10.1073/pnas.76.11.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. A hypothesis for the role of dithiol-disulfide interchange in solute transport and energy-transducing processes. Eur J Biochem. 1982 Oct;127(3):597–604. doi: 10.1111/j.1432-1033.1982.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., Bendall M. R., Egan A. F., Vink R., Rogers P. J. High-field phosphorus NMR studies of the stoichiometry of the lactate/proton carrier in Streptococcus faecalis. Eur J Biochem. 1983 Oct 17;136(1):63–69. doi: 10.1111/j.1432-1033.1983.tb07705.x. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Torchia D. A. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J Bacteriol. 1984 Jun;158(3):791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Brown T. R., den Hollander J. A., Glynn P., Shulman R. G. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R., Bendall M. R., Simpson S. J., Rogers P. J. Estimation of H+ to adenosine 5'-triphosphate stoichiometry of Escherichia coli ATP synthase using 31P NMR. Biochemistry. 1984 Jul 31;23(16):3667–3675. doi: 10.1021/bi00311a015. [DOI] [PubMed] [Google Scholar]