Abstract
Pectin lyase (PNL) and the bacteriocin carotovoricin (CTV) were induced in Erwinia carotovora subsp. carotovora 71 by the DNA-damaging agents mitomycin C, nalidixic acid, and UV light. To determine whether the recA product was involved in the expression of these damage-inducible phenotypes, we cloned the E. carotovora subsp. carotovora recA+ gene, inactivated it by Tn5 insertion, and constructed an E. carotovora subsp. carotovora recA::Tn5 strain by gene replacement via homologous recombination. The RecA- strain was more sensitive to methyl methanesulfonate, nitroquinoline oxide, and UV light than its RecA+ parent. The recA mutation did not affect the production of pectate lyase, polygalacturonase, cellulase, and protease or the ability to cause soft rot of potato tubers. With this mutant, unlike with the RecA+ parent strain, PNL and CTV were not induced by mitomycin C or detected in potato tuber tissue. The RecA+ phenotype, including the inducibility of PNL and CTV, could, however, be restored in the mutant in trans by the recA+ gene from either E. carotovora subsp. carotovora or Escherichia coli. We conclude that, in E. carotovora subsp. carotovora, the recA product is required in the induction of PNL and CTV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Mylroie J. R., Friello D. A., Vacca J. G. Transformation of Pseudomonas putida and Escherichia coli with plasmid-linked drug-resistance factor DNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3647–3651. doi: 10.1073/pnas.72.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K. Acceptance by Erwinia spp. of R plasmid R68.45 and its ability to mobilize the chromosome of Erwinia chrysanthemi. J Bacteriol. 1980 Apr;142(1):111–119. doi: 10.1128/jb.142.1.111-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Brown M. A. Generalized transduction in the enterobacterial phytopathogen Erwinia chrysanthemi. J Bacteriol. 1980 Sep;143(3):1444–1449. doi: 10.1128/jb.143.3.1444-1449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Thurn K. K., Feese D. A. Tn5-Induced Mutations in the Enterobacterial Phytopathogen Erwinia chrysanthemi. Appl Environ Microbiol. 1983 Feb;45(2):644–650. doi: 10.1128/aem.45.2.644-650.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer A., Whalen C. H., Beer S. V., Bateman D. F. An exo-poly-alpha-D-galacturonosidase implicated in the regulation of extracellular pectate lyase production in Erwinia chrysanthemi. J Bacteriol. 1982 Feb;149(2):626–634. doi: 10.1128/jb.149.2.626-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Nakazawa A. Direct participation of lexA protein in repression of colicin E1 synthesis. J Bacteriol. 1982 Jun;150(3):1479–1481. doi: 10.1128/jb.150.3.1479-1481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. R., Beringer J. E. A physical map of pPH1JI and pJB4JI. Plasmid. 1984 Sep;12(2):139–141. doi: 10.1016/0147-619x(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Nakatani T., Hase T., Matsubara H., Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981 Dec;27(3 Pt 2):515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
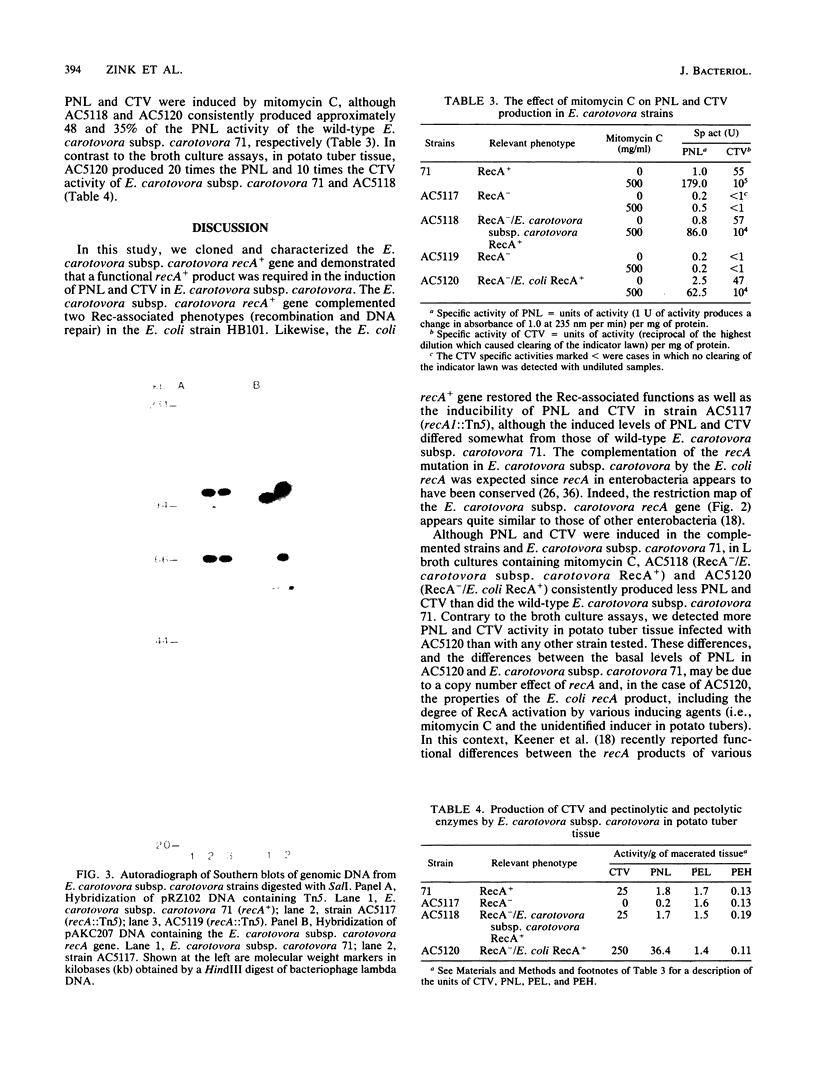
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Pierré A., Paoletti C. Purification and characterization of recA protein from salmonella typhimurium. J Biol Chem. 1983 Mar 10;258(5):2870–2874. [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- West S. C., Countryman J. K., Howard-Flanders P. Purification and properties of the recA protein of Proteus mirabilis. Comparison with Escherichia coli recA protein; specificity of interaction with single strand binding protein. J Biol Chem. 1983 Apr 10;258(7):4648–4654. [PubMed] [Google Scholar]
- Zink R. T., Chatterjee A. K. Cloning and expression in Escherichia coli of pectinase genes of Erwinia carotovora subsp. carotovora. Appl Environ Microbiol. 1985 Mar;49(3):714–717. doi: 10.1128/aem.49.3.714-717.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink R. T., Kemble R. J., Chatterjee A. K. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J Bacteriol. 1984 Mar;157(3):809–814. doi: 10.1128/jb.157.3.809-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Maat J., Walters H. H., Veltkamp E., Nijkamp H. J. The nucleotide sequence of the bacteriocin promoters of plasmids Clo DF13 and Co1 E1: role of lexA repressor and cAMP in the regulation of promoter activity. Nucleic Acids Res. 1982 Mar 25;10(6):1913–1928. doi: 10.1093/nar/10.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]