Abstract
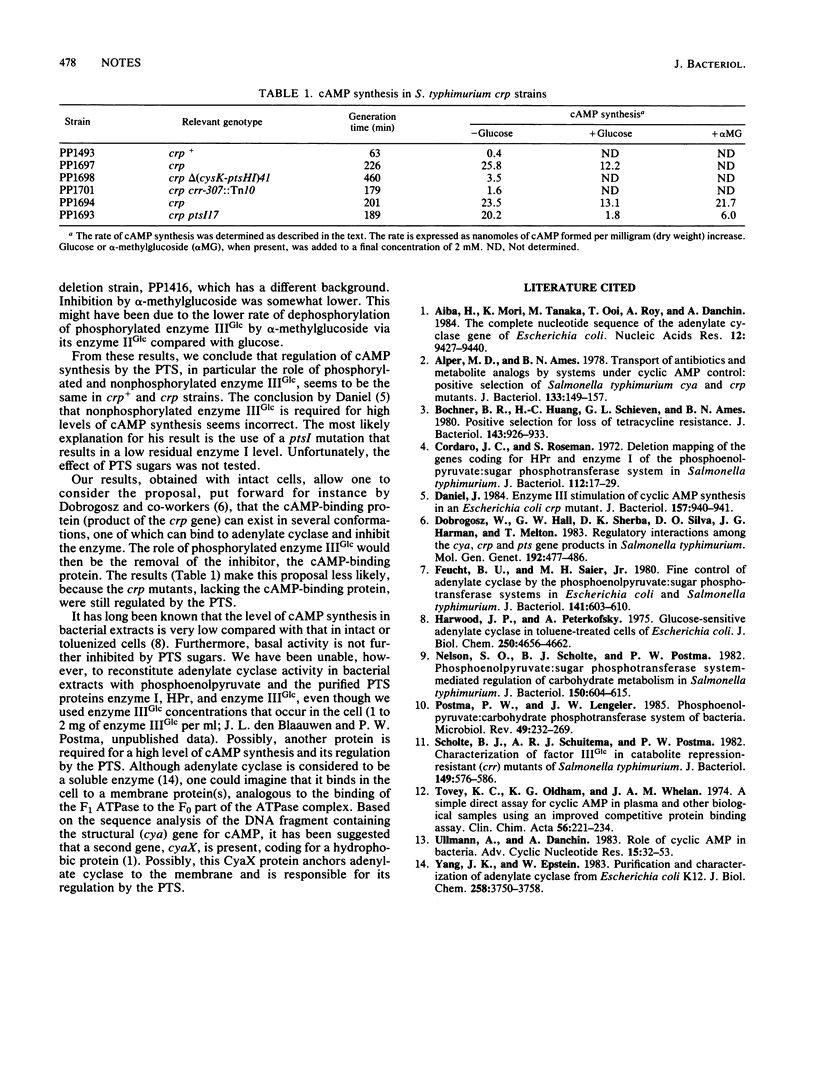
We investigated the claim (J. Daniel, J. Bacteriol. 157:940-941, 1984) that nonphosphorylated enzyme IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system is required for full synthesis of bacterial cyclic AMP (cAMP). In crp strains of Salmonella typhimurium, cAMP synthesis by intact cells was regulated by the phosphorylation state of enzyme IIIGlc. Introduction of either a pstHI deletion mutation or a crr::Tn10 mutation resulted in a low level of cAMP synthesis. In contrast, crp strains containing a leaky pstI mutation exhibited a high level of cAMP synthesis which was inhibited by phosphotransferase system carbohydrates. From these results, we conclude that phosphorylated enzyme IIIGlc rather than nonphosphorylated enzyme IIIGlc is required for full cAMP synthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mori K., Tanaka M., Ooi T., Roy A., Danchin A. The complete nucleotide sequence of the adenylate cyclase gene of Escherichia coli. Nucleic Acids Res. 1984 Dec 21;12(24):9427–9440. doi: 10.1093/nar/12.24.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. Enzyme III stimulation of cyclic AMP synthesis in an Escherichia coli crp mutant. J Bacteriol. 1984 Mar;157(3):940–941. doi: 10.1128/jb.157.3.940-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrogosz W. J., Hall G. W., Sherba D. K., Silva D. O., Harman J. G., Melton T. Regulatory interactions among the cya, crp and pts gene products in Salmonella typhimurium. Mol Gen Genet. 1983;192(3):477–486. doi: 10.1007/BF00392194. [DOI] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Yang J. K., Epstein W. Purification and characterization of adenylate cyclase from Escherichia coli K12. J Biol Chem. 1983 Mar 25;258(6):3750–3758. [PubMed] [Google Scholar]


