Abstract
Erythroid Krüppel-like factor (EKLF) is a red cell-specific transcriptional activator that is crucial for consolidating the switch to high levels of adult β-globin expression during erythroid ontogeny. EKLF is required for integrity of the chromatin structure at the β-like globin locus, and it interacts with a positive-acting factor in vivo. We find that EKLF is an acetylated transcription factor, and that it interacts in vivo with CBP, p300, and P/CAF. However, its interactions with these histone acetyltransferases are not equivalent, as CBP and p300, but not P/CAF, utilize EKLF as a substrate for in vitro acetylation within its trans-activation region. The functional effects of these interactions are that CBP and p300, but not P/CAF, enhance EKLF’s transcriptional activation of the β-globin promoter in erythroid cells. These results establish EKLF as a tissue-specific transcription factor that undergoes post-translational acetylation and suggest a mechanism by which EKLF is able to alter chromatin structure and induce β-globin expression within the β-like globin cluster.
Expression of the β-like globin cluster relies on the controlled interplay of cellular components at a number of levels during erythroid ontogeny (1–7). First, the appropriate combination of general and cell-specific DNA-binding factors directs transcription in a tissue- and developmental-specific fashion. Second, these transcriptional controls are placed within a chromatin context that itself exhibits tissue- and developmental-specific changes in structure. Structural analyses of the locus, genetic analyses of relevant hemoglobinopathies, the isolation of red cell-specific transcriptional players, and the ability to reproduce correct tissue and developmental regulation of the β-like globin cluster in transgenic mice have led to the identification of cis- and trans-acting components that act together to accomplish this feat. However, the molecular details of how such exquisite long-range regulation is attained are far from resolved, although recent studies of protein–protein interactions and the use of compound homozygous mice have provided some additional insights (8).
Erythroid Krüppel-like factor (EKLF) is a red cell-specific transcription factor that activates the β-globin promoter by means of its high-affinity binding to the CACCC element located at −90 (9). It contains three C2H2 zinc fingers that discriminate between CACCC elements present at erythroid promoters (10, 11). Its preferential binding to the adult β CACCC element over the elements at the murine and human embryonic (ɛ) or human fetal (γ) globin genes raised the possibility that EKLF may be involved in the developmental switch from embryonic/fetal to adult globin expression (12). Molecular and genetic analyses verified that EKLF is absolutely critical for the onset in expression of the adult β-globin gene, as homozygous EKLF-null mice die at the time of the switch to adult expression because of a profound β-thalassemia, and EKLF-null embryonic stem cells do not contribute to the red cell population in chimeric mice (12–15).
However, further analyses of EKLF-null mice, particularly after crossing with a mouse line that contained a single copy of the human β-like globin locus, revealed not only that human β-globin transcripts were absent from the mouse fetal liver but also that γ-globin transcripts were 5-fold higher and persisted beyond the level seen in the presence of EKLF (16, 17). Additionally, the absence of EKLF led to a complete lack of DNase-hypersensitive site formation at both the transgenic and endogenous β-globin promoters, and diminution of HS3 at the globin locus control region (LCR) (17). These data indicate that EKLF is a major player in activating adult β-globin expression, not only by its transcriptional activation properties but also by its ability to generate the proper chromatin configuration within part of the β-like globin locus and thus facilitate the silencing of the γ-globin promoter.
Part of these effects are likely because of the EKLF transactivation domain, which bears no homology to other proteins but is proline rich and is required for cell- and promoter-specific inducible expression (18). This domain is multipartite, containing a cis-acting inhibitory domain next to the zinc fingers, and a minimal activation domain that, by in vivo competition analyses, overlaps the EKLF trans-acting interaction domain (19). These studies revealed that EKLF interacts with a positive-acting cellular factor.
The molecular attributes of EKLF, in the context of its biological properties, led us to consider whether EKLF might be associating with molecules that alter chromatin structure (20). We focused on the particular class of coactivators that exhibit histone acetyltransferase (HAT) activity for a number of reasons. First, histone acetylation and transcriptional activation are intimately associated (21, 22). Second, the β-like globin locus is enriched in acetylated histones in erythroid cells (23). Third, HATs utilize multiple mechanisms to exert a range of effects that could account for some of the in vivo properties exhibited by EKLF. For example, they can serve as bridging molecules between activators and the basal transcription machinery (24, 25), their intrinsic HAT activity can disrupt the nucleosome structure and allow other DNA-binding proteins greater accessibility (26), and they can alter the molecular properties of at least one nonhistone transcription factor, p53 (27). Our results demonstrate that EKLF associates with HATs and that this interaction leads to protein acetylation in vitro and increased activity in vivo. EKLF thus becomes a tissue-specific example of the factor acetyltransferase (FAT) capability of p300 and CBP and their ability to modulate transcription factor function.
MATERIALS AND METHODS
Cell Culture.
K562 cells were grown in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS). COS7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS. All cells were cultured in a humidified incubator at 37°C in a 5% CO2/95% air atmosphere.
Antibodies.
Anti-CBP rabbit polyclonal antibody raised against amino acids 1736–2179 was purchased from Upstate Biotechnology. Anti-hemagglutinin (HA) monoclonal antibody was purchased from the Hybridoma Core Facility at Mt. Sinai School of Medicine. Purified anti-P/CAF rabbit polyclonal antibody was a kind gift of Yoshihiro Nakatani (28). Anti-FLAG M5 monoclonal antibody was from Kodak-IBI. 4B9 and 6B3 are two mouse monoclonal antibodies against EKLF made in this laboratory. We used a rabbit anti-peptide polyclonal antibody (29) or the 4B9 antibody for Western blot analysis and the 6B3 antibody for immunoprecipitation of EKLF.
Plasmid Constructions.
pSG5/EKLF (9), pCX/FLAG-P/CAF (28), pCMVβ-HA-P300 (30), pRc/RSV/CBP (31), and pHS2/β/luc (32) were as described. The pCX empty vector was generated from EcoRI/KpnI-digested pCX/FLAG-P/CAF. pSG5/CBP was generated by insertion of the BamHI fragment of pRc/RSV/CBP into pSG5. GST-EKLF constructs (GST, glutathione S-transferase) were as described (9, 18) except as follows. GST-EKLF(20–376) was generated by PCR of pSG5/EKLF using 5′-GGCCGGATCCATGGCCTCAGCTGAGACT-3′ and 5′-CGCGGAATTCTCACTCAGAGGTGACGCTTC-3′ as primers, digestion with BamHI and EcoRI, and insertion into pGEX-2TK. GST-EKLF(20–79) was made by digestion of GST-EKLF(20–376) with BspEI and EcoRI and ligation of the filled-in ends. GST-EKLF(Δ172–272) was generated by digestion of GST-EKLF(20–376) with SmaI, removal of the insert, and self-ligation. GST-EKLF(272–376) was constructed by digestion of GST-EKLF(20–376) with SmaI followed by EcoRI digestion; a 300-bp fragment of EKLF was isolated and inserted into SmaI and EcoRI sites of pGEX-2TK vector. GST-EKLF(20–124) was constructed by insertion of the BamHI piece from pGAL/EKLF(20–124) (19) into pGEX-3X.
Transfection, Immunoprecipitation, and Western Blot Analysis.
For immunoprecipitation (IP), 60% confluent COS7 cells were transfected with 10 μg of pSG5-EKLF, pCX-pCAF, pRc/RSV/CBP, pCMVβ/p300, or a combination of 10 μg of pSG5-EKLF plus either 10 μg of pCX-pCAF, pRc/RSV/CBP, or pCMVβ/p300 by using DMRIE C (GIBCO/BRL). Thirty-six hours after transfection, cells were washed twice with cold PBS, lysed with LSB (10 mM Hepes, pH 7.6/250 mM NaCl/5 mM EDTA/0.1% Nonidet P-40) supplemented with protease inhibitors, and incubated on ice for 20 min. Extracts were cleared by Microfuge centrifugation at 18,000 rpm for 20 minutes at 4°C. Supernatants were subjected to immunoprecipitation with the specific antibodies by rocking for 1 hr at 4°C followed by addition of 30 μl of a 50% slurry of protein A or G agarose beads (Sigma) and further incubation for 1 hr. For anti-CBP, anti-HA, anti-FLAG M5, and anti-pCAF antibodies, protein A agarose beads were used; for 6B3 anti-EKLF antibody, protein G agarose beads were used. Precipitated complexes were pelleted, washed four times with LSB, and resolved by electrophoresis on an SDS/10% polyacrylamide gel. Proteins were electrotransferred onto nitrocellulose membrane (Protran, Schleicher & Schuell) and probed with the appropriate antibodies. Chemiluminescent detection (Pierce) relied on horseradish peroxidase (HRP)-conjugated secondary antibodies.
IP-HAT Assay.
After transfection and IP as above, an IP-HAT assay was conducted as described (33) with slight modification. Briefly, a series of GST and GST-EKLF fusion proteins were purified with glutathione beads from BL21 expression bacterial lysates after a 2-hr induction with 1 mM isopropyl β-d-thiogalactoside (IPTG). Five micrograms of purified GST-EKLF fusions was added to each tube of IP complex together with 1 μl of 0.25 mCi/ml [3H]acetyl-CoA (5.1 Ci/mmol; Amersham; 1 Ci = 37 GBq). Mixtures were incubated at 30°C for 1 hr and electrophoresed in an SDS/10% polyacrylamide gel. The gel was then fixed in 30% methanol/10% acetic acid/60% water (vol/vol) for 40 min, immersed into EN3HANCE solution (DuPont) for 45 min, and precipitated for another 30 min in water. The gel was then dried and exposed to x-ray film for 3 days at −80°C. The same samples were also electrophoresed on a separate SDS/10% polyacrylamide gel and stained.
In Vivo Labeling of EKLF.
Ten micrograms of pSG5/EKLF or pSG5 was transfected as described above into one 10-cm dish of COS7 cells per test. After 30 hr, the DMEM was replaced with DMEM containing 1 mCi/ml sodium [3H]acetate (ICN) and 2 μM trichostatin A (TSA; Wako) for 1 hr. Extracts were prepared and processed as described above, using the 6B3 monoclonal antibody. One group of transfections were labeled, while a parallel group was analyzed by Western blotting.
Cotransfections and Luciferase and Growth Hormone Assays.
K562 cells were transfected with desired plasmids by using DMRIE C (GIBCO/BRL). In short, 2 × 105 cells were added to a 1-ml mixture of DMRIE C reagent and DNA in 35-mm dishes. After 5 hr, 2 ml of RPMI medium 1640 containing 20% FBS was added into the dish. TSA (2 μM) was added as appropriate. All transfections were normalized by cotransfecting pXGH5 growth hormone plasmid as an internal control. Thirty-six hours after transfection, cells were harvested for the luciferase assay (Promega kit), using equivalent amounts of extracted protein; media supernatants were used for the growth hormone assay (Nichols Institute). Luciferase levels are plotted after normalization to cotransfected growth hormone levels. All experiments were repeated three times in duplicate.
RESULTS
EKLF Associates with CBP, p300, and P/CAF in Vivo.
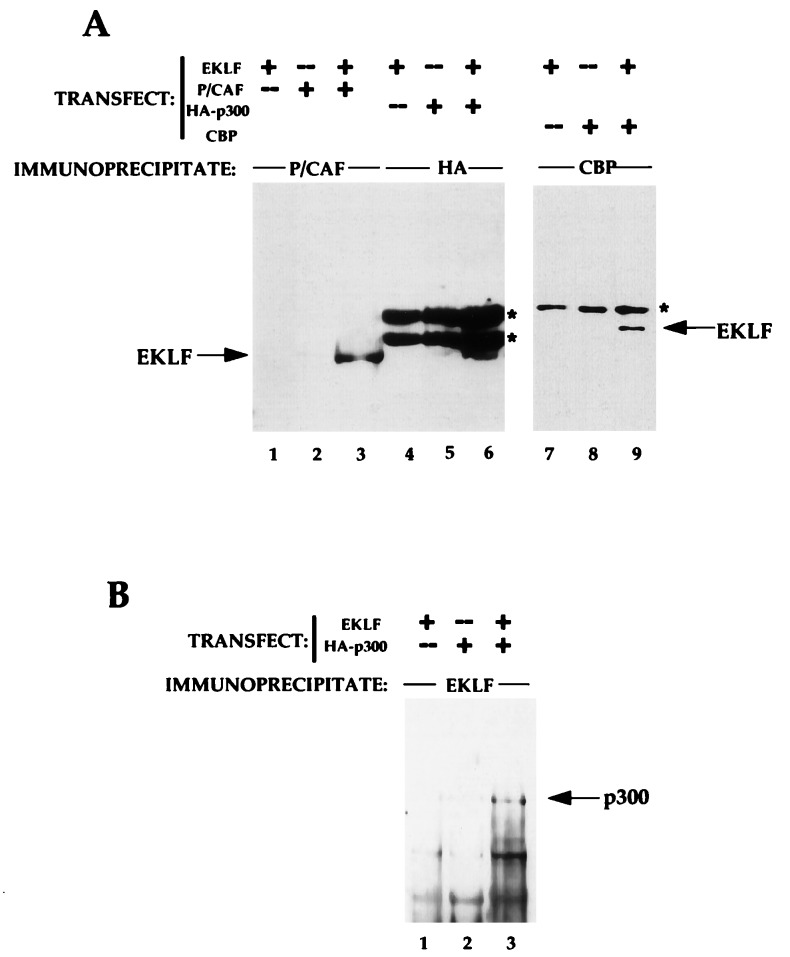
We began our studies by determining whether EKLF could interact in vivo with three proteins that contain HAT activity: CBP, p300, and P/CAF. Constructs that drive expression of each of these (pRSV-CBP, pCMVβ/HA-p300, or pCX/FLAG-P/CAF) were cotransfected with the EKLF expression vector (pSG5-EKLF) into COS7 cells. After 36 hr, cell extracts were prepared and immunoprecipitated with anti-CBP, anti-HA (for p300), or anti-P/CAF antibodies, and the material was monitored for the presence of EKLF after electrophoresis, transfer, and Western blot analysis with the anti-EKLF (4B9) antibody. As shown in Fig. 1A, antibodies to each of the HAT proteins are able to coprecipitate EKLF from extracts (lanes 3, 6, 9). This result requires cotransfection of HAT protein and EKLF (lanes 1, 2, 4, 5, 7, 8). This interaction was confirmed by the reverse experiment, i.e., immunoprecipitation with anti-EKLF (6B3) antibody followed by blotting with anti-HA also reveals the p300/EKLF interaction (Fig. 1B). We conclude that CBP, p300, and P/CAF can all interact with EKLF in vivo.
Figure 1.
Tests of EKLF interaction with CBP, p300, and P/CAF in vivo. Combinations of EKLF and HAT coactivator expression plasmids (10 μg each) as indicated were transfected into COS7 cells and whole cell extracts were subjected to immunoprecipitation with anti-CBP, anti-HA, anti-P/CAF, or anti-EKLF antibodies. (A) Immunoprecipitated complexes were resolved, blotted, and probed with anti-EKLF monoclonal antibody 4B9. Location of co-electrophoresed EKLF in each gel is shown. Asterisks indicate nonspecific (Ig heavy chain) signals from the immunoprecipitating antibodies. (B) Immunoprecipitated complexes were resolved, blotted, and probed with anti-HA monoclonal antibody for detection of p300. Location of co-electrophoresed HA-p300 is shown.
CBP and p300, but Not P/CAF, Acetylate EKLF in Vitro.
Although CBP and p300 were originally isolated as co-activators that act as bridging proteins between upstream activators and the basal transcription apparatus (34), the recent demonstration of their FAT activity on p53 (27) and TFIIEβ and TFIIF (35) prompted us to determine if EKLF is a substrate for acetylation by CBP, p300, or P/CAF. We performed these experiments in vitro by using purified recombinant GST-EKLF fusion proteins as substrates for immunoprecipitated HAT molecules derived from transfected COS7 cells. Fig. 2A demonstrates that CBP and p300 are both able to acetylate a GST-EKLF(76–376) construct in vitro (lanes 1, 2, 4, 5); however, P/CAF is not able to do so (lanes 6, 7). Autoacetylated CBP, p300, and P/CAF bands indicate that the HAT molecule in each immunoprecipitated complex is enzymatically active. In addition, IP of nontransfected COS7 cells did not yield any acetylated EKLF (lanes 3, 8), and EKLF did not autoacetylate (lane 9). These data demonstrate that EKLF is specifically acetylated only by p300 and CBP, and not by P/CAF.
Figure 2.
Tests of EKLF acetylation by CBP, p300, and P/CAF in vitro. (A) COS7 cells were transfected and extracts were immunoprecipitated with the indicated antibodies. IP-HAT assays utilized 5 μg of GST-EKLF(76–376). Samples were electrophoresed and the dried gel was exposed in autoradiography (Upper). GST-EKLF(76–376) alone was tested for autoacetylation in lane 9. Locations of p300/CBP, P/CAF, and GST-EKLF are indicated. Protein samples were also stained to show that equivalent amounts were used (Lower). (B) Endogenous CBP from COS7 cells was immunoprecipitated, and IP-HAT assays were performed with 5 μg of various GST-EKLF fusion proteins as diagrammed on the Right, which also shows the locations and sequences of lysines conserved between mouse and human EKLF [amino acid residues 47, 74, 177, 279, 288; mouse numbering is based on initiator methionine being residue 19 (9)]. Proteins were resolved and subjected to autoradiography (Upper Left) or stained for protein (Lower Left). Asterisks show the location of nondegraded GST-EKLF fusion proteins. Molecular mass markers (on the left) are 70, 55, 33, 25, and 15 kDa (top to bottom).
We used the robust signal generated by CBP acetylation in vitro to map potential target sites within EKLF. A series of GST fusion proteins harboring different EKLF deletions were used as substrates (Fig. 2B). The EKLF trans-activation region contains five lysines that are conserved in the human and the murine orthologues (9, 36). Analysis of the substrate suitability of the EKLF fragments (Fig. 2B) indicates that Lys-47 and Lys-71 are not used, but that Lys-279 and Lys-288 are likely target sites. Both of these are located within the EKLF inhibitory domain immediately on the amino side of the zinc fingers.
EKLF Is an Acetylated Protein in Vivo.
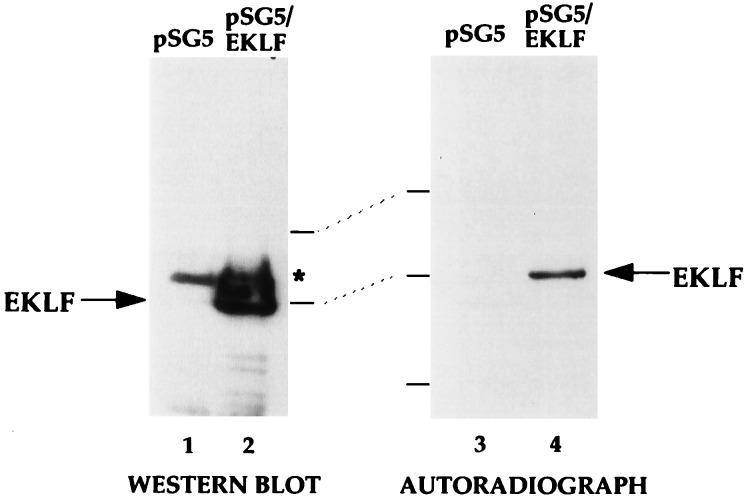
We next examined whether EKLF is acetylated in vivo by immunoprecipitating EKLF from transfected cells that had been labeled for 1 hr with sodium [3H]acetate in the presence of a histone deacetylase inhibitor (TSA) prior to lysis. Western blot analysis (Fig. 3, lanes 1 and 2) of a portion of the immunoprecipitated samples indicates that EKLF is recovered from EKLF-transfected but not empty vector-transfected cells, and autoradiography (Fig. 3, lanes 3 and 4) indicates that the immunoprecipitated EKLF is the only labeled protein in either extract. These data directly demonstrate that EKLF is an acetylated transcription factor in vivo.
Figure 3.
Status of EKLF acetylation in vivo. COS7 cells transfected with 10 μg of pSG5 or pSG5/EKLF as indicated were labeled with sodium [3H]acetate in the presence of TSA and extracts were immunoprecipitated with anti-EKLF monoclonal antibody 6B3. Immunoprecipitated samples were resolved on SDS/PAGE and blotted and probed with anti-EKLF (Left) or processed and exposed in autoradiography (Right). Asterisk indicates a nonspecific signal from the immunoprecipitating antibody. Locations of molecular mass markers (70, 50, and 33 kDa, top to bottom) and EKLF are shown.
CBP and p300, but Not P/CAF, Enhance EKLF Trans-activation in Erythroid Cells.
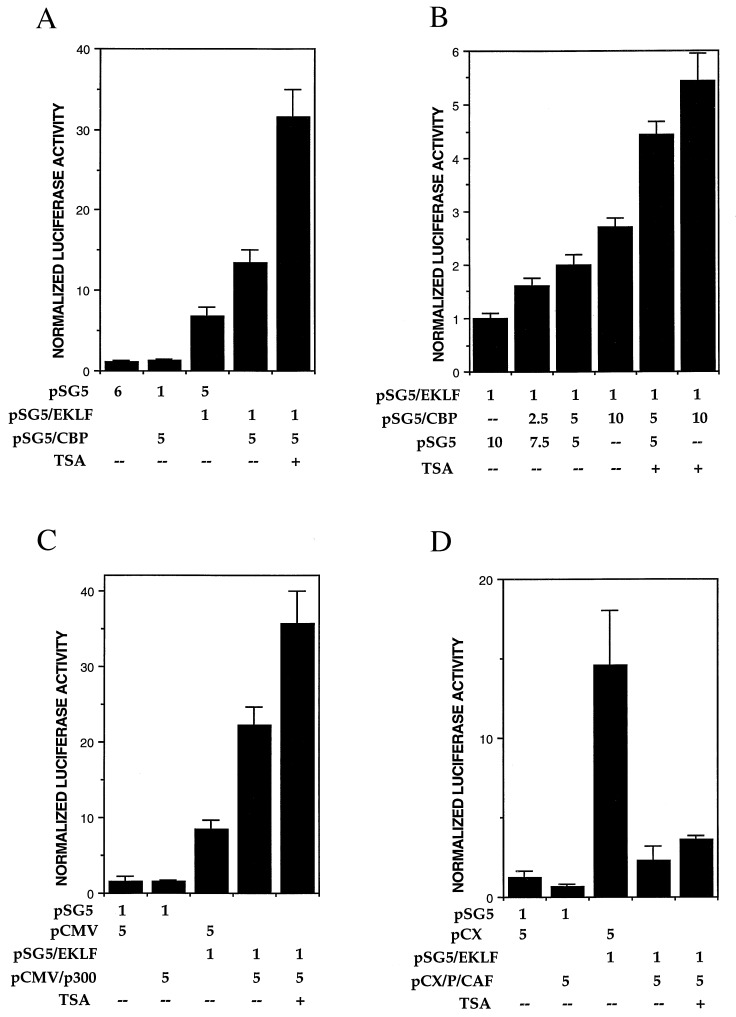
To investigate the functional consequence of physical interaction and acetylation of EKLF by HAT molecules, we tested the effect of HAT proteins on the ability of EKLF to transactivate the β-globin promoter, which is its normal activation target (9). For this experiment we used K562, which is a human erythroleukemic cell line that does not express EKLF (36). K562 cells express the fetal γ-globin gene but not the endogenous or a transfected adult β-globin gene. However, cotransfection of EKLF with a β-globin gene reporter construct switches on its expression (12). We reasoned that use of the natural β-globin promoter in an erythroid cell would provide a stringent test for any effect of HAT proteins upon EKLF trans-activation. Cotransfection of pSG5-EKLF and the pHS2/β/luc reporter were performed with pSG5-CBP, pCMVβ/HA-p300, pCX/FLAG-P/CAF, or their corresponding empty vectors. The results (Fig. 4) indicate that EKLF activates the β-globin reporter as expected, and that CBP and p300 can further boost this high level of activity an additional 2- to 3-fold in a dose-dependent fashion (Fig. 4 A–C). However, P/CAF significantly inhibits EKLF trans-activation by 6-fold (Fig. 4D). Trans-activation in the presence of TSA can further potentiate the effects of CBP and p300, but not P/CAF, activation. These data suggest that the functional consequence of interaction between EKLF and CBP or p300 is to positively affect its transactivational ability, maybe in part as a result of EKLF acetylation.
Figure 4.
Analyses of EKLF transactivation of the β-globin promoter. K562 erythroleukemic cells were transfected with 1 μg of HS2/β/luc reporter and the indicated amounts (μg) of expression or vector plasmids, and extracts were processed for luciferase activity. TSA was added where indicated to a final concentration of 2 μM at the time of transfection. Multiple experiments were averaged after normalization of luciferase activity to cotransfected growth hormone levels. The effects of CBP (A), p300 (C), and P/CAF (D) are shown along with a dose–response test of CBP (B).
DISCUSSION
EKLF is an erythroid transcription factor that plays direct or indirect roles in activation of β-globin transcription (12–14), consolidation of the switch from γ- to β-globin (16, 17), and generation of an intact chromatin structure at the β-like globin locus (17). As EKLF interacts with other proteins in vivo (19), we investigated whether HATs might play a role in helping coordinate EKLF’s diverse effects on expression.
Specificity of EKLF–HAT Interaction.
There are two levels of target specificity by HATs upon EKLF. First, not all potential lysines are acetylated in EKLF. Second, not all HATs utilize EKLF as substrate. The inability of P/CAF to acetylate EKLF is not due to limitation of its substrate specificity to histones, as it can acetylate itself, TFIIEβ, and TFIIF (35). Although P/CAF was originally isolated by its interaction with CBP (28), these two molecules do not always play the same role together, for example during muscle cell differentiation (37) and in RAR-, STAT1-, or CREB-dependent transcription (38). In the present case, the ability of P/CAF to interact with, yet not acetylate, EKLF is not a passive event, but instead leads to inhibition of EKLF trans-activation. This may be functionally significant, as P/CAF levels are altered in terminally differentiating tissues (28, 37). An alternative explanation is that a high level of P/CAF expression simply interferes with EKLF’s interaction with CBP or p300. P/CAF interacts with CBP within the same region as E1A (28), and such interference would imply that EKLF interacts there also.
Although we have not directly investigated which regions of EKLF associate with p300/CBP, the interaction must be complex, as GST constructs that contain only the trans-activation region (amino acids 20–291) or primarily the zinc finger region (amino acids 287–376) are each suitable substrates for acetylation in vitro.
The putative acetylated lysines 279 and 288 map to the EKLF inhibitory domain, which acts in cis and is the highly basic portion of the trans-activation region (19). These lysines also overlap the nuclear localization signal that is common to EKLF, gut-enriched Krüppel-like factor (GKLF), and lung Krüppel-like factor (LKLF) (39). As a consequence, not only is the distance between these two lysines constant in this related family, but the locations of flanking arginines (amino acid residues 280, 282, 283, 289) are also conserved. Intriguingly, the distance between acetylated lysines in p53 (K373 and K382) is identical to that of EKLF, and this region also contains a conserved arginine residue (27). Although we have not observed any effect of acetylation on EKLF DNA binding in vitro by gel-shift analysis (unpublished observations), such effects may be subtle and not readily apparent when using GST-EKLF fusions. Whether acetylation affects nuclear translocation awaits analysis, as these two processes have not been linked previously.
It is now clear that EKLF undergoes two types of post-translational modifications: phosphorylation and acetylation. Phosphorylation of Thr-41 within the activation domain is critical for EKLF trans-activation in vivo (40). Unlike the inhibitory domain, the minimal activation domain acts in trans, is acidic, and is located at the amino terminus of the EKLF transactivation region (19). Our present studies show that EKLF activity may also be augmented by acetylation. Altered levels of EKLF phosphorylation and acetylation may provide two distinct yet overlapping forms of cellular control over its activity during erythroid ontogeny. If these types of control mechanisms are primarily operative in definitive cells, this may explain how EKLF is required for completion of the definitive erythroid program, even though it is also expressed in primitive erythroid cells (29) and in multipotent progenitors (41, 42).
Biological Implications of EKLF Acetylation.
HATs have been implicated in maintenance of the open chromatin configuration seen at the β-globin locus, as acetylated histones are preferentially distributed throughout the region in erythroid cells (23). In addition, treatment of erythroid cells with butyrate (43) or TSA (44) alters the expression profile of the globin genes within the locus. The present experiments reveal that HATs may exert a second effect on expression within the locus by their ability to acetylate critical erythroid-specific transcription factors such as EKLF and affect their function. Consistent with this idea, TSA acts as an inducer of globin synthesis in MEL cells (45), and conversely, interference (by E1A repression) of CBP activity in MEL cells prevents induction of globin expression (46).
Our present experiments utilized transient transfection to test for functional effects of HATs on EKLF activity. Although the actual chromatin status of these templates is not known, it is likely to be a loose and disorganized structure (47). In this sense, one may speculate that acetylation of histones is not the dominant cause of EKLF superactivity in transient assays, and that the effect of p300 and CBP on EKLF activity arises from their function as bridging factors and/or as FATs. However, CBP/p300 may also be affecting other parts of the transcriptional process, including bridging with and/or modification of other target proteins important for full activity of the β-globin promoter.
Although the functional effects of TFIIEβ and TFIIH modification are not known, acetylation of p53 alters its conformation, increasing its DNA-binding affinity (27). Acetylation of histones decreases the affinity of their amino-terminal tails for DNA, altering chromatin structure (reviewed in ref. 21). By analogy, EKLF acetylation would be predicted to affect its protein–protein or protein–DNA interactions as a result of neutralizing positively charged lysines. Given that EKLF is acetylated and that its transcriptional activity is stimulated by CBP/p300, it is more likely that intra- or intermolecular protein interactions, rather than those with DNA, are affected. This may result in a cascade of interactions that would explain the requirement of EKLF for formation of DNase-sensitive sites at the adult β-globin promoter and at HS3 (17). For example, EKLF may recruit CBP/p300 to the β-globin promoter, resulting in modification of the local core histones and of EKLF, which may then be further able to recruit a chromatin remodeling complex that decondenses the area and renders it transcriptionally competent. In support of such an idea, the ability of EKLF to activate in vitro transcription of a chromatin-repressed β-globin promoter requires a SWI/SNF-like remodeling complex (J. Armstrong, J.J.B., and B. Emerson, unpublished results). Indeed, the histone acetylase ADA/GCN5 and the chromatin remodeling SWI/SNF complex are thought to act in concert to modify chromatin structure at specific target genes in yeast (48). In addition, CBP/p300 interacts with BRG1, a human homolog of SWI2 (49). Such a coordinate acetylation/remodeling mechanism has been postulated to be involved in the long-distance regulation of the β-like globin cluster (50). Clearly, future experiments will be directed at resolving whether and how EKLF acts as an integrator of these various signals at the adult β-globin locus.
Acknowledgments
We thank Drs. Xiaoyong Chen and Checco Ramirez for comments on the manuscript, Dr. Tom Moran for help in raising anti-EKLF monoclonal antibodies, and Drs. Liaohan Ouyang, Yoshihiro Nakatani, Richard Goodman, Ronald Kwok, David Livingston, and Tim Townes for reagents. This work was supported by Public Health Service Grant DK46865 to J.J.B., who is a Scholar of the Leukemia Society of America. This is manuscript 267 from the Brookdale Center for Molecular Biology at the Mount Sinai School of Medicine.
ABBREVIATIONS
- EKLF
erythroid Krüppel-like factor
- CBP
CREB-binding protein
- P/CAF
p300/CBP-associated factor
- HAT
histone acetyltransferase
- FAT
factor acetyltransferase
- TSA
trichostatin A
- HA
hemagglutinin
- GST
glutathione S-transferase
- IP
immunoprecipitation
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Townes T M, Behringer R R. Trends Genet. 1990;6:219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- 2.Felsenfeld G. Nature (London) 1992;355:219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- 3.Engel J D. Trends Genet. 1993;9:304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- 4.Orkin S H. Eur J Biochem. 1995;231:271–281. doi: 10.1111/j.1432-1033.1995.tb20697.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin D I K, Fiering S, Groudine M. Curr Opin Genet Dev. 1996;6:488–495. doi: 10.1016/s0959-437x(96)80072-4. [DOI] [PubMed] [Google Scholar]
- 6.Baron M H. Biochim Biophys Acta. 1997;1351:51–72. doi: 10.1016/s0167-4781(96)00195-9. [DOI] [PubMed] [Google Scholar]
- 7.Fraser P, Gribnau J, Trimborn T. Curr Opin Hematol. 1998;5:139–144. doi: 10.1097/00062752-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bieker J J. Curr Opin Hematol. 1998;5:145–150. doi: 10.1097/00062752-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Miller I J, Bieker J J. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieker J J. In: Molecular Biology of Hemoglobin Switching. Stamatoyannopoulos G, editor. Vol. 1. Andover, U.K.: Intercept; 1994. pp. 231–241. [Google Scholar]
- 11.Feng W C, Southwood C M, Bieker J J. J Biol Chem. 1994;269:1493–1500. [PubMed] [Google Scholar]
- 12.Donze D, Townes T M, Bieker J J. J Biol Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 13.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Nature (London) 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 14.Perkins A C, Sharpe A H, Orkin S H. Nature (London) 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 15.Lim S K, Bieker J J, Lin C S, Costantini F. Blood. 1997;90:1291–1299. [PubMed] [Google Scholar]
- 16.Perkins A C, Gaensler K M L, Orkin S H. Proc Natl Acad Sci USA. 1996;93:12267–12271. doi: 10.1073/pnas.93.22.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 18.Bieker J J, Southwood C M. Mol Cell Biol. 1995;15:852–860. doi: 10.1128/mcb.15.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Bieker J J. EMBO J. 1996;15:5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 21.Wade P A, Pruss D, Wolffe A P. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 22.Struhl K. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 23.Hebbes T R, Clayton A L, Thorne A W, Crane-Robinson C. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Nature (London) 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 25.Janknecht R, Hunter T. Nature (London) 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 26.Wolffe A P, Pruss D. Cell. 1996;84:817–819. doi: 10.1016/s0092-8674(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 27.Gu W, Roeder R G. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 28.Yang X J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. Nature (London) 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 29.Southwood C M, Downs K M, Bieker J J. Dev Dyn. 1996;206:248–259. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 31.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Nature (London) 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 32.Caterina J J, Ciavatta D J, Donze D, Behringer R R, Townes T M. Nucleic Acids Res. 1994;22:1006–1011. doi: 10.1093/nar/22.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bannister A J, Kouzarides T. Nature (London) 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 34.Shikama N, Lyon J, LaThangue N B. Trends Cell Biol. 1997;7:230–237. [Google Scholar]
- 35.Imhof A, Yang X J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 36.Bieker J J. DNA Cell Biol. 1996;15:347–352. doi: 10.1089/dna.1996.15.347. [DOI] [PubMed] [Google Scholar]
- 37.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y J, Graessmann A, Nakatani Y, Levrero M. Molecular Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 38.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 39.Shields J M, Yang V W. J Biol Chem. 1997;272:18504–18507. doi: 10.1074/jbc.272.29.18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang, L., Chen, X. & Bieker, J. J. (1998) J. Biol. Chem.273, in press. [DOI] [PubMed]
- 41.Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 42.Reese T T, Gregory R C, Sharlow E R, Pacifici R E, Crouse J A, Todokoro K, Wojchowski D M. Growth Factors. 1997;14:161–176. doi: 10.3109/08977199709021518. [DOI] [PubMed] [Google Scholar]
- 43.Stamatoyannopoulos G, Nienhuis A W. Annu Rev Med. 1992;43:497–521. doi: 10.1146/annurev.me.43.020192.002433. [DOI] [PubMed] [Google Scholar]
- 44.McCaffrey P G, Newsome D A, Fibach E, Yoshida M, Su M S S. Blood. 1997;90:2075–2083. [PubMed] [Google Scholar]
- 45.Yoshida M, Nomura S, Beppu T. Cancer Res. 1987;47:3688–3691. [PubMed] [Google Scholar]
- 46.Blobel G A, Nakajima T, Eckner R, Montminy M, Orkin S H. Proc Natl Acad Sci USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith C L, Hager G L. J Biol Chem. 1997;272:27493–27496. doi: 10.1074/jbc.272.44.27493. [DOI] [PubMed] [Google Scholar]
- 48.Pollard K J, Peterson C L. Mol Cell Biol. 1997;17:6212–6222. doi: 10.1128/mcb.17.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dallas P B, Yaciuk P, Moran E. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bresnick E H, Versaw W K, Lam L T, Forsberg E C, Eisenman H C. In: Gene Therapy and Molecular Biology: From Basic Mechanisms to Clinical Applications. Boulikas T, editor. Vol. 1. Palo Alto, CA: Gene Therapy Press; 1997. pp. 483–494. [Google Scholar]