Abstract
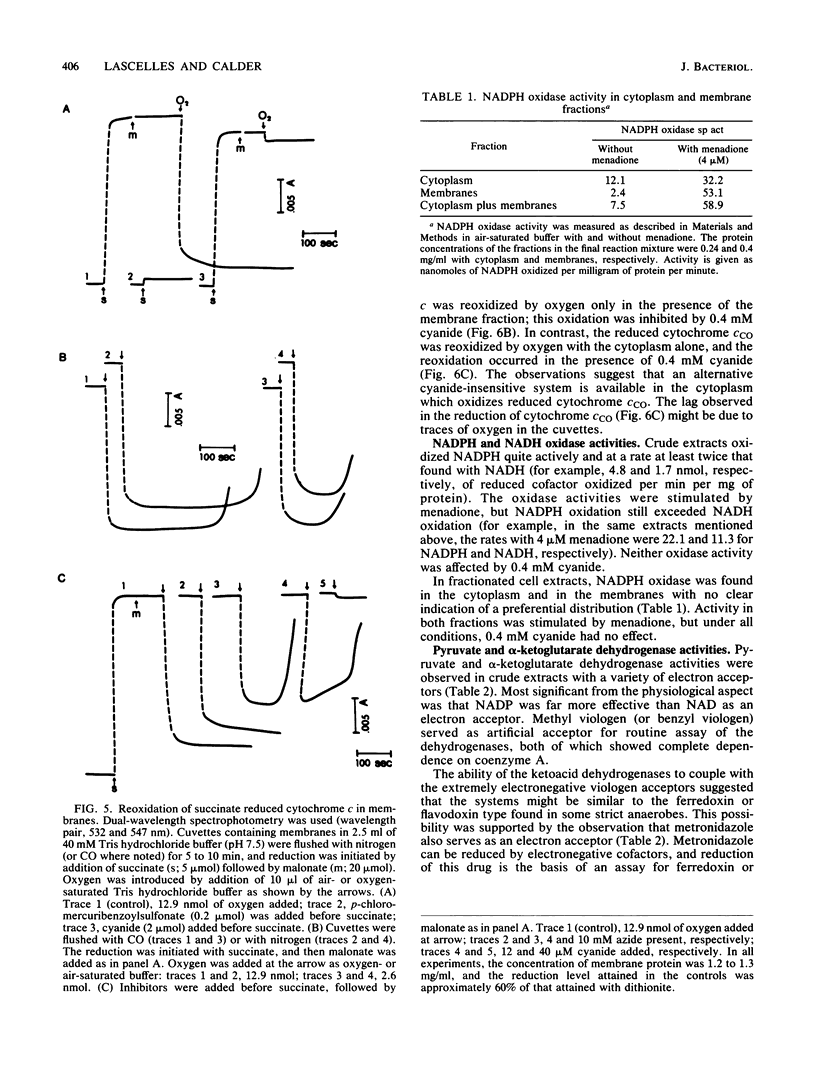
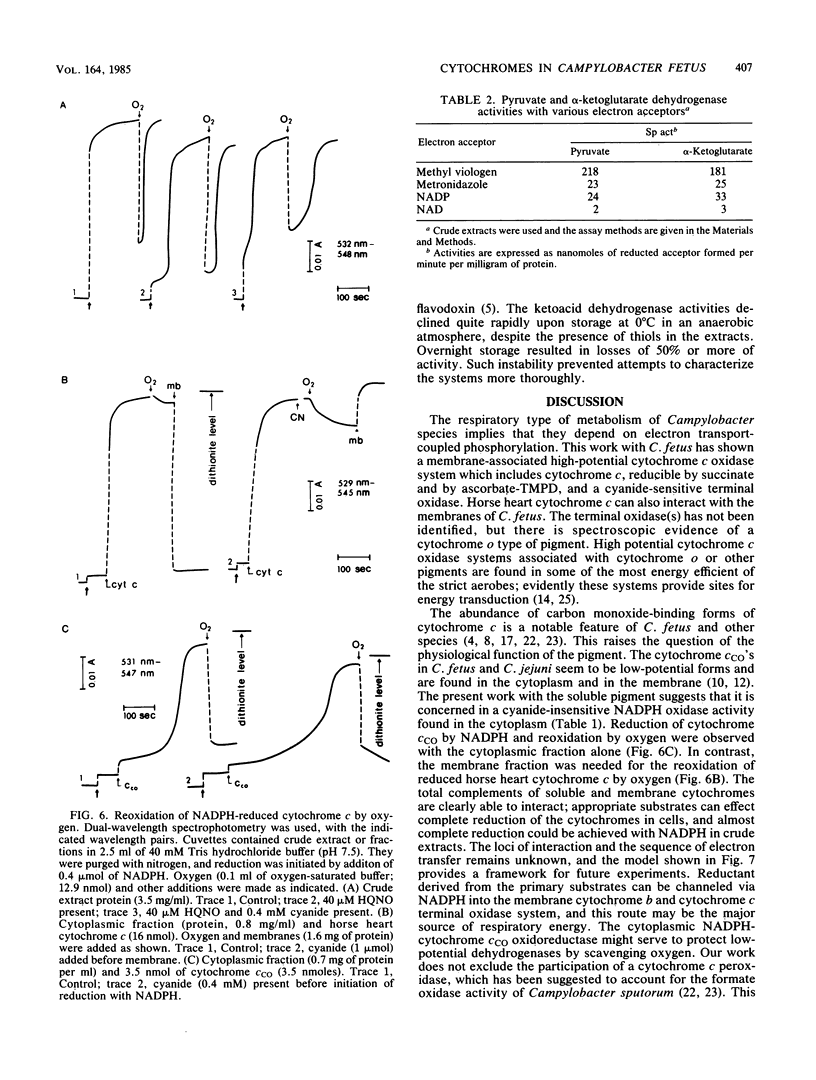
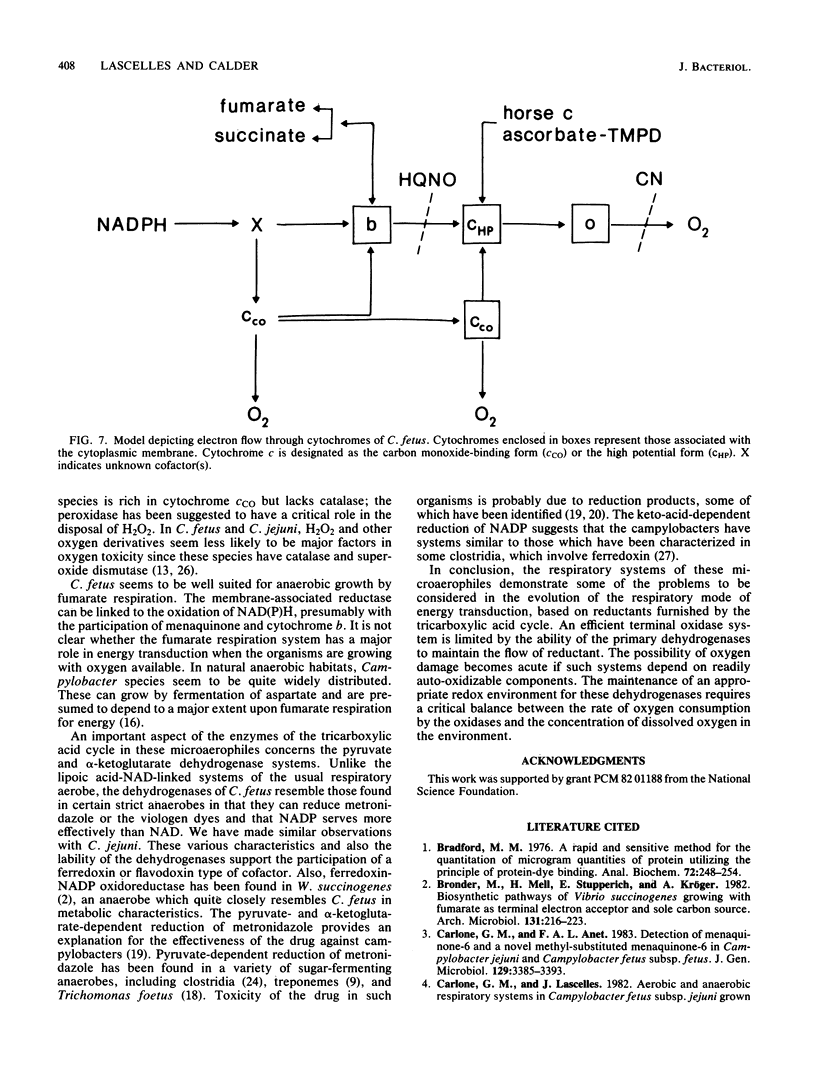
Campylobacter species are rich in c-type cytochromes, including forms which bind carbon monoxide. The role of the various forms of cytochromes in Campylobacter fetus has been examined in cell-free preparations by using physiological electron donor and acceptor systems. Under anaerobic conditions, NADPH reduced essentially all of the cytochrome c in crude cell extracts, whereas the reduction level with succinate was 50 to 60%. The carbon monoxide spectrum with NADPH was predominated by the cytochrome c complex; evidence of a cytochrome o type was seen in the succinate-reduced extracts and in membrane fractions. Succinate-reduced cytochrome c was oxidized by oxygen via a cyanide-sensitive, membrane-associated system. NADPH-reduced cytochrome c was oxidized by a cyanide-insensitive system. Partially purified carbon monoxide-binding cytochrome c, isolated from the cytoplasm, could serve as electron acceptor for NADPH-cytochrome c oxidoreductase; the reduced cytochrome was oxidized by oxygen by a cyanide-insensitive system present in the cytoplasmic fraction. Horse heart cytochrome c was also reducible by NADPH and by succinate; the reduced cytochrome was oxidized by a cyanide-sensitive system in the membrane fraction. NADPH and NADH oxidase activities were observed aerobically and under anaerobic conditions with fumarate. NADPH was more active than NADH. NADP was also more effective than NAD as an electron acceptor for the coenzyme A-dependent pyruvate and alpha-ketoglutarate dehydrogenase activities found in crude extracts. These dehydrogenases used methyl viologen and metronidazole as electron acceptors; they could be loci for oxygen inhibition of growth. It is proposed that energy provision via the high-potential cytochrome c oxidase system in the cytoplasmic membrane is limited by oxygen-sensitive primary dehydrogenases and that the carbon monoxide-binding cytochrome c may have a role as an oxygen scavenger.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronder M., Mell H., Stupperich E., Kröger A. Biosynthetic Pathways of Vibrio succinogenes growing with fumarate as terminal electron acceptor and sole carbon source. Arch Microbiol. 1982 May;131(3):216–223. doi: 10.1007/BF00405882. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Anet F. A. Detection of menaquinone-6 and a novel methyl-substituted menaquinone-6 in Campylobacter jejuni and Campylobacter fetus subsp. fetus. J Gen Microbiol. 1983 Nov;129(11):3385–3393. doi: 10.1099/00221287-129-11-3385. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Lascelles J. Aerobic and anaerobic respiratory systems in Campylobacter fetus subsp. jejuni grown in atmospheres containing hydrogen. J Bacteriol. 1982 Oct;152(1):306–314. doi: 10.1128/jb.152.1.306-314.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Blanchard D. K. A simple hydrogenase-linked assay for ferredoxin and flavodoxin. Anal Biochem. 1979 Feb;93(1):216–222. [PubMed] [Google Scholar]
- Collins M. D., Costas M., Owen R. J. Isoprenoid quinone composition of representatives of the genus Campylobacter. Arch Microbiol. 1984 Feb;137(2):168–170. doi: 10.1007/BF00414461. [DOI] [PubMed] [Google Scholar]
- Doyle M. P., Roman D. J. Response of Campylobacter jejuni to sodium chloride. Appl Environ Microbiol. 1982 Mar;43(3):561–565. doi: 10.1128/aem.43.3.561-565.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Smibert R. M. Pyruvate oxidation by the Reiter strain of Treponema phagedenis. J Bacteriol. 1982 Dec;152(3):1060–1065. doi: 10.1128/jb.152.3.1060-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Lascelles J. Respiratory systems and cytochromes in Campylobacter fetus subsp. intestinalis. J Bacteriol. 1980 Dec;144(3):917–922. doi: 10.1128/jb.144.3.917-922.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Goodman T. G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982 Apr;150(1):319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. I. Physiological aspects of enhanced aerotolerance. Can J Microbiol. 1979 Jan;25(1):1–7. doi: 10.1139/m79-001. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Edwards C. The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol. 1977 Oct 24;115(1):85–93. doi: 10.1007/BF00427850. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Lambers J. T., de Vos W. M., Veldkamp H. L-Aspartate fermentation by a free-living Campylobacter species. Arch Microbiol. 1978 Apr 27;117(1):109–114. doi: 10.1007/BF00689359. [DOI] [PubMed] [Google Scholar]
- Marczak R., Gorrell T. E., Müller M. Hydrogenosomal ferredoxin of the anaerobic protozoon, Tritrichomonas foetus. J Biol Chem. 1983 Oct 25;258(20):12427–12433. [PubMed] [Google Scholar]
- Molavi A., LeFrock J. L., Prince R. A. Metronidazole. Med Clin North Am. 1982 Jan;66(1):121–133. doi: 10.1016/s0025-7125(16)31446-8. [DOI] [PubMed] [Google Scholar]
- Moreno S. N., Mason R. P., Docampo R. Distinct reduction of nitrofurans and metronidazole to free radical metabolites by Tritrichomonas foetus hydrogenosomal and cytosolic enzymes. J Biol Chem. 1984 Jul 10;259(13):8252–8259. [PubMed] [Google Scholar]
- Moss C. W., Kai A., Lambert M. A., Patton C. Isoprenoid quinone content and cellular fatty acid composition of Campylobacter species. J Clin Microbiol. 1984 Jun;19(6):772–776. doi: 10.1128/jcm.19.6.772-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekus H. G., van Doorn E., Stouthamer A. H. Oxygen consumption by Campylobacter sputorum subspecies Bubulus with formate as substrate. Arch Microbiol. 1980 Sep;127(2):137–143. doi: 10.1007/BF00428017. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum. Arch Mikrobiol. 1972;84(3):225–233. doi: 10.1007/BF00425200. [DOI] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]