Abstract
Fragments encompassing the apical domain of GroEL, called minichaperones, facilitate the refolding of several proteins in vitro without requiring GroES, ATP, or the cage-like structure of multimeric GroEL. We have identified the smallest minichaperone that is active in vitro in chaperoning the refolding of rhodanese and cyclophilin A: GroEL(193–335). This finding raises the question of whether the minichaperones are active under more stringent conditions in vivo. The smallest minichaperones complement two temperature-sensitive Escherichia coli groEL alleles, EL44 and EL673, at 43°C. Although they cannot replace GroEL in cells in which the chromosomal groEL gene has been deleted by P1 transduction, GroEL(193–335) enhances the colony-forming ability of such cells when limiting amounts of GroEL are expressed from a tightly regulated plasmid. Surprisingly, we found that overexpression of GroEL prevents plaque formation by bacteriophage λ and inhibits replication of the λ origin-dependent plasmid, Lorist6. The minichaperones also inhibit Lorist6 replication, but less markedly. The complex quaternary structure of GroEL, its central cavity, and the structural allosteric changes that take place on the binding of nucleotides and GroES are not essential for all of its functions in vivo.
Keywords: protein folding/heat shock/protein biosynthesis/hsp60/cpn60
The GroE complex of Escherichia coli is essential for protein biosynthesis and the recovery of proteins from heat shock (1). It is a complex molecular machine, consisting of fourteen 57.5-kDa subunits of GroEL and seven 10-kDa GroES subunits and is fueled by ATP hydrolysis to prevent protein misfolding and aggregation (2, 3). The mechanism of the molecular chaperone GroEL has engendered much speculation (4–6). Of particular interest is whether its large central cavity (7, 8) acts as a biological test tube or “folding cage” in which proteins can fold productively by avoiding contact with other polypeptide chains and so prevent aggregation and precipitation (4, 9). One approach to tackling the problem has been to dissect the active site of GroEL and examine it in isolation from the complex tetradecameric structure of the intact molecular chaperone (10, 11). Functionally active monomeric minichaperones containing part of the polypeptide binding domain of GroEL (8, 11–13) have been produced that are effective in vitro in solution (10) or monodispersed on a solid support (14). The cage is clearly not necessary for these chaperoning activities tested in vitro. This finding raises the question of whether the minichaperones are active in vivo.
The complex GroE machine is essential for bacterial growth (4, 9), both under normal and under stress growth conditions (15). However, why GroE proteins are essential for cell survival is unknown. Only 10% of all newly synthesized polypeptides are thought to interact with GroEL (5, 16). Different GroEL activities could facilitate the folding of only a few essential cellular proteins (17, 18). The first example of an essential GroE-dependent E. coli protein has been reported recently (19).
Here we investigate whether the cage structure is essential for GroEL function in vivo by searching for minichaperone constructs that are active in vivo. Because the larger minichaperones are less active than the smaller, we have searched first for the smallest construct that is active in vitro. We then examined the in vivo activities of the GroEL minichaperones in three ways. The first was to complement two thermosensitive (ts) groEL mutants, EL44 (Glu-191→Gly) and EL673(Gly-173 → Asp and Gly-337 → Asp), of E. coli strains SV2 and SV6 (20) at 43°C. In a second series of experiments, we analyzed the effect of minichaperone overexpression on growth of E. coli AI90. In this strain, the chromosomal groEL gene has been deleted, and GroEL is expressed instead from a tightly regulated plasmid (21). Finally, we determined whether the minichaperones inhibit the replication of Lorist6 plasmid (22), which uses the bacteriophage λ origin, because, as we show here, overexpression of GroEL alone inhibits λ plaque formation and Lorist6 replication.
MATERIALS AND METHODS
Bacterial and Bacteriophage Strains.
The E. coli strains used in this study were: C41(DE3), a mutant of BL21(DE3) capable of expressing toxic genes (23); SV2 (B178groEL44) and SV6 (B178groEL673), isogenic strains carrying temperature-sensitive alleles of groEL; SV1(=B178) (24), AI90 (ΔgroEL∷kanR) [pBAD-EL] (21), and TG1 (25). λ b2cI (20) was used, according to standard methods (26). Plaque formation was assayed at 30°C.
Plasmid Construction.
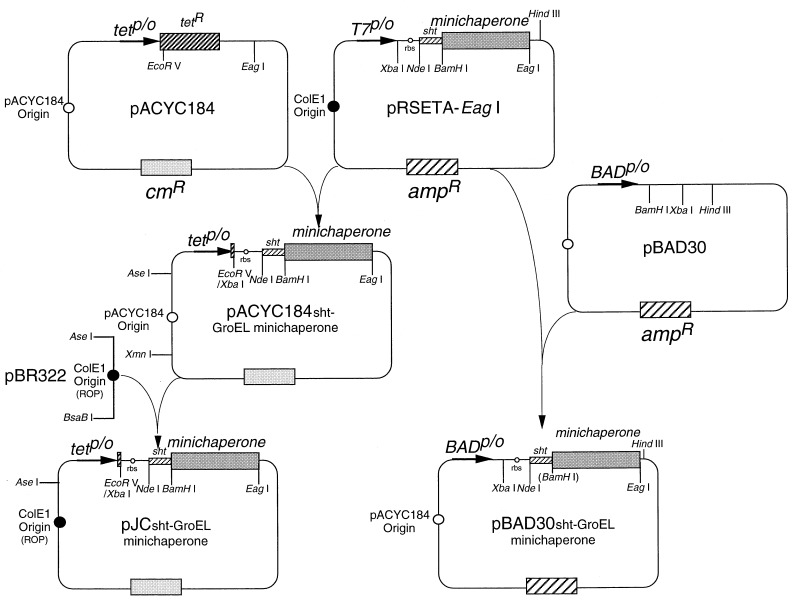
Standard molecular biology procedures were used (27). The principal steps of plasmid construction are summarized in Fig. 1.
Figure 1.
Schematic representation of the plasmid construction and organization. amp, β-lactamase; cm, chloramphenicol acetyl transferase; R, resistance; rbs, ribosome binding site; p/o, promoter/operator: tet, tetracycline. Relevant restriction sites are indicated.
Genomic DNA was extracted from E. coli TG1 (25) by using a commercial kit (Qiagen). PCR using two oligonucleotides 5′-ATT CAT ATG AAT ATT CGT CCA TTG CAT GAT CG-3′ and 5′-AA CGG CCG TTA ATT AAG GTG CAC CGA AAG ATT TAT CCA GAA CTA CG-3′ produced a 494-bp DNA carrying the complete 294 bp of groES gene and unique sites for NdeI and EagI (underlined). This fragment also contains the 44-bp intergenic ES-EL region and the first 123 bp of groEL, including the unique ApaLI site (bold characters) of the groE operon (1). groEL gene was PCR-amplified by using two oligonucleotides, 5′-T AGC TGC CAT ATG GCA GCT AAA GAC GTA AAA TTC GG-3′ and 5′-ATG TAA CGG CCG TTA CAT CAT GCC GCC CAT GCC ACC-3′, producing a 1,659-bp DNA with unique sites for NdeI and EagI (underlined). These fragments were cloned into pRSETA-EagI, allowing expression of GroE proteins under control of a T7 promoter. The unique EagI recognition site (underlined) was created by replacing the EcoRI–HindIII fragment of pRSETA (Invitrogen) by using a synthetic DNA cassette consisting of two oligos: 5′-AAT TCA A CGG CCG TTA-3′ and 5′-AGC TTA ACG GCC GTT G-3′. The pRSETA-EagI (GroESL) vector was generated by subcloning the NdeI–ApaLI fragment from pRSETA-EagI (GroES) into pRSETA-EagI (GroEL) ΔNdeI–ApaLI. Various N- and C-terminally truncated fragments of the apical domain of GroEL were cloned by PCR into BamHI and EagI sites of pRSETA-EagI vector encoding an N-terminal histidine-tail [17 amino acids; a short histidine tail (sht)], which contained an engineered thrombin cleavage site (10). The mutation Y203E was introduced into GroEL by PCR, as described (28), by using oligonucleotides 5′-ATT CAT CAA CAA GCC-3′ and 5′-TCA GGA GAC AGG TAG CC-3′, creating a unique EcoRI site (bold characters).
A modified pACYC184 vector (New England Biolabs) was constructed. The different pRSETA-EagI-based vectors were digested by XbaI, the recessed 3′ ends filled in with Klenow enzyme and then digested by EagI. The XbaI blunt-ended EagI fragments, containing the ribosome binding site of pRSETA, were ligated into pACYC184 EcoRV/EagI-digested and alkaline phosphatase-treated plasmid. pJC vector was generated by replacing the XmnI–AseI fragment of pACYC184 by the AseI–BsaBI of pBR322 prepared in the dam− dcm− JM110 E. coli strain (29). The XbaI–HindIII fragments from pRSETA-EagI-based vectors were cloned into pBAD30 XbaI/HindIII-digested and alkaline phosphatase-treated plasmid. Alternatively, the different groE genes were subcloned into the unique NdeI and EagI sites of pACYC184 or pJC and pBAD30 (30) vectors. Plasmids containing no groE inserts were generated by ligated filled-in recessed 3′ ends of pACYC184 or pJC and pBAD30 ΔNdeI–EagI vectors.
A colony-based PCR procedure (31) was performed to identify the positive clones by using T7 promoter and 3′ reverse cloning oligonucleotides. PCR cycle sequencing using fluorescent dideoxy chain terminators (Applied Biosystems) were performed. Sequencing reactions were analyzed on an Applied Biosystems 373A Automated DNA. All PCR-amplified DNA fragments were sequenced after cloning. The groEL gene differs from the sequence in the database (1) by the substitutions A262L and I267M as described (20).
Protein Production and Characterization.
GroE proteins, ≈57.5-kDa GroEL and ≈10-kDa GroES, were expressed by inducing the T7 promoter of pRSETA-EagI-based vectors with isopropyl-β-d-thiogalactoside in E. coli C41(DE3) (23). Purification was performed as previously described (32). The overexpression and purification of minichaperones in E. coli C41(DE3) cells were carried out essentially as previously described (10). Proteins were analyzed by electrospray mass spectrometry. Protein concentration was determined by absorbance at 276 nm by using the method of Gill and von Hippel (33) and confirmed by quantitative amino acid analysis. Constitutive expression under the control of the tetracycline-resistance gene promoter/operator was obtained either by using the low copy number pACYC184 or the high copy number pJC vectors. pBAD30 vector allows inducible expression with 0.2–0.5% arabinose controlled by the PBAD promoter and its regulatory gene, araC (30). The level of expression of GroEL minichaperones was analyzed by 15% SDS/PAGE under nonreducing conditions followed by Western blotting. After separation of proteins, GroEL molecules were detected with rabbit anti-GroEL antibodies (Sigma) followed by anti-rabbit Igs horseradish peroxidase conjugate antibodies (Sigma). Bromochloroindolyl phosphate/nitro blue tetrazolium chromogens (Sigma) were used as substrate.
In Vitro Refolding Experiments.
Refolding assays of rhodanese (34) and cyclophilin A (10) were carried out as described.
In Vivo Complementation Experiments.
Complementation experiments at 43°C were performed by transforming the ts E. coli strains SV2 or SV6 (20) with the pJC or pBAD30 series of expression vectors. Three-milliliter cultures of appropriately transformed cells were grown overnight at 30°C in Luria–Bertani (LB) medium. Cells (A600 = 0.1) were serially diluted 10-fold in sterile 0.85% (wt/vol) NaCl. Five microliters from each 10-fold dilution was spotted onto each of two LB plates. One plate was incubated overnight at 30°C and the other overnight at 43°C. The number of viable cells/ml of culture was deduced from the number of colonies in the lowest dilution to give single colonies.
P1 transduction (35), using strain AI90 (ΔgroEL∷kanR) [pBAD-EL] as donor (21), was used to delete the groEL gene of TG1 cells transfected by the different pACYC184 or pJC or pBAD30 (sht-GroEL minichaperones) vectors. Transductants were selected on LB plates containing 10 μg/ml of kanamycin at 37°C. Approximately 25 colonies were transferred onto plates containing kanamycin at 50 μg/ml. After incubation for 24 h at 37°C, colonies that grew were screened by PCR as described (21). AI90 (ΔgroEL∷kanR) [pBAD-EL] cells were transformed with the pJC(sht-GroEL minichaperones) vectors. Transformants were selected at 37°C on LB supplemented with 50 μg/ml of kanamycin, 120 μg/ml of ampicillin, 25 μg/ml of chloramphenicol, and 0.2% l(+)-arabinose. Depletion of GroEL protein was analyzed at 37°C by plating the same quantity of AI90 [pBAD-EL + pJC (sht-GroEL minichaperones)] cells on LB plates containing 1% d(+)-glucose or various amounts of arabinose.
Each experiment was performed in triplicate. Plasmids carrying no groE genes or encoding the GroE proteins were used as negative or positive controls, respectively.
Effect on Lorist6 Replication of Overexpressing GroE Proteins or the Minichaperones.
TG1 (25) cells carrying the bacteriophage λ origin vector, Lorist6 that encodes kanamycin resistance (22), were transformed with the pBAD30 series of plasmids. Transformants were selected at 37°C on LB supplemented with 50 μg/ml of kanamycin, 120 μg/ml of ampicillin, and 1.0% D(+)glucose. Ten-fold serial dilutions of overnight cultures were plated onto kanamycin + ampicillin LB with or without 0.2% L(+)arabinose. The effect of overexpressing GroE proteins or minichaperones on Lorist6 replication was determined by comparing the number of colonies forming units (cfu) per ml resistant to kanamycin on plates containing arabinose relative to the number formed on plates lacking arabinose at 37°C.
RESULTS AND DISCUSSION
Identification of Minimal GroEL Minichaperone GroEL(193–335).
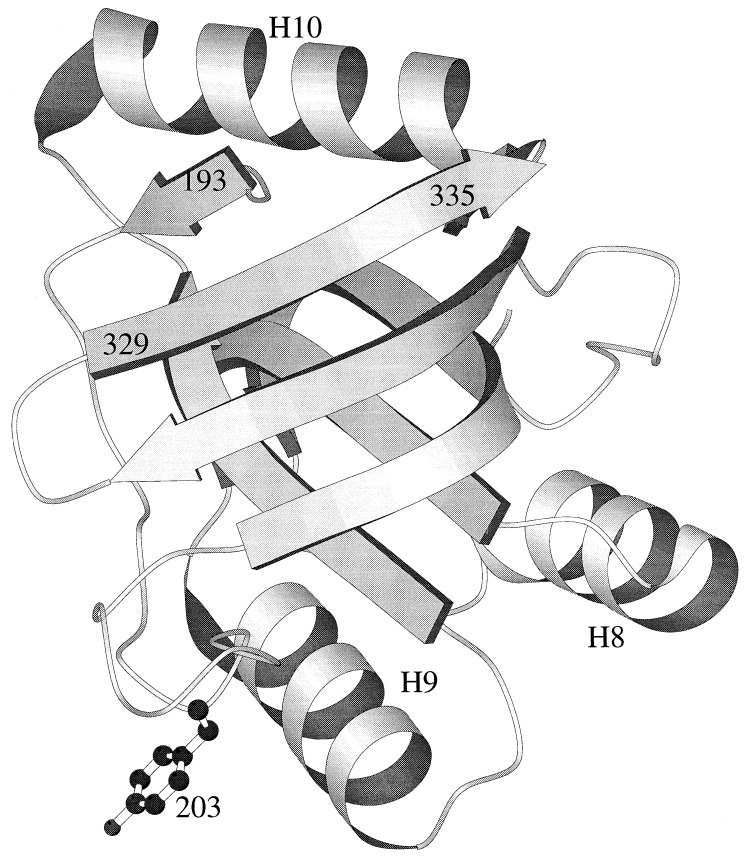
The crystal structure of the active minichaperone GroEL(191–345), solved at 2.5 Å, displays a well-ordered domain with the same fold as in intact GroEL chaperonin (10) (Fig. 2). No electron density was observed for the C-terminal residues 337–345, corresponding to the first half of α-helix H11 (10). Residue Gly-335 is at the end of a β-strand. Further truncation before residue 329 leads to inclusion-body formation, indicating considerable destabilization of the overexpressed GroEL fragment (10). Residue Glu-191 protrudes from the protein surface, making no interactions. Gly-192 NH C-caps α-helix H12, which is absent in the functional monomeric minichaperone GroEL(191–345). The N-terminal β-strand starts at Met-193 in the hydrophobic core. The integrity of the hydrophobic core is considered to stabilize the polypeptide-binding site composed by α-helices H8 and H9 and the surrounding loops (11, 12). On structural grounds, it was predicted that GroEL(193–335) should be the minimal active chaperone unit (Fig. 2).
Figure 2.
Three-dimensional structure of minichaperone GroEL(191–345) solved at 2.5 Å (10). Secondary structure representation is drawn with molscript (41). Positions mentioned in the text are indicated (residues numbered as in ref. 1).
Various N- and C-terminally truncated fragments of the apical domain of GroEL were amplified and cloned downstream of the T7 promoter of pRSETAsht-EagI vector. The minichaperones sht-GroEL(191–345) and sht-GroEL(191–376) were previously expressed with a sht for ease of purification (10). After sonication, the soluble fractions of isopropyl-β-d-thiogalactoside-induced transfected C41(DE3) cells were analyzed by SDS/PAGE. sht-GroEL(193–335) was overexpressed in C41(DE3) cells (23) to give ≈150 mg of purified protein per liter of culture. Further truncations lead to severe aggregation in C41(DE3) cells. Fragments (all with sht) 193–335, 193–336, 193–337, 193–345, 191–335, 191–336, 191–337, and 191–345 all are expressed at 100–150 mg/liter of culture after purification and are highly soluble. 193–330, 195–330 and 195–335 all are poorly expressed and have low solubility. sht-GroEL(193–335) purified by gel filtration is monomeric at μM-mM concentrations, as determined by light scattering (data not shown) and NMR experiments (J.-L. Neira, J.C., and A.R.F., unpublished results). The minichaperone GroEL(193–335), lacking the N-terminal histidine tail was generated by thrombin cleavage of purified sht-GroEL(193–335). The circular dichroism spectra of GroEL(193–335) with or without the sht indicated significant α-helical structure (data not shown). sht-GroEL(193–335) has been found by differential scanning calorimetry experiments to be the most thermostable of the minichaperones with a Tm at about 70°C. The calorimetric data are also consistent with the unfolding of the minichaperone sht-GroEL(193–335) as a monomer (C. M. Johnson, J.C., and A.R.F, unpublished data).
In Vitro Activity of Minichaperone GroEL(193–335).
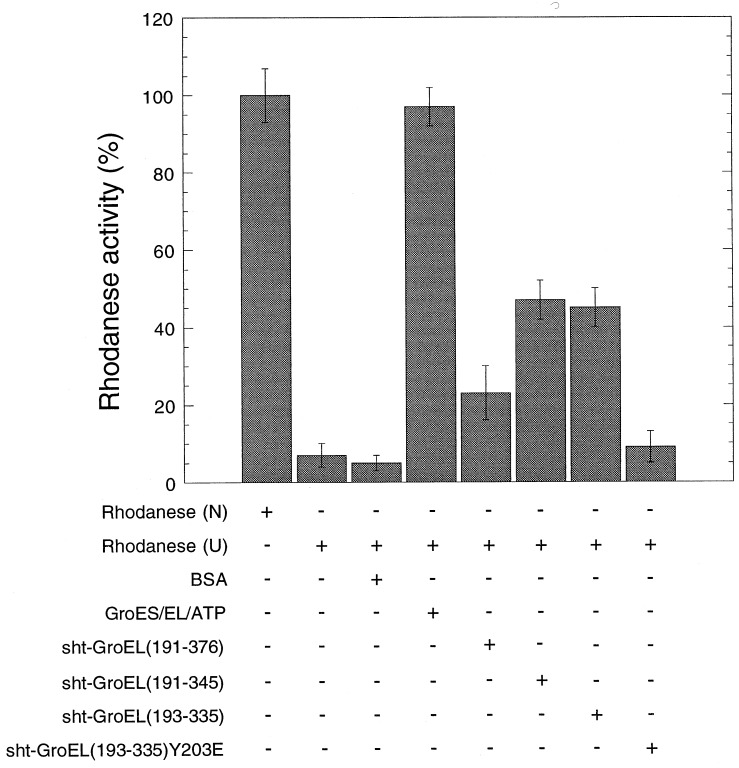
In vitro, rhodanese refolds in high yield only in the presence of GroEL, ATP, and the cochaperonin GroES (36). Minichaperone sht-GroEL(193–335) is as active in vitro as sht-GroEL(191–345) and more active than sht-GroEL(191–376) in chaperoning the folding of rhodanese (Fig. 3). The presence of the N-terminal histidine tail does not abolish binding activity. Residue Y203 is in the polypeptide-binding site of GroEL(191–376) minichaperone (Fig. 2) (11). The mutation Y203E prevents the binding of intact GroEL to the human ornithine transcarbamylase (12). The mutation Y203E similarly abolishes the chaperone activity of sht-GroEL(193–335) (Fig. 3). This finding suggests that the recognition of denatured polypeptide substrates by GroEL and minichaperones uses the same residues.
Figure 3.
In vitro refolding of rhodanese in the presence of GroEL minichaperones. Relative enzymatic activity of rhodanese (0.1 μM) after refolding in the presence (+) or absence (−) of GroEL (2.5 μM monomer), GroES (2.5 μM monomer), ATP (2 mM), sht-GroEL191–376 (2.5 μM), sht-GroEL191–345 (2.5 μM), sht-GroEL193–335 wild-type and mutant Y203E (2.5 μM), or BSA (45 μg/ml), from 8 M urea (U). The yield of refolding activity was measured after 15 min at 25°C. One-hundred percent activity was obtained with native rhodanese (N). Standard error bars are shown.
In Vivo Complementation of ts Alleles by GroEL Minichaperones.
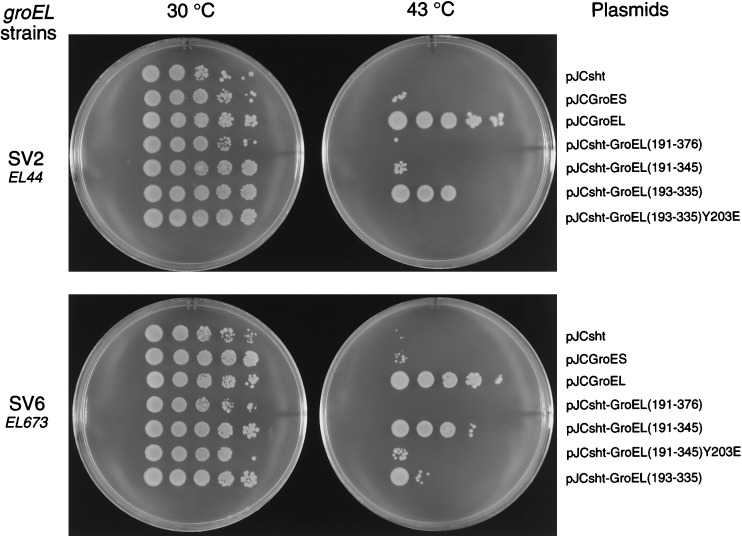
To determine whether GroEL minichaperones could supplement defective GroEL in general cell growth, we sought complementation of two ts groEL mutants of E. coli at 43°C (Fig. 4). E. coli SV2 has the mutation Glu-191 → Gly in GroEL corresponding to groEL44 allele (20), whereas SV6 carries the EL673 allele, which has two mutations, Gly-173 → Asp and Gly-337 → Asp (20). The thermosensitivity at 43°C of the SV2 and SV6 strains is suppressed by transformation of pJCGroESL containing the groE operon (data not shown) or by GroEL alone (Fig. 4). Only the minichaperone sht-GroEL(193–335) complements the defect in SV2. About 45% of the cells, transformed by the vector encoding the minichaperone sht-GroEL(193–335) that grows at the permissive temperature of 37°C, also grow at 43°C (Fig. 4). In contrast, the defective groEL in SV6 was complemented by expression of the minichaperone sht-GroEL(191–345), and less well by sht-GroEL(193–335) (see Fig. 4). About 65% of the cells SV6 transformed with pJCsht-GroEL(191–345), which grow at 37°C, also grow at 43°C (Fig. 4). Colony-forming units were not observed for either strain at 43°C with vectors either lacking inserts or containing the mutation, Y203E, in the minichaperone gene. Thus, the mutation Y203E, which prevents the growth of LG6 E. coli strain (12), also inactivates the minichaperones in vivo. Similar results were obtained by using the pBAD30(sht-GroELminichaperone) expression system (data not shown).
Figure 4.
Suppression of the temperature-sensitive phenotypes of E. coli SV2 (groEL44) and SV6 (groEL673) strains by overexpression of GroEL or minichaperones. The vectors are indicated on the right.
In Vivo Complementation at 37°C.
The effects of minichaperones on the growth at 37°C of a strain of E. coli in which the chromosomal groEL gene had been deleted were analyzed in two ways. First, we attempted to delete the groEL gene of TG1, which had been transformed with the different pJC or pBAD30 (sht-GroEL minichaperone) vectors. This was done by using P1 transduction from the strain AI90 where the chromosomal groEL gene has been precisely replaced by a kanamycin resistance (kanR) cassette (21). However, no kanR transductants could be obtained where the groEL gene had been deleted, unless intact GroEL was expressed from the complementing plasmid. This finding is consistent with the known essential role of GroEL (15).
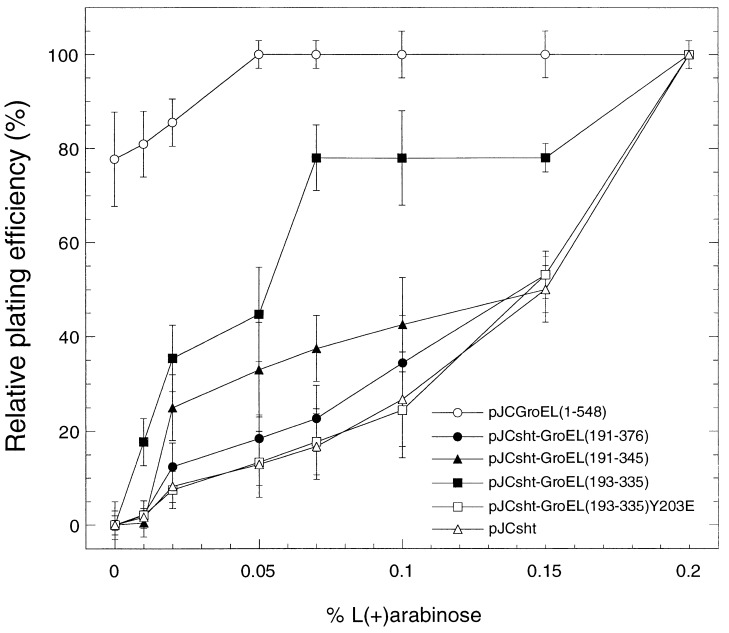
In a second series of experiments, we analyzed the complementation of AI90 (ΔgroEL∷kanR) [pBAD-EL] E. coli strain (21). In this strain, the chromosomal groEL gene has been deleted and GroEL is expressed exclusively from a plasmid-borne copy of the gene, which can be tightly regulated by the arabinose PBAD promoter and its regulatory gene, araC (30). AraC protein acts as either a repressor or an activator depending on the carbon source used. PBAD is activated by arabinose but repressed by glucose (30). The AI90[pBAD-EL] cells cannot grow on medium supplemented with glucose at 37°C (21). None of the minichaperones were able to suppress this groEL growth defect. We then determined whether the constructs could supplement low levels of GroEL from transfected AI90[pBAD-EL] cells by progressively switching on the plasmid-borne groEL gene by increasing arabinose from 0 to 0.2% (Fig. 5). In the absence of arabinose, the only cells that form colonies are those carrying pJC expressing GroEL. About 80% of the cells form colonies compared with the number produced in the presence of 0.2% arabinose, at which concentration all cells are fully induced (100% viability). At 0.01% arabinose, cells transfected with pJC expressing sht alone, sht-GroEL(191–376), sht-GroEL(191–345), or sht-GroEL(193–335)(Y203E) showed little colony-forming ability. But those containing pJC[sht-GroEL(193–335)] produced 15–20% of the number produced in the presence of 0.2% arabinose. At 0.07% arabinose, cells containing pJCGroEL produce as many colonies as those fully induced by 0.2%; pJCsht-GroEL[193–335] 75–80%; pJCsht-GroEL[191–345]; 30–40%; pJCsht-GroEL[191–376] ≈20%; and the two controls, pJCsht-GroEL[193–335(Y203E)] and pJCsht, ≈15%. Thus, pJCsht-GroEL[193–335] can significantly supplement depleted levels of GroEL.
Figure 5.
Supplementation of low levels of GroEL in E. coli by minichaperones. E. coli AI90[pBAD-EL] has the chromosomal groEL gene deleted by P1 transduction (21), and essential GroEL is provided by a plasmid-borne copy of the gene pBAD-EL, which can be tightly regulated by arabinose (30). In the absence of arabinose, the cells are not viable, unless pJCGroEL(1–548) is present, which expresses intact GroEL (○). pJCsht (▵) and pJCGroELsht-(193–335)Y203E (□) plasmids are controls, and cells containing these show increased viability with increasing arabinose concentrations. Overexpression of sht-GroEL(191–376) (•) marginally increases viability; sht-GroEL(191–345) (▴), and (193–335) (■) minichaperones both give significant increases. Each value recorded is the plating efficiency relative to that in the presence of 0.2% arabinose (100%).
Effect on λ Replication of Overexpressing GroEL or the Minichaperones.
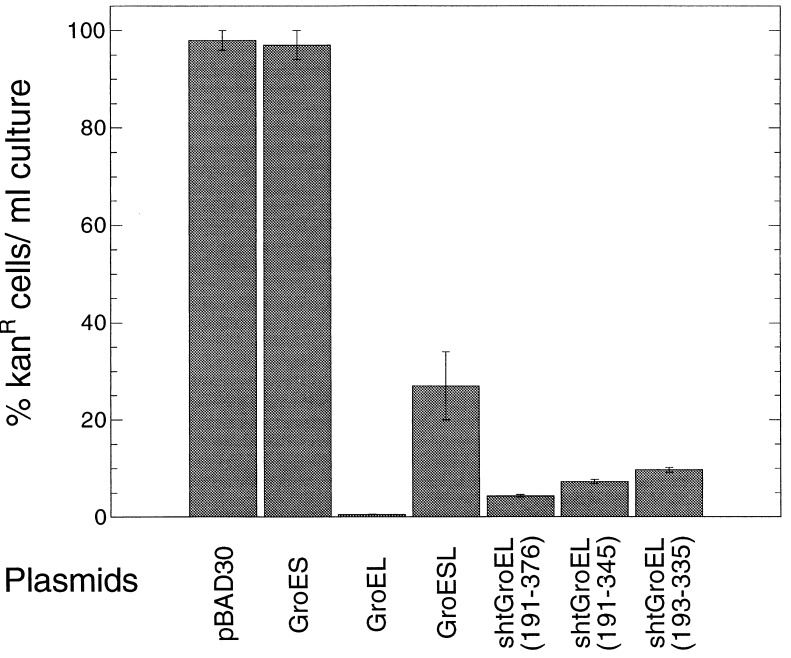
Heat induction of the groE operon has been shown to decrease burst size of λ bacteriophage in E. coli (37). In contrast, we have found that the overexpression of GroEL alone prevents plaque formation by λ in wild-type strains, including SV1. This effect was specific because neither overexpression of GroES alone or together with GroEL caused a significant drop in number of plaques formed. No effects on plaque counts from overexpressing the different minichaperones were found (data not shown). Although the groE operon was named for its effects on the E protein of λ (24), it seems that the main effect of GroEL overexpression is mediated through the λ origin. Both GroEL and the minichaperones inhibit replication of the Lorist6 plasmid (22), which uses the bacteriophage λ origin. A series of cultures of TG1 carrying Lorist6, encoding kanamycin resistance, and each of the pBAD30 vectors were titered on LB plates containing kanamycin and ampicillin in presence or absence of 0.2% arabinose, which induces expression from the PBAD promoter (Fig. 6). The percentage of cells forming kanamycin-resistant colonies in presence of arabinose compared with the number formed in the absence of arabinose (100%) is shown for each of the expression vectors. Loss of kanamycin resistance reflects inhibition of Lorist6 replication. Overexpression of GroES alone has no effect, whereas GroEL decrease the number of kanamycin-resistant colonies 200-fold (Fig. 6). Each of the minichaperones inhibited Lorist6 replication; kanamycin-resistant colonies were decreased by 10- to 23-fold (Fig. 6).
Figure 6.
Effect of overexpressing GroEL or the minichaperones on Lorist6 replication. Cultures of TG1 carrying Lorist6 and one of the pBAD30 series were titered on LB plates containing kanamycin and ampicillin in the presence or absence of 0.2% arabinose at 37°C. The percentage of cells forming kanamycin-resistant colonies in the presence of arabinose compared with the number formed in the absence of arabinose (100%) is shown for each of the pBAD30 series of expression vectors. Lorist6 plasmid encodes resistance to kanamycin and uses the bacteriophage λ origin of replication (22). Arabinose induces expression of the pBAD30 series vectors; loss of kanamycin resistance reflects inhibition of Lorist6 replication.
CONCLUSION
The minimal monomeric minichaperone corresponds to residues 193–335 of the polypeptide-binding fragment of GroEL. It has an activity in vitro facilitating the refolding of cyclophilin A (data not shown) and of rhodanese in the absence of both GroES and ATP. This minimal minichaperone GroEL(193–335) complements the ts groEL44 allele, whereas GroEL(191–345) complements a second ts allele groEL673, at the nonpermissive temperature, 43°C, where different model substrate proteins fail to fold correctly in the cell (38). Complementation at 43°C is a very stringent test of GroEL function, because some known groEL mutants or homologues that complement other ts E. coli mutants well at 37°C do so only very weakly at 43°C (21). In addition, minichaperone GroEL(193–335) significantly complements cells depleted of GroEL (Fig. 5). The mutation Y203E abolishes the activity of minichaperone sht-GroEL(193–335) in vitro and in vivo, just as it has been shown to prevent the binding of GroEL to human ornithine transcarbamylase and the growth of LG6 E. coli strain (12).
We do not yet know why the longest minichaperone GroEL(191–376) is unable to suppress groEL growth defects, whereas the shorter GroEL(191–345) and GroEL(193–335) are effective. All minichaperones have comparable levels of expression at 37°C or 43°C (determined by Western blot, data not shown). But, the results parallel the previous study that showed that the longer constructs are less active in vitro (10). We currently are conducting detailed structural and biophysical analysis to investigate further the differences in their properties.
The groES and groEL genes of E. coli were discovered because mutants interfered with the morphogenesis of bacteriophage λ (39). We found that overexpression of GroEL alone inhibits replication of, and prevents plaque formation by, bacteriophage λ. Although the minichaperones do not inhibit plaque formation, the possibility exists for selecting an improved minichaperone, after mutagenesis, which could confer λ resistance. Overexpression of minichaperones GroEL(191–376/345 or 193–335) inhibits the replication of bacteriophage λ origin dependent plasmids, but to a lesser degree than overexpression of the full-length GroEL (Fig. 6). Clearly the minichaperones are active in vivo, although less active than the full-length wild-type GroEL. The replication of bacteriophage λ origin dependent plasmids requires two proteins, encoded by the bacteriophage genome: O and P. It will be of interest to see whether mutants in either gene O or P can overcome the inhibition of plaque formation by excess GroEL.
It is likely that GroEL has a variety of activities in vivo, and that the minichaperones can substitute for some, but not all of these. It is possible, for example, that the mutations in the ts strains SV2 and SV6 block the heat shock activity of GroEL but that the mutants still can take part in other activities. The central cavity of GroEL and the structural changes seen on binding of nucleotides (40) are thus not strictly essential for all its function in vivo but may be refinements for optimal activity. The main function of GroEL in facilitating folding must be to provide the hydrophobic binding site (10, 11), thus preventing aggregation of folding intermediates and causing the unfolding of compact states (36). The activity of the minichaperones may represent an evolutionary vestige; GroEL may have evolved from an archetypal minichaperone. This vestigial activity is reflected in the specificity of action of minichaperones in solution in vitro. We find that they can enhance the refolding of some, but not all, proteins. For example, proteins that have a significant background of spontaneous refolding, such as rhodanese, cyclophilin, and glyceraldehyde-3-phosphate dehydrogenase (unpublished) have their yields of refolding considerably enhanced. Conversely, other proteins that are refolded under nonpermissive conditions, such as malate dehydrogenase, β-galactosidase, and green fluorescent protein are little affected by the minichaperones (unpublished). Some of the vestigial activity may result just from the exposed hydrophobic surface. Other proteins with similar abilities to bind hydrophobic or denatured substrates may also have a chaperoning activity with the first set of proteins. For example, we find that calmodulin, which binds hydrophobic peptides, can also mimic some of the activities of GroEL and minichaperones (unpublished).
Acknowledgments
We thank Dr. Saskia van der Vies (University of Geneva) for the gift of bacterial and bacteriophage strains. We also thank Dr. Ashley Buckle for preparing Fig. 2. Postdoctoral fellowships from the Federation of European Biochemical Societies (until June 1997) and the European Union (from July 1997) to J.C. are gratefully acknowledged. The Isaac Newton Trust supported F.H.
ABBREVIATIONS
- sht
short histidine tail
- ts
thermosensitive
- LB
Luria–Bertani
References
- 1.Hemmingsen S M, Woolford C, van der Vies S, Tilly K, Dennis D T, Georgopoulos C P, Hendrix R W, Ellis R J. Nature (London) 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 2.Todd M J, Viitanen P V, Lorimer G H. Science. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 3.Weissman J S, Kashi Y, Fenton W A, Horwich A L. Cell. 1994;78:693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 4.Hartl F U. Nature (London) 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 5.Lorimer G H. FASEB J. 1996;10:5–9. doi: 10.1096/fasebj.10.1.8566548. [DOI] [PubMed] [Google Scholar]
- 6.Fenton W A, Horwich A L. Protein Sci. 1997;6:743–760. doi: 10.1002/pro.5560060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saibil H R, Zheng D, Roseman A M, Hunter A S, Watson G M F, Chen S, auf der Mauer A, O’Hara B P, Wood S P, Mann N H, et al. Curr Opin Biol. 1993;3:265–273. doi: 10.1016/0960-9822(93)90176-o. [DOI] [PubMed] [Google Scholar]
- 8.Braig K, Otwinowski Z, Hegde R, Boisvert D C, Joachimiak A, Horwich A L, Sigler P B. Nature (London) 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 9.Ellis R J, Hartl F U. FASEB J. 1996;10:20–26. doi: 10.1096/fasebj.10.1.8566542. [DOI] [PubMed] [Google Scholar]
- 10.Zahn R, Buckle A M, Perret S, Johnson C M J, Corrales F J, Golbik R, Fersht A R. Proc Natl Acad Sci USA. 1996;93:15024–15029. doi: 10.1073/pnas.93.26.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckle A M, Zahn R, Fersht A R. Proc Natl Acad Sci USA. 1997;94:3571–3575. doi: 10.1073/pnas.94.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenton W A, Kashi Y, Furtak K, Horwich A L. Nature (London) 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- 13.Braig K, Adams P D, Brünger A T. Nat Struct Biol. 1995;2:1083–1094. doi: 10.1038/nsb1295-1083. [DOI] [PubMed] [Google Scholar]
- 14.Altamirano M M, Golbik R, Zahn R, Buckle A M, Fersht A R. Proc Natl Acad Sci USA. 1997;94:3576–3578. doi: 10.1073/pnas.94.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayet O, Ziegelhoffer T, Georgopoulos C. J Bacteriol. 1989;171:1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewalt K L, Hendrick J P, Houry W A, Hartl F U. Cell. 1997;90:491–500. doi: 10.1016/s0092-8674(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 17.Zeilstra-Ryalls J, Fayet O, Georgopoulos C. J Bacteriol. 1994;176:6558–6565. doi: 10.1128/jb.176.21.6558-6565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeilstra-Ryalls J, Fayet O, Georgopoulos C. FASEB J. 1996;10:148–152. doi: 10.1096/fasebj.10.1.8566535. [DOI] [PubMed] [Google Scholar]
- 19.McLennan N, Masters M. Nature (London) 1998;392:139. doi: 10.1038/32317. [DOI] [PubMed] [Google Scholar]
- 20.Zeilstra-Ryalls J, Fayet O, Baird L, Georgopoulos C. J Bacteriol. 1993;175:1134–1143. doi: 10.1128/jb.175.4.1134-1143.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivic A, Olden D, Wallington E J, Lund P A. Gene. 1997;194:1–8. doi: 10.1016/s0378-1119(97)00087-5. [DOI] [PubMed] [Google Scholar]
- 22.Gibson T J, Rosenthal A, Waterston R H. Gene. 1987;53:283–286. doi: 10.1016/0378-1119(87)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Miroux B, Walker J E. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos C, Hendrix R W, Casjens S R, Kaiser A D. J Mol Biol. 1973;76:45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- 25.Gibson T J. Ph.D. thesis. United Kingdom: University of Cambridge; 1984. [Google Scholar]
- 26.Arber W, Enquist L, Hohn B, Murray N E, Murray K. In: Lambda II. Hendrix R, Roberts J, Stahl F, Weisberg R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1983. pp. 433–466. [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Hemsley A, Arnheim N, Toney M D, Cortopassi G, Galas D J. Nucleic Acids Res. 1989;17:6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Viera J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 30.Guzman L-M, Belin D, Carson M J, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güssow D, Clackson T. Nucleic Acids Res. 1989;17:4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrales F J, Fersht A R. Folding Design. 1996;1:265–273. doi: 10.1016/s1359-0278(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 33.Gill S C, von Hippel P H. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz P M. In: Chaperonin-Assisted Protein Folding of the Enzyme Rhodanese by GroEL/GroES. Shirley B A, editor. Vol. 40. Clifton, NJ: Humana; 1995. pp. 361–368. [DOI] [PubMed] [Google Scholar]
- 35.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 36.Zahn R, Perrett S, Stenberg G, Fersht A R. Science. 1996;271:642–645. doi: 10.1126/science.271.5249.642. [DOI] [PubMed] [Google Scholar]
- 37.Wegrzyn A, Wegrzyn G, Taylor K. Virology. 1996;217:594–597. doi: 10.1006/viro.1996.0154. [DOI] [PubMed] [Google Scholar]
- 38.Horwich A L, Low K B, Fenton W A, Hirshfield I N, Furtak K. Cell. 1993;74:909–917. doi: 10.1016/0092-8674(93)90470-b. [DOI] [PubMed] [Google Scholar]
- 39.Zeilstra-Ryalls J, Fayet O, Georgopoulos C. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- 40.Roseman A M, Chen S, White H, Braig K, Saibil H R. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- 41.Kraulis P. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]