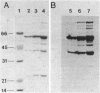
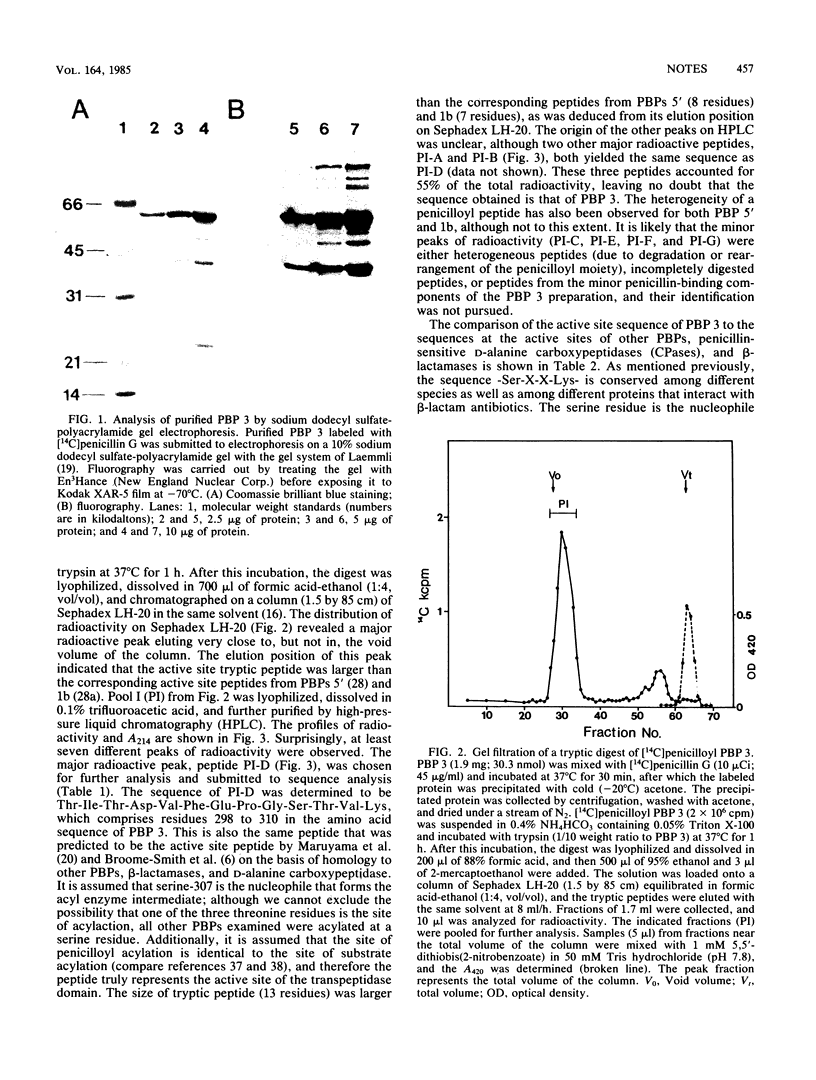
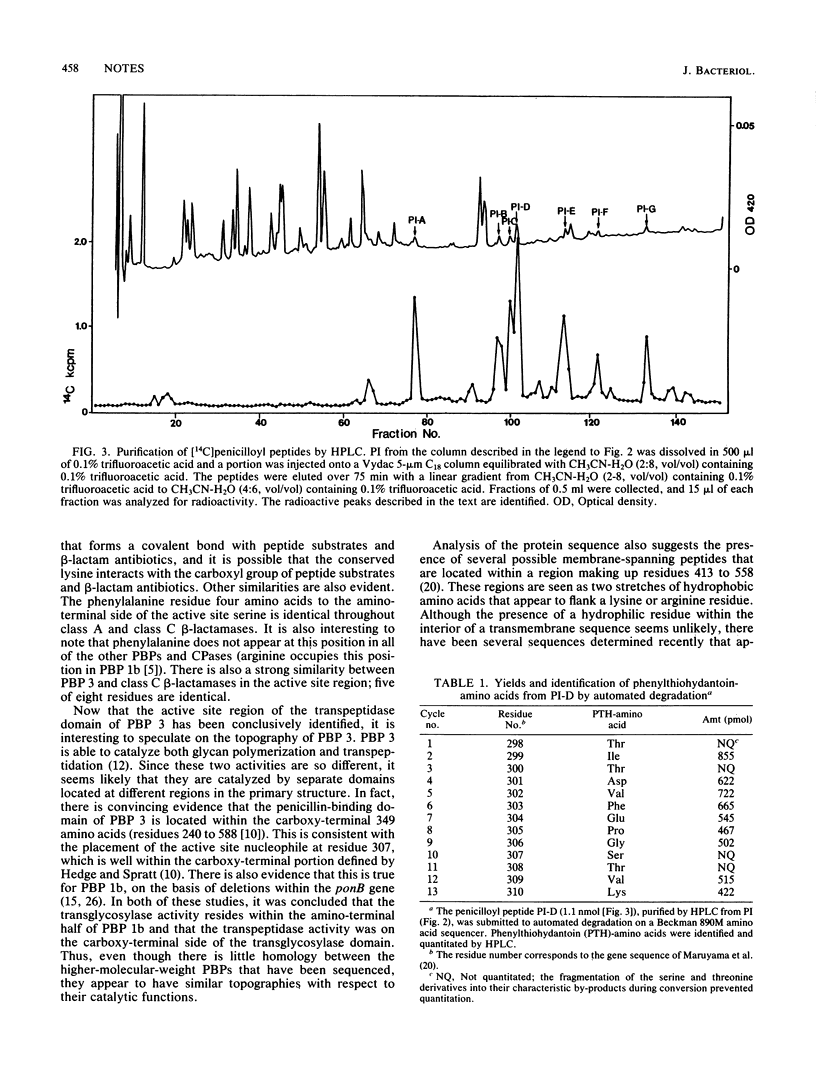
Abstract
We report the sequence of the active site tryptic peptide of penicillin-binding protein 3 from Escherichia coli. Purified penicillin-binding protein 3 was labeled with [14C]penicillin G and digested with trypsin, and the resulting radioactive peptides were isolated by a combination of gel filtration and high-pressure liquid chromatography. The major radioactive peak from high-pressure liquid chromatography was sequenced, and the peptide Thr-Ile-Thr-Asp-Val-Phe-Glu-Pro-Gly-Ser-Thr-Val-Lys, which comprises residues 298 to 310 in the amino acid sequence, was identified. This sequence is compared with the active site sequences from other penicillin-binding proteins and beta-lactamases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Strominger J. L. Purification and properties of penicillin-binding proteins 5 and 6 from Escherichia coli membranes. J Biol Chem. 1980 Dec 10;255(23):11173–11180. [PubMed] [Google Scholar]
- Amanuma H., Strominger J. L. Purification and properties of penicillin-binding proteins 5 and 6 from the dacA mutant strain of Escherichia coli (JE 11191). J Biol Chem. 1984 Jan 25;259(2):1294–1298. [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Edelman A., Yousif S., Spratt B. G. The nucleotide sequences of the ponA and ponB genes encoding penicillin-binding protein 1A and 1B of Escherichia coli K12. Eur J Biochem. 1985 Mar 1;147(2):437–446. doi: 10.1111/j.1432-1033.1985.tb08768.x. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Hedge P. J., Spratt B. G. Production of thiol-penicillin-binding protein 3 of Escherichia coli using a two primer method of site-directed mutagenesis. EMBO J. 1985 Jan;4(1):231–235. doi: 10.1002/j.1460-2075.1985.tb02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Hedge P. J., Spratt B. G. A gene fusion that localises the penicillin-binding domain of penicillin-binding protein 3 of Escherichia coli. FEBS Lett. 1984 Oct 15;176(1):179–184. doi: 10.1016/0014-5793(84)80936-9. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Ishino F., Mitsui K., Tamaki S., Matsuhashi M. Dual enzyme activities of cell wall peptidoglycan synthesis, peptidoglycan transglycosylase and penicillin-sensitive transpeptidase, in purified preparations of Escherichia coli penicillin-binding protein 1A. Biochem Biophys Res Commun. 1980 Nov 17;97(1):287–293. doi: 10.1016/s0006-291x(80)80166-5. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Overlapping of the coding regions for alpha and gamma components of penicillin-binding protein 1 b in Escherichia coli. Mol Gen Genet. 1984;196(3):449–457. doi: 10.1007/BF00436192. [DOI] [PubMed] [Google Scholar]
- Keck W., Glauner B., Schwarz U., Broome-Smith J. K., Spratt B. G. Sequences of the active-site peptides of three of the high-Mr penicillin-binding proteins of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1999–2003. doi: 10.1073/pnas.82.7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Jayatilake G. S., Waley S. G., Jaurin B., Grundström T. Active sites of beta-lactamases. The chromosomal beta-lactamases of Pseudomonas aeruginosa and Escherichia coli. Biochem J. 1982 Mar 1;201(3):621–627. doi: 10.1042/bj2010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Maruyama I. N., Takagaki Y., Tamaki S., Nishimura Y., Hirota Y. Isolation of a mutant of Escherichia coli lacking penicillin-sensitive D-alanine carboxypeptidase IA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2631–2635. doi: 10.1073/pnas.75.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Tamaki S., Curtis S. J., Strominger J. L. Mutational evidence for identity of penicillin-binding protein 5 in Escherichia coli with the major D-alanine carboxypeptidase IA activity. J Bacteriol. 1979 Jan;137(1):644–647. doi: 10.1128/jb.137.1.644-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa J., Tamaki S., Tomioka S., Matsuhashi M. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J Biol Chem. 1984 Nov 25;259(22):13937–13946. [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Nicholas R. A., Ishino F., Park W., Matsuhashi M., Strominger J. L. Purification and sequencing of the active site tryptic peptide from penicillin-binding protein 5 from the dacA mutant strain of Escherichia coli (TMRL 1222). J Biol Chem. 1985 May 25;260(10):6394–6397. [PubMed] [Google Scholar]
- Nicholas R. A., Suzuki H., Hirota Y., Strominger J. L. Purification and sequencing of the active site tryptic peptide from penicillin-binding protein 1b of Escherichia coli. Biochemistry. 1985 Jul 2;24(14):3448–3453. doi: 10.1021/bi00335a009. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Nishimura A., Nishimura Y., Yamada M., Yasuda S., Suzuki H., Hirota Y. Synthetic ColE1 Plasmids carrying genes for penicillin-binding proteins in Escherichia coli. Plasmid. 1981 Jul;6(1):86–98. doi: 10.1016/0147-619x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Tamura T., Suzuki H., Nishimura Y., Mizoguchi J., Hirota Y. On the process of cellular division in Escherichia coli: isolation and characterization of penicillin-binding proteins 1a, 1b, and 3. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4499–4503. doi: 10.1073/pnas.77.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Sequence of active site peptides from the penicillin-sensitive D-alanine carboxypeptidase of Bacillus subtilis. Mechanism of penicillin action and sequence homology to beta-lactamases. J Biol Chem. 1980 May 10;255(9):3964–3976. [PubMed] [Google Scholar]
- Yocum R. R., Rasmussen J. R., Strominger J. L. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem. 1980 May 10;255(9):3977–3986. [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]